Introduction
With its increasing incidence, endometrial carcinoma
(EC) is now the most common gynecological type of cancer in
developed countries (1). The
incidence of EC has also increased in China over the past 20 years,
with an increase of over 100% in the overall mortality rate from EC
during this time (2). Type I
(endometrioid) EC is usually considered to be low risk and is
normally treatable. Conversely, type II EC has a non-endometrioid
histology with a high incidence of deep myometrial invasion and
lymph node metastasis, which leads to a poor prognosis and
significant mortality (3).
Identification of the mechanisms underlying the
invasion of EC is likely to assist in the development of novel
therapeutic approaches. The process of metastasis of EC cells is
complicated. During the process, matrix metalloproteinase (MMP)-2
and -9 play significant roles by degrading the extracellular matrix
(ECM) (4). MMP-2 and -9 also play key
roles in the metastasis of EC and are closely correlated with the
progression of EC (5,6).
Curcumin, an active component of the spice turmeric
(Curcuma longa), has chemopreventive and therapeutic
properties against numerous tumors in vitro and in
vivo (7). However, to date there
have been no studies into the anti-metastatic effect of curcumin on
EC.
In the present study, we aim to elucidate the
anti-metastatic effect of curcumin on EC and its associated
mechanisms.
Materials and methods
Cell lines and cell culture
HEC-1B and Ishikawa cell lines were obtained from
the cell bank of the Chinese Academy of Science (Shanghai, China).
Cells were all cultured in Dulbecco's modified Eagle's medium
(DMEM; Life Technologies, Darmstadt, Germany) or DMEM-F12 with 10%
fetal bovine serum (FBS; Life Technologies, Darmstadt, Germany) and
incubated in a humidified atmosphere of 5% CO2 at
37°C.
Assessment of cell viability
Cell viability was determined by a colorimetric
3-(4,5-dimethylthiazol-2-yl) 2,5-diphenyltetrazolium bromide (MTT)
assay in accordance with previously described protocols. Briefly,
cells were plated in 96-well culture plates (2×104 cells
per well) then treated for 24, 48 and 72 h with various
concentrations of curcumin. The cells were then washed twice with
phosphate-buffered saline (PBS) and incubated with 5 mg/ml MTT
(Sigma-Aldrich, St. Louis, MO, USA) for 4 h. Then, cells were
washed with PBS and solubilized with dimethyl sulfoxide.
Migration and invasion assay
Cells were seeded into a six-well plate and cultured
to 70% confluence in medium containing 10% FBS. Cell monolayers
were scratched with a plastic tip (1 mm) and then incubated in
serum-containing medium (1% serum) with 0, 10, 20 and 30 µM
curcumin for 24 h. The migration distance of the cells was measured
at three sites using Photoshop software (Adobe Systems, Inc., San
Jose, CA, USA). The migration rate was expressed as a percentage of
the control.
The invasion potential of the cancer cells was
assessed in vitro using Transwell chambers (Corning
Incorporated, Corning, New York, NY, USA). First, the upper
chambers were coated with Matrigel (BD Biosciences, San Jose, CA,
USA), then 1×106 cells in serum-free medium were added
to the upper chambers, and FBS (10%) was added to the bottom
chambers. Cells on the bottom side of the filter were fixed,
stained and counted. For transfection experiments, cells were
seeded 24 h after transfection. The rate of invasive cells was
expressed as a percentage of the control.
Construction of expression plasmids
and transfection
The expression plasmids were provided by Dr Chen and
the transfection was performed as described previously (4). Briefly, the full-length pcDNA3.1
(Invitrogen Life Technologies, Carlsbad, CA, USA) MEK1 vector was
prepared by cloning the full-length polymerase chain reaction
product of MEK1 with KOD® DNA polymerase (Toyobo, Osaka, Japan).
DNA sequencing was used to confirm the plasmid sequences. For
transient transfection experiments, cells were plated 24 h prior to
transfection in a six-well plate at a density of 2×105
cells per well. Lipofectamine 2000 (Invitrogen) was used with 4.0
µg pcDNA3.1(+)-MEK1 vector or 4.0 µg pcDNA3.1(+) empty vector as a
negative control in accordance with the manufacturer's
instructions.
Western blotting analysis
Following treatment with various concentrations of
curcumin or U0126 (Sigma), 2×106 cells were suspended in
200 µl lysis buffer (1 mmol/l EDTA, 40 mmol/l Tris-HCl, 150 mmol/l
KCl, 1% Triton X-100, 100 mmol/l NaVO3 and 1 mmol/l
phenylmethylsulfonyl fluoride; pH 7.5). The proteins (80 µg) were
separated by 10 or 12% sodium dodecyl sulphate-polyacrylamide gel
electrophoresis (SDS-PAGE) and then transferred onto polyvinylidene
fluoride membranes. After being blocked in defatted milk (5% in
Tris-buffered saline with Tween-20 buffer) at 37°C, the membranes
were incubated with various antibodies against MMP-2, MMP-9,
extracellular signal-regulated kinase (ERK) 1/2, p-ERK1/2 or
β-actin (all from Cell Signaling Technology, Inc., Danvers, MA,
USA) overnight at 4°C. The membranes were then incubated with
appropriate secondary antibodies for 1 h at room temperature. The
bands were detected and expressed as arbitrary units. Densitometric
analysis was performed using ImageQuant TL software (GE Healthcare,
Chalfont, Bucks, UK).
