Introduction
Leukemia is a malignant disease of the hematopoietic
system and is one of the ten most common causes of
cancer-associated mortality in the Chinese population, particularly
amongst individuals aged <35 years old (1). Chemotherapy is the most commonly
utilized strategy for the treatment of leukemia (2). Although 50–70% patients achieve
remission following treatment with conventional chemotherapy
regimens, the majority of patients subsequently relapse and may
develop chemoresistance (3–9). An improved understanding of the
molecular mechanisms underlying leukemia may aid the identification
of novel chemotherapeutic regimens, and improve the efficiency of
treatments, therefore prolonging patient survival times. Thus, the
mechanisms underlying leukemia require further investigation.
Previously, by comparing the gene expression and
molecular activity of leukemic stem cells and healthy hematopoietic
stem cells, a number of molecules (interleukin-3R, cluster of
differentiation 47, cluster of differentiation 44) and signaling
pathways [Nuclear factor-κB (NF-κB) (10), Wnt/β-catenin (11)] have been identified to be highly
expressed or activated only in leukemic stem cells. Due to these
results, the NF-κB signaling pathway has attracted the attention of
researchers. NF-κB activity is detectable in the majority of
leukemic cells, and its expression is significantly increased in
leukemic cells compared with that of healthy bone marrow cells
(12). Furthermore, in vitro
studies have identified that NF-κB inhibition is capable of
inducing apoptosis in leukemic stem cells, and apoptosis levels are
significantly increased these cells compared with those of normal
hematopoietic stem cells (10). Due
to the ability of NF-κB inhibitor to induce apoptosis of leukemic
cells, specifically leukemic stem cells, NF-κB inhibitors may
present a potential anti-leukemic therapy (13–15).
However, there are a number of potential side effects of NF-κB
inhibition in the treatment of leukemia, which may limit its
clinical application (16). NF-κB
inhibition not only induces apoptosis in leukemia cells, it
additionally acts on normal cells, inducing inflammatory molecules,
particularly tumor necrosis factor-α (TNF-α), to increase
sensitivity to cell death signals (17). It has been reported that the toxicity
induced by NF-κB inhibition was significantly suppressed in TNF-α
or TNF-α receptor (TnfR) knockout mice (18). Therefore, a combination of NF-κB and
TNF-α inhibition may attenuate the side-effects associated with
inhibition of NF-κB alone (18–24).
Furthermore, TNF-α induces NF-κB-dependent and -independent
survival signaling, therefore promoting proliferation of leukemia
cells (25,26). Based on these previous results, the
present study hypothesized that inhibition of TNF-α signaling may
be able to protect healthy hematopoietic cells and other healthy
tissue cells, while enhancing the anti-leukemic effects of NF-κB
inhibition. A combination of NF-κB and TNF-α inhibition may be a
potential specific and effective novel therapeutic strategy for the
treatment of leukemia. The present study aimed to provide insight
into the synergistic effects of NF-κB and TNF-α inhibition on
leukemia cells. In addition, the underlying molecular mechanisms
were investigated.
Materials and methods
Chemicals
Roswell Park Memorial Institute (RPMI)-1640 and
fetal bovine serum (FBS) were purchased from Hyclone (GE Healthcare
Life Sciences, Logan, UT, USA). Rabbit monoclonal anti-protein
kinase B (Akt) primary antibody used at 1:1,000, and rabbit
anti-TNF-α antibody (TNF-α inhibitor) were obtained from Abcam
(Cambridge, MA, USA). Rabbit polyclonal anti-caspase 9 primary
antibody used at 1:1,000 was purchased from Cell Signaling
Technology, Inc. (Danvers, MA, USA). NF-κB inhibitor (MG-132) was
purchased from Beyotime Institute of Biotechnology (Beijing,
China). Annexin V-fluorescein isothiocyanate (FITC) apoptosis
detection kit was obtained from Beijing Baosai Biotechnology Co.,
Ltd. (Beijing, China).