Zymography
The assay was performed as previously described
(8). Following treatment with various
concentrations of curcumin for 24 h, samples of conditioned cell
media were collected and separated by 0.1% gelatin 8% SDS-PAGE
electrophoresis. Then, the gels were washed twice in 2.5% Triton
X-100 for 45 min at room temperature and then incubated in reaction
buffer (40 mM Tris-HCl, 10 mM CaCl2 and 0.01%
NaN3; pH 8.0) at 37°C for 14 h. The gels were stained
with Coomassie brilliant blue R-250 gel stain. The intensities of
bands on the gels were calculated using an image analysis system
(Bio-Rad Laboratories, Richmond, CA, USA). The final volumes of the
samples were adjusted by counting the viable cell number.
Statistical analysis
Experiments were repeated three times, and dates
were analyzed using Student's t-test. All statistical tests
and corresponding P-values were two-sided. P<0.05 was considered
to indicate a statistically significant difference. Correlation
analysis was performed using the Z-test.
Results
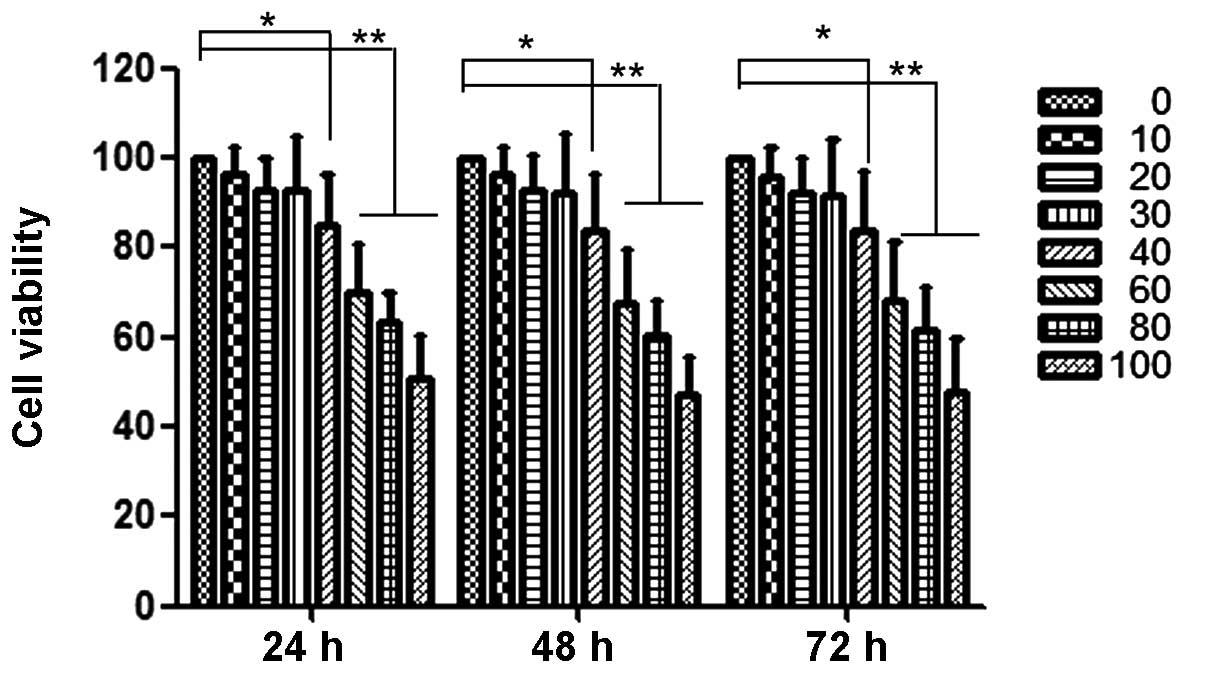
Curcumin inhibits proliferation of
HEC-1B cells
The anti-proliferation effects of curcumin at
various concentrations (0 to 100 µM) on HEC-1B cells are shown in
Fig. 1. At 40 µM, curcumin
significantly inhibited the proliferation of HEC-1B cells. At
concentrations below 40 µM, the anti-proliferative effect was not
evident. Thus, a concentration range of curcumin lower than this
was selected for all subsequent experiments.