Cell culture and drug treatment
The HL-60, K562 and K562 with doxorubicin resistance
(K562-ADM) human leukemia cell lines (Shanghai Insititutes for
Biological Sciences, Shanghai, China) were grown in RPMI-1640
medium supplemented with 10% FBS, and maintained in humidified 5%
CO2 at 37°C.
For treatment with anti-TNF-α antibody and MG-132,
cells were plated at a density of 1.5×105 cells/well in
2 ml RPMI-1640 in a 6-well plate. Twenty-four hours later, the
medium was replaced and anti-TNF-α antibody (10 ng/ml) and MG-132
(3 µM) were added, alone or in combination. Cells were incubated at
37°C for 48 h and subsequently used for further experiments.
Detection of apoptosis by flow
cytometry (FCM) and microscopy
In order to evaluate the apoptotic rate the various
cell types, an Annexin V-FITC apoptosis detection kit was used
according to the manufacturer's protocol. Briefly, cells were
collected and resuspended in 200 µl binding buffer(Beijing Baitaike
Biology Company (Beijing, China). Subsequently, 10 µl Annexin
V-FITC and 5 µl propidium iodide (PI) were added to the suspension
and allowed to react for 15 min at room temperature. Following
incubation, 300 µl binding buffer was added and the apoptotic rate
was determined using a FACSCCanto II flow cytometer (BD
Biosciences, Franklin Lakes, NJ, USA). In addition, cell morphology
was observed using a U-TV0 phase contrast microscope (Olympus
Corporation, Tokyo, Japan).
RNA isolation and reverse
transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted using the TRIzol®
(Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA)
method and reverse transcribed using an Invitrogen SuperScript III
First-Strand Synthesis system (Thermo Fisher Scientific, Inc.) to
generate complementary DNA, according to the manufacturer's
protocol. Transcription of GAPDH was examined using the following
primers (Shanghai Sangong Pharmaceutical Co., Ltd., Shanghai,
China): F 5′-AGTCAACGGATTTGGTCGTATT-3′ and R
5′-AATGAGCCCCAGCCTTCT-3′. Primers for Akt were, F
5′-AAGACGACGAGGACTGCTATG-3′ and R 5′-ACCCTTCCGCTTATCCACTGA-3′. PCR
steps were performed using an Applied Biosystems GeneAmp® PCR
System 9700 thermocycler (Thermo Fisher Scientific, Inc.).
RT-PCR was performed using a LightCycler® 480 SYBR
Green I Master (Roche Diagnostics, Burgess Hill, UK) according to
the manufacturer's instructions. Transcription of β-actin was
examined with primers, 5′-TGGCACCCAGCACAATGAA-3′ and
5′-CTAAGTCATAGTCCGCCTAGAAGCA-3′. Primers for Akt were,
5′-CTTCGTCCTCCTCCTCACAC-3′ and, 5′-GCCTGCTTCTCCAACAACA-3′; and
primers for caspase 9 were, 5′-AACAGGCAAGCAGCAAAGTT-3′ and,
5′-CCTCCAGAACCAATGTCCAC-3′. Following an initial denaturation step
at 95°C for 5 min, 40 cycles of amplification for each primer pair
were performed. Each cycle included a denaturation step, 10 sec at
95°C; an annealing step, 20 sec at 60°C; and an elongation step, 10
sec at 72°C. A final elongation phase was performed at 65°C for 1
min. Relative gene expression levels were measured using a
LightCycler 480 (Roche Diagnostics) according to the manufacturer's
protocol. Relative changes in the gene expression levels of caspase
9 and Akt were normalized against the levels of β-actin gene
expression in each sample. Experiments were performed at least in
duplicate for each data point.