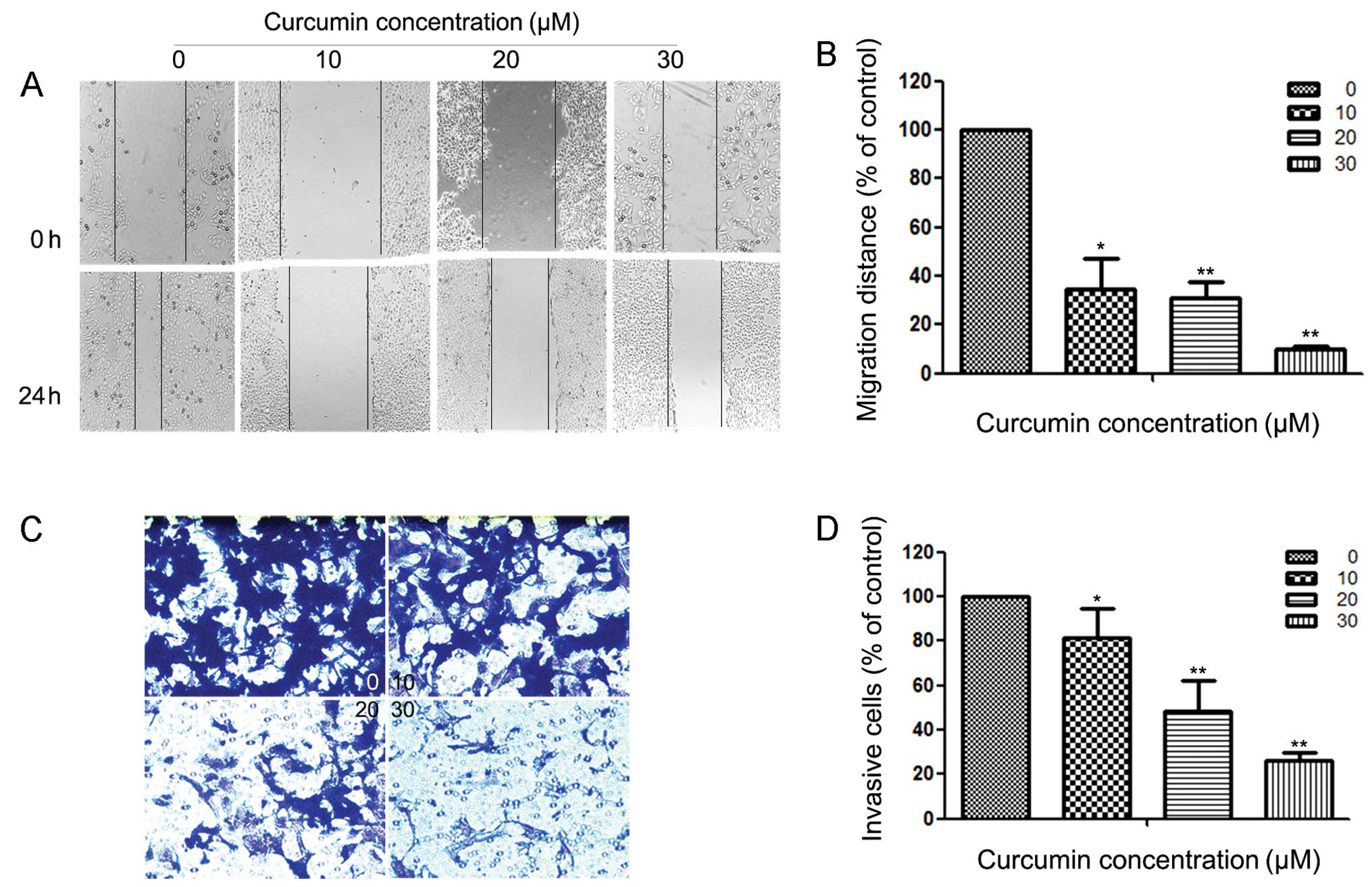
Curcumin inhibits motility and
invasion of HEC-1B cells
Since cell motility is a measure of the metastatic
potential of cancer cells, the motility of HEC-1B cells was
examined. As shown in Fig. 2A, a
continuous rapid movement was observed in control cells. The
movement of HEC-1B cells was significantly reduced following
treatment with curcumin in a concentration-dependent manner; the
inhibition rates were ~65.33, 69.23 and 90.07% at 24 h with 10, 20
and 30 µM curcumin, respectively (Fig.
2B). Fig. 2C reveals the effect
of curcumin on the invasiveness of HEC-1B cells that were treated
with 0, 10, 20 and 30 µM of curcumin for 24 h. Curcumin reduced the
invasion of HEC-1B cells substantially in a concentration-dependent
manner. A similar anti-metastatic effect of curcumin was observed
in Ishikawa cells (results not shown). Quantification analysis
indicated that the invasiveness of HEC-1B cells was reduced by
18.58, 51.89 and 73.58% when cells were treated with 10, 20 and 30
µM of curcumin (Fig. 2D),
respectively. Curcumin also inhibits the invasion of Ishikawa cells
(results not shown).
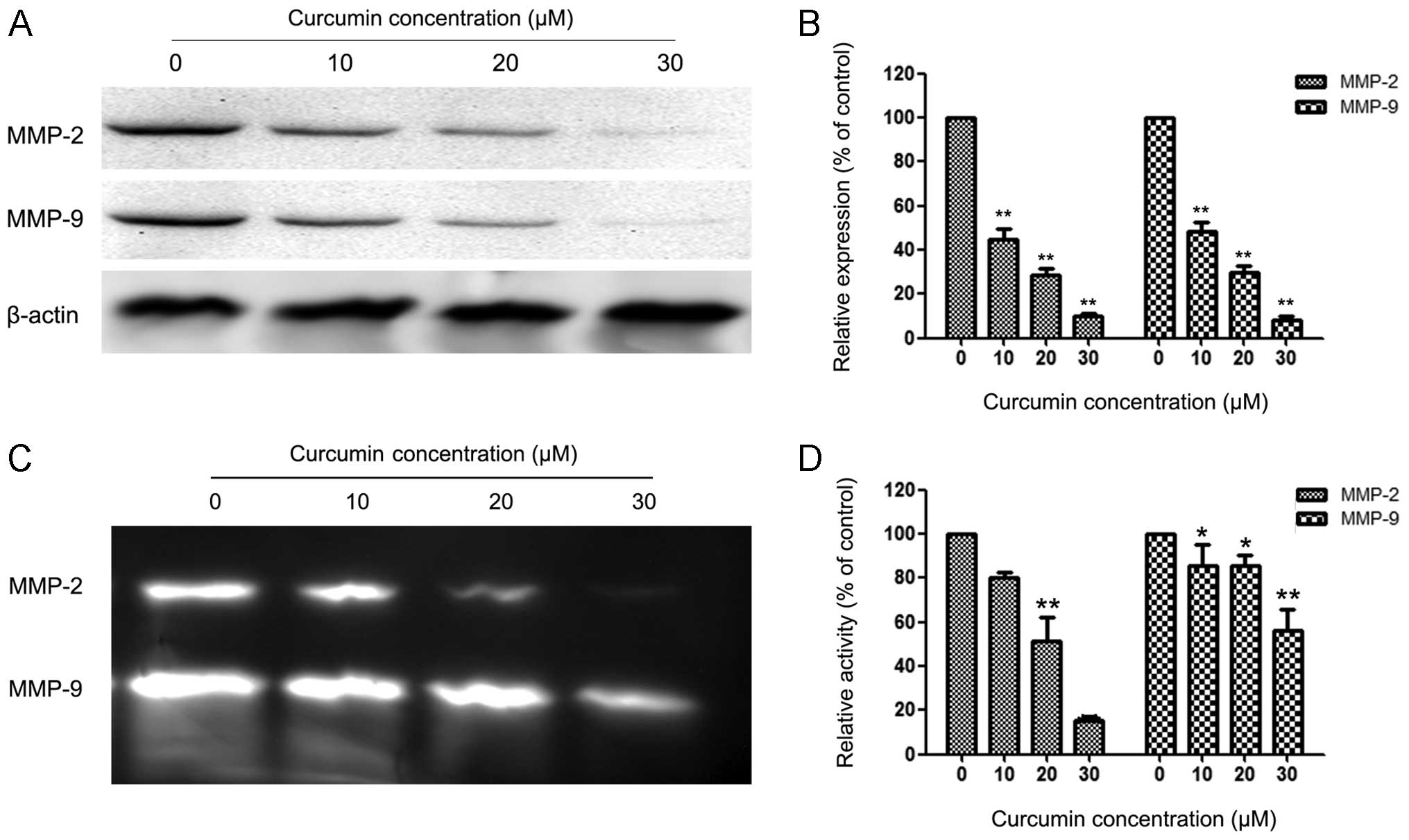
Curcumin suppressed expression and
activity of MMP-2 and MMP-9
MMPs are crucial to cell invasion, so the expression
of MMP-2/-9 in EC cells that were exposed to various concentrations
of curcumin was measured. HEC-1B cells were treated with 0, 10, 20
and 30 µM curcumin for 24 h, and then subjected to western
blotting. Fig. 3A and B reveal that
curcumin significantly reduced the protein levels of MMP-2/-9 in a
concentration-dependent manner compared with the control group in
HEC-1B cells. Fig. 3C and D reveal
similar results for the activity of MMP-2/-9 in HEC-1B cells.