Western blotting
Total cells were lysed with buffer (1% sodium
dodecyl sulfate, 10 mm Tris-Cl, pH 7.6, 20 g/ml aprotinin, 20 g/ml
leupeptin and 1 mm 4-(2-aminoethyl) benzenesulfonyl fluoride
hydrochloride; Shanghai Sangong Pharmaceutical Co., Ltd.). Protein
concentrations were determined using the Bradford method. Protein
(20 µg) was separated on 12% SDS-PAGE gels and transferred to
polyvinylidene difluoride membranes (EMD Millipore, Billerica, MA,
USA). Following blocking with 10% non-fat milk, membranes were
incubated with anti-AKT and anti-caspase 9 antibodies (as mentioned
in the Chemicals sections, above) at 4°C overnight. Following
washing three times with TBS, the membranes were incubated with
horseradish peroxidase-conjugated goat anti-rabbit IgG at 1:200
dilution (Abcam, Cambridge, UK) at room temperature for 1 h. The
signals were developed using an enhanced chemiluminescence kit
(Applygen Technologies, Inc., Beijing, China) and rabbit polyclonal
anti-β-actin antibody (dilution, 1:1,000; cat no. ab8227, Abcam)
served as an internal control.
Statistical analysis
All statistical comparisons were performed using
SPSS version 16.0 (SPSS, Inc., Chicago, IL, USA). Student's t-test
was utilized to compare the differences or association between the
two groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Inhibition of TNF-α enhances apoptosis
induced by NF-κB inhibition in leukemia cells
K562 cells were treated with anti-TNF-α antibody
(TNF-α inhibitor) and MG-132 (NF-κB inhibitor) (27), respectively or in combination.
Following treatment, the apoptotic morphology and rate were
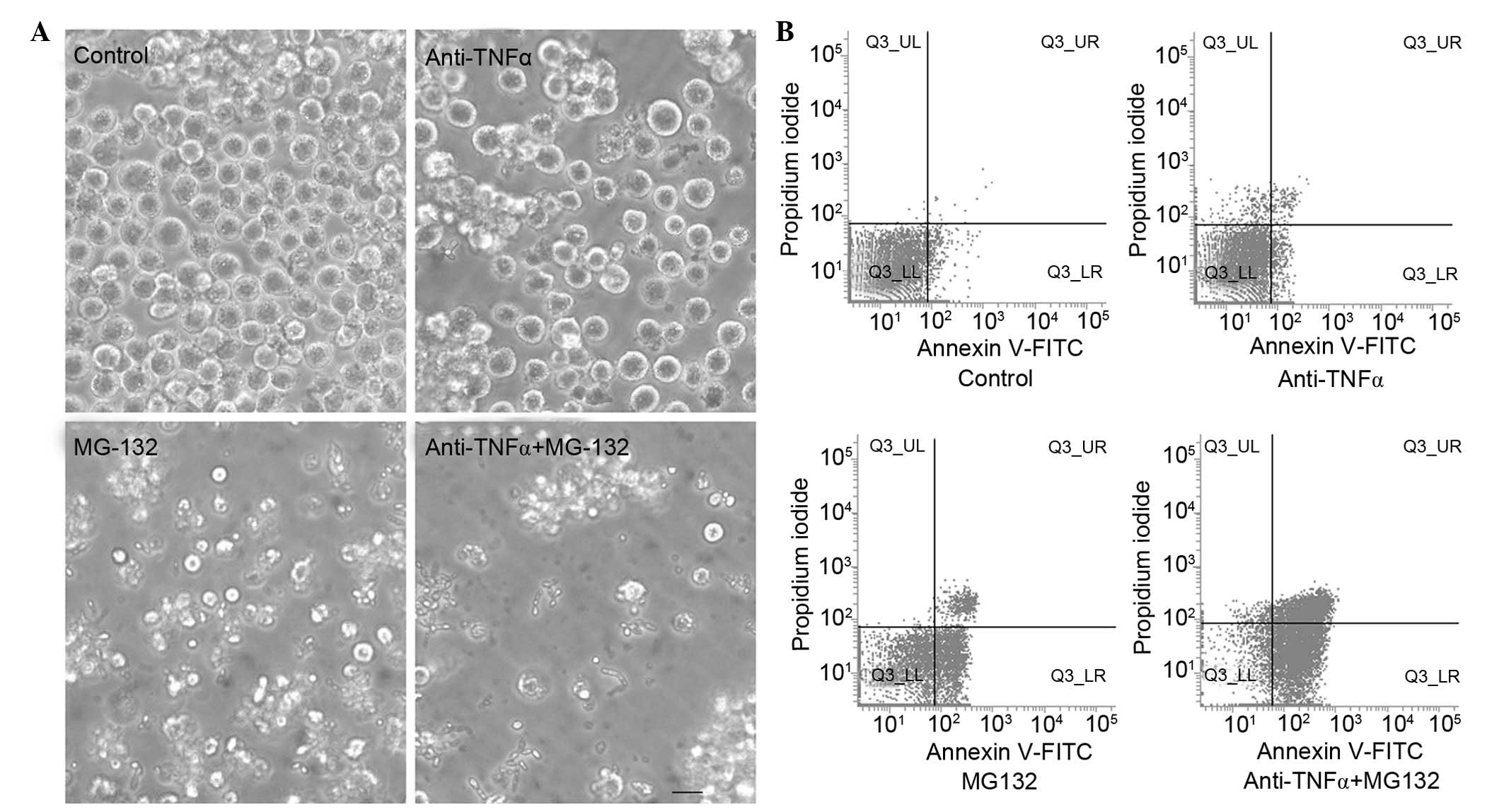
compared with those of the control group. Cell morphology was
observed by phase contrast microscopy. Compared with the control
group, anti-TNF-α antibody or MG-132 treatment induced a typical
apoptotic cell morphology (intense staining, fragmented and
condensed nuclei) in K562 cells, and anti-TNF-α antibody and MG-132
co-treated cells displayed more marked apoptotic morphology
compared with that of the groups treated with anti-TNF-α antibody
or MG-132 separately (Fig. 1A).
Subsequently, the apoptotic rates of the anti-TNF-α
antibody and MG-132 combined treatment group, and the separately
treated groups, were detected using FCM and Annexin V-FITC and PI
immunofluorescence (Fig. 1B). The
results indicated that the apoptotic rate of the combined treatment
group (68.31±4.15%) was increased compared with that of the groups
that were treated separately (anti-TNF-α antibody treatment,
30.5±3.11%; MG-132 treatment, 50.53±2.85%) and the control group
(2.23±1.23%; P<0.05).
In order to confirm that the effects of co-treatment
with TNF-α inhibitor and NF-κB inhibitor were not restricted to
K562 cells, additional types of leukemia cell, including K562-ADM
and HL-60, were investigated (Fig.
2). These cells were also treated with anti-TNF-α antibody and
MG-132, respectively or in combination. Following treatment, the
apoptotic rate was detected via FCM. The apoptotic rates of the
combined treatment group in K562-ADM cells (72.38±2.57%) were
increased compared with those of the separately treated groups
(anti-TNF-α antibody treatment, 18.37±1.35%; MG-132 treatment,
46.35±3.65%) and the control group (3.25±2.33%; P<0.05). The
apoptotic rate of the combined treatment group in HL-60 cells
(78.65±3.72%) was also increased compared with the separately
treated groups (anti-TNF-α antibody treatment, 6.85±1.42%; MG-132
treatment, 57.44±2.46%) and the control group (1.46±2.28%;
P<0.05).