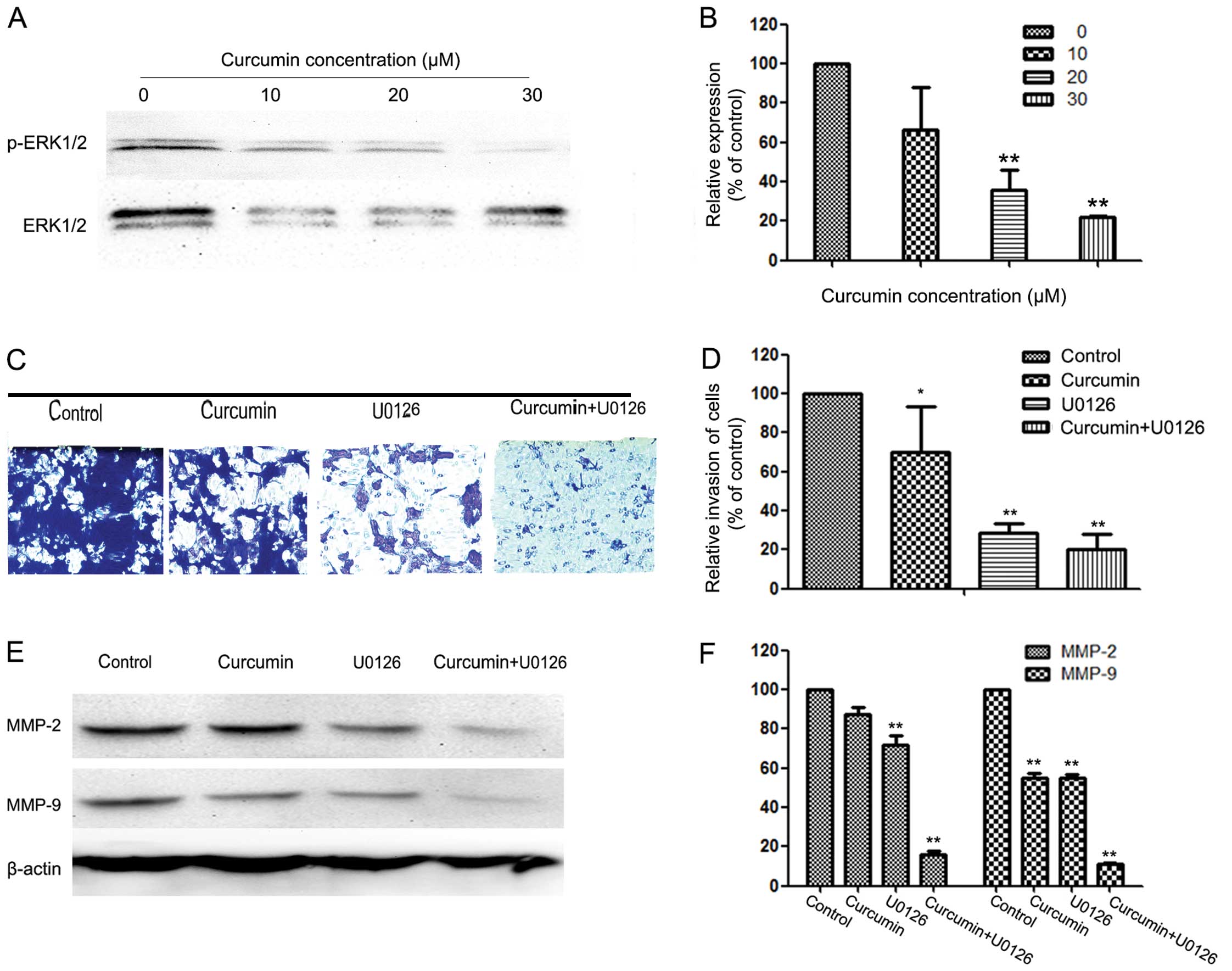
ERK pathway is involved in the
anti-metastatic mechanism of curcumin
To further investigate the mechanisms underlying the
anti-metastatic effect of curcumin, western blotting was used to
detect the expression of ERK1/2 and p-ERK1/2 in HEC-1B cells.
Western blotting revealed that curcumin reduced the phosphorylation
of ERK1/2 in a concentration-dependent manner (Fig. 4A and B).
To further analyze the exact effect of curcumin on
the ERK pathway, we transfected HEC-1B cells with a plasmid
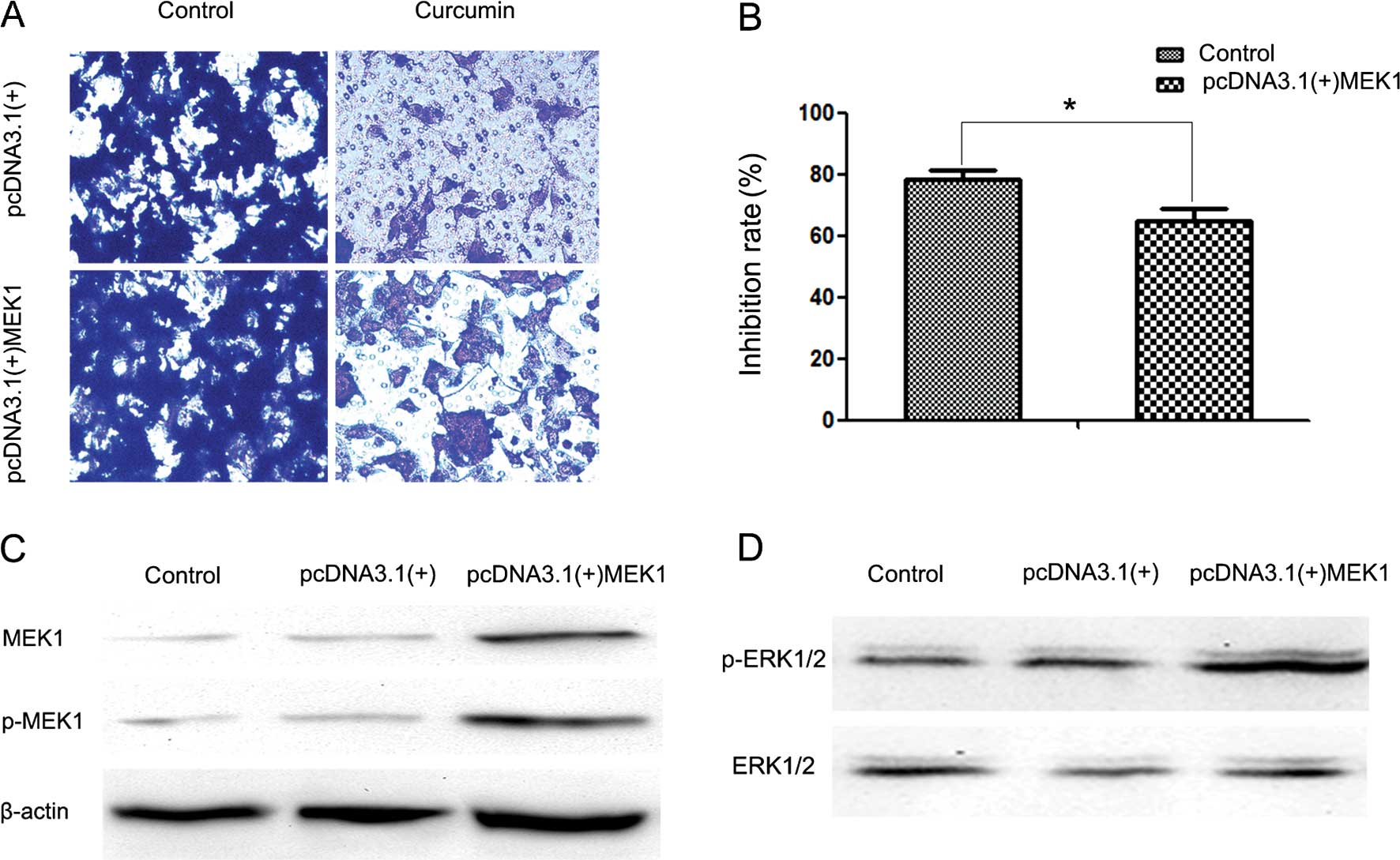
(pcDNA3.1(+)-MEK1) expressing human MEK1 (Fig. 5D). Following transfection, activity of
the ERK signaling pathway was increased (Fig. 5C). It was also observed that MEK1
reversed the inhibitory effect of curcumin on cell invasion
(Fig. 5A and B). The reduction in the
inhibition rate in the pcDNA3.1(+) group and the pcDNA3.1(+)-MEK1
group was ~78.4 and 64.92% following 24 h of treatment with 30 µM
curcumin, respectively.
To further investigate whether the inhibitory effect
of curcumin on cell invasion and MMP-2/-9 expression was correlated
with inhibition of the ERK pathway, HEC-1B cells were pretreated
with an ERK inhibitor (U0126; 10 µM) for 30 min and then incubated
in the presence or absence of curcumin (10 µM) for 24 h. The cells
with the indicated pretreatment were then subjected to in
vitro invasion assay. The results reveal that treatment with
U0126 and curcumin significantly reduced cell invasion (Fig. 4C and D) as well as MMP-2 and -9
protein expression (Fig. 4E and
F).
Discussion
In this study, curcumin was demonstrated to inhibit
the migration and invasion of EC cells by inhibiting the expression
and activity of MMP-2 and -9 through regulation of the ERK
signaling pathway. Curcumin has been demonstrated to possess an
antitumor effect in numerous types of cancer (9–11).
Treatment with either letrozole or curcumin inhibited the
xenografted endometrial tumor growth by inducing apoptosis in tumor
cells, and the combination of letrozole and curcumin further
strengthened the inhibitory effect on tumor growth (12). Another study also identified that
curcumin induced the apoptosis of EC cells (13). However, until now there have been no
studies on the anti-metastatic of effect curcumin on EC cells.