Treatment with a combination of
anti-TNF-α antibody and MG-132 promotes Akt expression
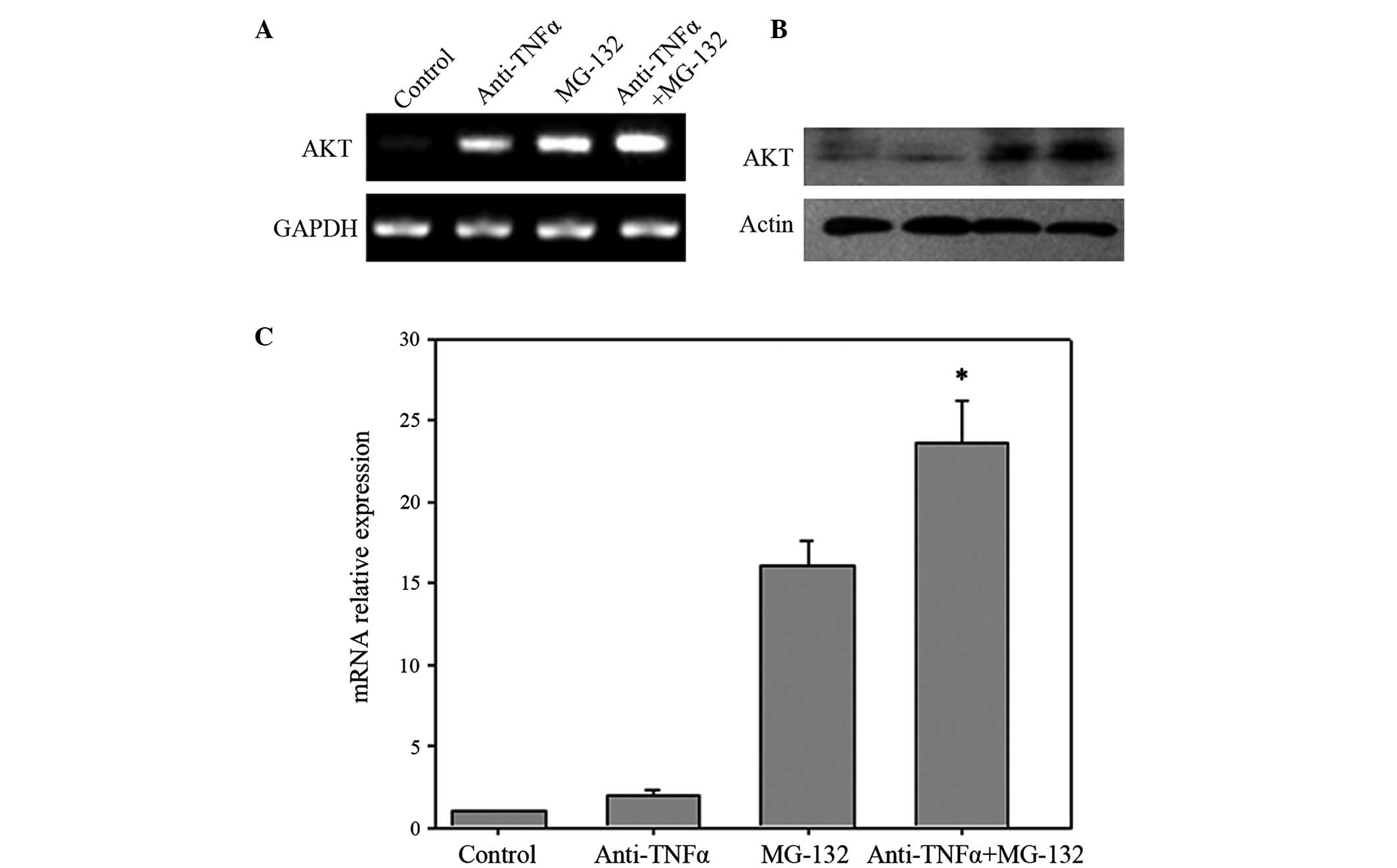
Akt is located downstream of TNF-α and NF-κB
signaling (28). The messenger RNA
(mRNA) and protein expression levels of Akt in anti-TNF-α antibody
and MG-132 co-treated cells, separately treated cells and control
cells were therefore detected by RT-PCR (Fig. 3A), western blotting (Fig. 3B) and RT-quantitative (q) PCR
(Fig. 3C). The results of the present
study revealed that the expression levels of Akt were increased in
anti-TNF-α antibody or MG-132 treated cells, while co-treated cells
expressed increased levels of Akt compared with those of the
separately treated cells or control cells, in terms of mRNA and
protein.
Combined treatment with anti-TNF-α
antibody and MG-132 promotes caspase 9 activation
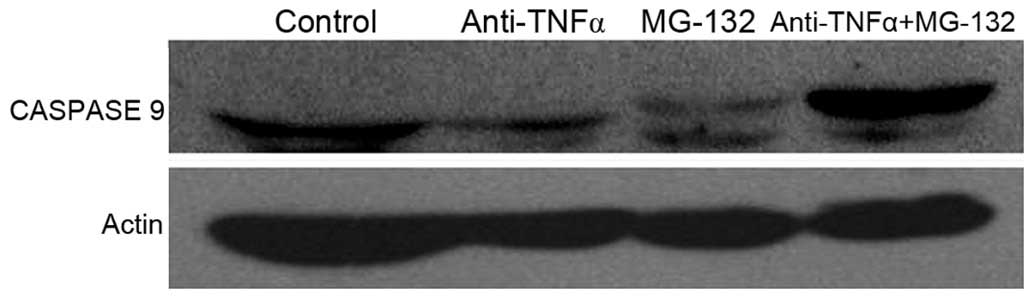
Caspase 9 is activated in the intrinsic apoptotic
pathway (29), therefore in the
present study, expression and activation of caspase 9 in anti-TNF-α
antibody and MG-132 co-treated, separately-treated and control
cells, was investigated using western blotting. Caspase 9 was
identified to be highly expressed and activated in co-treated
cells, compared with control cells and separately treated cells
(Fig. 4). Caspase 9 activation was
promoted following TNF-α and NF-κB inhibitor co-treatment.
Discussion
NF-κB is a nuclear transcription factor, which may
mediate survival pathways in a number of types of cancer, including
leukemia (30). NF-κB induces the
expression of genes involved in cell proliferation, angiogenesis
and metastasis, and possesses significant roles in carcinogenesis
and chemoresistance (31). NF-κB
activity is detectable in the majority of leukemic cells, and its
expression has been observed to be significantly increased in
leukemic cells compared with that of healthy bone marrow cells
(32,33). NF-κB inhibition exerts antitumor
activity in leukemia cells (27).
Cosimo et al (34) reported
that inhibition of NF-κB was able to induce apoptosis in chronic
lymphocytic leukemia cells. Therefore, inhibition of the activity
of NF-κB may be a potential novel therapeutic strategy for the
treatment of leukemia. However, a significant limitation of NF-κB
inhibition in the treatment of leukemia is the low efficiency.
NF-κB inhibition induces apoptosis in leukemia cells, however
additionally causes inflammatory molecules (particularly TNF-α) to
increase the sensitivity of normal hematopoietic cells to cell
death signals (18), which limit its
clinical application.
TNF-α is a central regulator of inflammation, which
exerts various functions in leukemia and normal hematopoietic cells
(35). TNF-α is continually expressed
in a number of types of leukemia cell (36), and has been demonstrated to upregulate
certain molecules involved in cell growth and proliferation via the
NF-κB-dependent or -independent pathway in leukemia (37). TNF-α is critical for the growth and
survival of leukemic cells. However, in healthy hematopoietic
cells, TNF-α induces death signaling (20). TNF-α-induced death signaling has been
observed to be enhanced by NF-κB inhibition in healthy
hematopoietic cells. The toxicity induced by NF-κB inhibition was
observed to be significantly suppressed in TNF-α or TnfR knockout
mice (25). In the present study, it
was suggested that inhibition of TNF-α may be capable of enhancing
NF-κB inhibitor-induced apoptosis in leukemia cells. Combined
treatment with NF-κB and TNF-α inhibitors may be a specific and
effective novel therapeutic strategy for the treatment of leukemia.