In the study, we observed that curcumin inhibited
the migration of EC cells at non-cytotoxic concentrations. Curcumin
was also observed to inhibit the invasion of EC cells at
non-cytotoxic concentrations. MMP-2 and -9 play a significant role
in the metastasis of tumor cells (14–16). By
degrading the ECM, MMP-2 and -9 make the metastasis of tumor cells
possible. A previous study established that curcumin inhibited the
expression and activity of MMP-2/-9 in tumor cells (17–20). In
the present study, we observed that curcumin inhibited the
expression and activity of MMP-2/-9 in EC cells. The results reveal
that the anti-metastatic effect of curcumin is correlated with
MMP-2/-9.
The ERK signaling pathway is overactivated in a
number of tumors (21). The ERK
signaling pathway plays a crucial role in regulating the
proliferation, survival and invasion of EC (22,23). The
synthesis of MMP-2 and -9 is regulated by a number of signaling
pathways, including the ERK signaling pathway (24–26).
Curcumin has been demonstrated to be capable of inhibiting the
activity of the ERK signaling pathway (27,28). Other
researchers have observed that curcumin inhibited the expression
and activity of MMPs by regulating the ERK signaling pathway
(29,30). In our study, curcumin was demonstrated
to inhibit the activity of the ERK signaling pathway. Combined
treatment with U0126 generated a synergistic effect on the
migration and invasion of EC cells, further reducing the expression
of MMP-2 and -9 in EC cells. Ectopic expression of MEK1 reversed
the inhibitory effect of curcumin on cell invasion.
In conclusion, curcumin inhibits the expression and
activity of MMP-2 and -9 by inhibiting the ERK signaling pathway.
These findings reveal a new potential therapeutic application of
curcumin in anti-metastatic therapy for EC.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (no. 81070536) and the Natural Science
Foundation of Shaanxi Province (no. 2014JM4143).
References
|
1
|
Kitchener HC and Trimble EL: Endometrial
cancer state of the science meeting. Int J Gynecol Cancer.
19:134–140. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sorosky JI: Endometrial cancer. Obstet
Gynecol. 111:436–447. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Barrena Medel NI, Bansal S, Miller DS,
Wright JD and Herzog TJ: Pharmacotherapy of endometrial cancer.
Expert Opin Pharmacother. 10:1939–1951. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen K, Zhang S, Ji Y, et al: Baicalein
inhibits the invasion and metastatic capabilities of hepatocellular
carcinoma cells via down-regulation of the ERK pathway. PLoS One.
8:e729272013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Weigel MT, Krämer J, Schem C, et al:
Differential expression of MMP-2, MMP-9 and PCNA in endometriosis
and endometrial carcinoma. Eur J Obstet Gynecol Reprod Biol.
160:74–78. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Karahan N, Güney M, Baspinar S, Oral B,
Kapucuoglu N and Mungan T: Expression of gelatinase (MMP-2 and
MMP-9) and cyclooxygenase-2 (COX-2) in endometrial carcinoma. Eur J
Gynaecol Oncol. 28:184–188. 2007.PubMed/NCBI
|
|
7
|
Naksuriya O, Okonogi S, Schiffelers RM and
Hennink WE: Curcumin nanoformulations: a review of pharmaceutical
properties and preclinical studies and clinical data related to
cancer treatment. Biomaterials. 35:3365–3383. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Likhter MS, Shelygin Iu A and Achkasov SI:
Multidisciplinary approach to the treatment of colorectal cancer,
complicated by urinary tract invasion. Khirurgiia (Mosk). 12:34–39.
2012.(In Russian). PubMed/NCBI
|
|
9
|
Chen J, Wang FL and Chen WD: Modulation of
apoptosis-related cell signalling pathways by curcumin as a
strategy to inhibit tumor progression. Mol Biol Rep. 41:4583–4594.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wong TF, Takeda T, Li B, et al: Curcumin
targets the AKT-mTOR pathway for uterine leiomyosarcoma tumor
growth suppression. Int J Clin Oncol. 19:354–363. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Ranjan AP, Mukerjee A, Helson L, Gupta R
and Vishwanatha JK: Efficacy of liposomal curcumin in a human
pancreatic tumor xenograft model: inhibition of tumor growth and
angiogenesis. Anticancer Res. 33:3603–3609. 2013.PubMed/NCBI
|
|
12
|
Liang YJ, Hao Q, Wu YZ, Wang QL, Wang JD
and Hu YL: Aromatase inhibitor letrozole in synergy with curcumin
in the inhibition of xenografted endometrial carcinoma growth. Int
J Gynecol Cancer. 19:1248–1252. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Yu Z and Shah DM: Curcumin down-regulates
Ets-1 and Bcl-2 expression in human endometrial carcinoma HEC-1-A
cells. Gynecol Oncol. 106:541–548. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sun W, Liu DB, Li WW, et al: Interleukin-6
promotes the migration and invasion of nasopharyngeal carcinoma
cell lines and upregulates the expression of MMP-2 and MMP-9. Int J
Oncol. 44:1551–1560. 2014.PubMed/NCBI
|
|
15
|
Park SL, Lee EJ, Kim WJ and Moon SK:
p27KIP1 is involved in ERK1/2-mediated MMP-9 expression via the
activation of NF-κB binding in the IL-7-induced migration and
invasion of 5637 cells. Int J Oncol. 44:1349–1356. 2014.PubMed/NCBI
|
|
16
|
Lee KR, Lee JS, Kim YR, Song IG and Hong
EK: Polysaccharide from Inonotus obliquus inhibits migration
and invasion in B16-F10 cells by suppressing MMP-2 and MMP-9 via
downregulation of NF-κB signaling pathway. Oncol Rep. 31:2447–2453.