Leukemia cells were treated with anti-TNF-α antibody and MG-132,
respectively or in combination, and the apoptotic rates of these
various treatment groups were compared. It was identified that
combination treatment with anti-TNF-α antibody and MG-132 enhanced
apoptosis in a number of leukemia cell types, including K562 and
HL-60 cells. K562-ADM is a doxorubicin-resistant leukemia cell
line. In K562-ADM cells, which are chemoresistant, a significant
increase in apoptosis was also observed following co-treatment with
MG-132 and anti-TNF-α antibody.
The molecular mechanism by which inhibition of TNF-α
and NF-κB promotes apoptosis in leukemia cells was additionally
elucidated. Previously, it was demonstrated that Akt is a
downstream molecule influenced by NF-κB signaling (38). In addition, TNF-α induced activation
of the phosphoinositide 3-kinase/Akt pathway, and therefore the
transcriptional activation of NF-κB, in C4HD murine mammary tumor
cells (28). In the present study,
the expression of Akt in the presence of anti-TNF-α antibody and
MG-132, respectively or in combination, was detected. The present
study identified that the expression of Akt was significantly
increased, at the mRNA and protein levels, when leukemia cells were
treated with anti-TNF-α antibody and MG-132 in combination. This
result further confirmed the involvement of Akt in the regulation
of TNF-α and NF-κB inhibition-induced apoptosis in K562 cells.
The results of the present study also revealed that
caspase 9 was activated following anti-TNF-α antibody and MG-132
co-treatment. However, low levels of cleavage of caspase 9 were
identified in cells treated with anti-TNF-α antibody or MG-132
alone. Caspase 9 is activated during the intrinsic apoptotic
pathway (29). In K562 cells,
inhibition of NF-κB and TNF-α signaling induced activation of the
intrinsic apoptotic pathway, during which caspase 9 was
activated.
In conclusion, the present study revealed that
inhibition of TNF-α enhanced NF-κB inhibition-induced apoptosis in
leukemia cells. Akt was significant in the regulation of TNF-α and
NF-κB inhibition-induced apoptosis. During this process, the
intrinsic apoptotic pathway was activated. A combination of
treatment with NF-κB inhibitor and TNF-α inhibitor may therefore
present a specific and effective novel therapeutic strategy for the
treatment of leukemia.
Acknowledgements
The present study was supported by the Fundamental
Research Fund for the Central Universities (grant no.
lzujbky-2013-150), the Science and Technology Plan Project in Gansu
Province (grant no. 1308RJYA071) and the Research Program of the
First Hospital of Lanzhou University (grant no.
ldyyynlc201101).
References
|
1
|
Elbahesh E, Patel N and Tabbara IA:
Treatment of acute promyelocytic leukemia. Anticancer Res.
34:1507–1517. 2014.PubMed/NCBI
|
|
2
|
Lynch RC and Medeiros BC: Chemotherapy
options for previously untreated acute myeloid leukemia. Expert
Opin Pharmacother. 16:2149–2162. 2015.PubMed/NCBI
|
|
3
|
Tallman MS, Gilliland DG and Rowe JM: Drug
therapy for acute myeloid leukemia. Blood. 106:1154–1163. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chomel JC, Bonnet ML, Sorel N, Bertrand A,
Meunier MC, Fichelson S, Melkus M, Bennaceur-Griscelli A, Guilhot F
and Turhan AG: Leukemic stem cell persistence in chronic myeloid
leukemia patients with sustained undetectable molecular residual
disease. Blood. 118:3657–3660. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Schrappe M, Valsecchi MG, Bartram CR,
Schrauder A, Panzer-Grümayer R, Möricke A, Parasole R, Zimmermann
M, Dworzak M, Buldini B, et al: Late MRD response determines
relapse risk overall and in subsets of childhood T-cell ALL:
Results of the AIEOP-BFM-ALL 2000 study. Blood. 118:2077–2084.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Oran B and de Lima M: Prevention and
treatment of acute myeloid leukemia relapse after allogeneic stem
cell transplantation. Curr Opin Hematol. 18:388–394. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Campana D: Progress of minimal residual