2014.PubMed/NCBI
|
|
17
|
Lin HJ, Su CC, Lu HF, et al: Curcumin
blocks migration and invasion of mouse-rat hybrid retina ganglion
cells (N18) through the inhibition of MMP-2, -9, FAK, Rho A and
Rock-1 gene expression. Oncol Rep. 23:665–670. 2010.PubMed/NCBI
|
|
18
|
Qi Q, Dai M, Fan H, Zhang B and Yuan X:
Inhibitory effect of curcumin on MMP-2 and MMP-9 expression induced
by polyethylene wear particles and its mechanism. Zhongguo Xiu Fu
Chong Jian Wai Ke Za Zhi. 23:677–682. 2009.(In Chinese). PubMed/NCBI
|
|
19
|
Mitra A, Chakrabarti J, Banerji A,
Chatterjee A and Das BR: Curcumin, a potential inhibitor of MMP-2
in human laryngeal squamous carcinoma cells HEp2. J Environ Pathol
Toxicol Oncol. 25:679–690. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Banerji A, Chakrabarti J, Mitra A and
Chatterjee A: Effect of curcumin on gelatinase A (MMP-2) activity
in B16F10 melanoma cells. Cancer Lett. 211:235–242. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Hoshino R, Chatani Y, Yamori T, et al:
Constitutive activation of the 41-/43-kDa mitogen-activated protein
kinase signaling pathway in human tumors. Oncogene. 18:813–822.
1999. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wang Y, Zhu Y, Zhang L, et al: Insulin
promotes proliferation, survival and invasion in endometrial
carcinoma by activating the MEK/ERK pathway. Cancer Lett.
322:223–231. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Tong JS, Zhang QH, Wang ZB, et al: ER-α36,
a novel variant of ER-α, mediates estrogen-stimulated proliferation
of endometrial carcinoma cells via the PKCδ/ERK pathway. PLoS One.
5:e154082010. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zhang H, Shen B, Swinarska JT, Li W, Xiao
K and He P: 9-Hydroxypheophorbide α-mediated photodynamic therapy
induces matrix metalloproteinase-2 (MMP-2) and MMP-9
down-regulation in Hep-2 cells via ROS-mediated suppression of the
ERK pathway. Photodiagnosis Photodyn Ther. 11:55–62. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Shi MD, Shih YW, Lee YS, Cheng YF and Tsai
LY: Suppression of 12-O-tetradecanoylphorbol-13-acetate-induced
MCF-7 breast adenocarcinoma cells invasion/migration by α-tomatine
through activating PKCα/ERK/NF-κB-dependent MMP-2/MMP-9
expressions. Cell Biochem Biophys. 66:161–174. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dong QZ, Wang Y, Tang ZP, et al: Derlin-1
is overexpressed in non-small cell lung cancer and promotes cancer
cell invasion via EGFR-ERK-mediated up-regulation of MMP-2 and
MMP-9. Am J Pathol. 182:954–964. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Guo Y, Shan Q, Gong Y, et al: Curcumin
induces apoptosis via simultaneously targeting AKT/mTOR and
RAF/MEK/ERK survival signaling pathways in human leukemia THP-1
cells. Pharmazie. 69:229–233. 2014.PubMed/NCBI
|
|
28
|
Xie YQ, Wu XB and Tang SQ: Curcumin
treatment alters ERK-1/2 signaling in vitro and inhibits
nasopharyngeal carcinoma proliferation in mouse xenografts. Int J
Clin Exp Med. 7:108–114. 2014.PubMed/NCBI
|
|
29
|
Mo N, Li ZQ, Li J and Cao YD: Curcumin
inhibits TGF-β1-induced MMP-9 and invasion through ERK and Smad
signaling in breast cancer MDA-MB-231 cells. Asian Pac J Cancer
Prev. 13:5709–5714. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Shin HK, Kim J, Lee EJ and Kim SH:
Inhibitory effect of curcumin on motility of human oral squamous
carcinoma YD-10B cells via suppression of ERK and NF-kappaB
activations. Phytother Res. 24:577–582. 2010.PubMed/NCBI
|