disease studies in childhood acute leukemia. Curr Hematol Malig
Rep. 5:169–176. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ravindranath Y: Recent advances in
pediatric acute lymphoblastic and myeloid leukemia. Curr Opin
Oncol. 15:23–35. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Rowe JM and Goldstone AH: How I treat
acute lymphocytic leukemia in adults. Blood. 110:2268–2275. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Guzman ML, Neering SJ, Upchurch D, Grimes
B, Howard DS, Rizzieri DA, Luger SM and Jordan CT: Nuclear
factor-kappaB is constitutively activated in primitive human acute
myelogenous leukemia cells. Blood. 98:2301–2307. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Wang Y, Krivtsov AV, Sinha AU, North TE,
Goessling W, Feng Z, Zon LI and Armstrong SA: The Wnt/beta-catenin
pathway is required for the development of leukemia stem cells in
AML. Science. 327:1650–1653. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bellavia D, Campese AF, Alesse E, Vacca A,
Felli MP, Balestri A, Stoppacciaro A, Tiveron C, Tatangelo L,
Giovarelli M, et al: Constitutive activation of NF-kappaB and
T-cell leukemia/lymphoma in Notch3 transgenic mice. Embo J.
19:3337–3348. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Hadian K and Krappmann D: Signals from the
nucleus: Activation of NF-kappaB by cytosolic ATM in the DNA damage
response. Sci Signal. 4:pe22011. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Miyamoto S: Nuclear initiated NF-κB
signaling: NEMO and ATM take center stage. Cell Res. 21:116–130.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Wu ZH and Miyamoto S: Induction of a
pro-apoptotic ATM-NF-kappaB pathway and its repression by ATR in
response to replication stress. EMBO J. 27:1963–1973. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sakurai T, Maeda S, Chang L and Karin M:
Loss of hepatic NF-kappa B activity enhances chemical
hepatocarcinogenesis through sustained c-Jun N-terminal kinase 1
activation. Proc Natl Acad Sci USA. 103:10544–10551. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Igarashi H, Baba Y, Nagai Y, Jimi E, Ghosh
S and Kincade PW: NF-kappaB is dispensable for normal lymphocyte
development in bone marrow but required for protection of
progenitors from TNFalpha. Int Immunol. 18:653–659. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wullaert A, van Loo G, Heyninck K and
Beyaert R: Hepatic tumor necrosis factor signaling and nuclear
factor-kappaB: Effects on liver homeostasis and beyond. Endocr Rev.
28:365–386. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Oguma K, Oshima H and Oshima M:
Inflammation, tumor necrosis factor and Wnt promotion in gastric
cancer development. Future Oncol. 6:515–526. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Balkwill F: Tumour necrosis factor and
cancer. Nat Rev Cancer. 9:361–371. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Geisler F, Algül H, Paxian S and Schmid
RM: Genetic inactivation of RelA/p65 sensitizes adult mouse
hepatocytes to TNF-induced apoptosis in vivo and in vitro.
Gastroenterology. 132:2489–2503. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hatano E: Tumor necrosis factor signaling
in hepatocyte apoptosis. J Gastroenterol Hepatol. 22(Suppl 1):
S43–S44. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Quante M and Wang TC: Inflammation and
stem cells in gastrointestinal carcinogenesis. Physiology
(Bethesda). 23:350–359. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee YR, Yu HN, Noh EM, Youn HJ, Song EK,
Han MK, Park CS, Kim BS, Park YS, Park BK, et al: TNF-alpha
upregulates PTEN via NF-kappaB signaling pathways in human leukemic
cells. Exp Mol Med. 39:121–127. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kagoya Y, Yoshimi A, Kataoka K, Nakagawa
M, Kumano K, Arai S, Kobayashi H, Saito T, Iwakura Y and Kurokawa
M: Positive feedback between NF-κB and TNF-α promotes
leukemia-initiating cell capacity. J Clin Invest. 124:528–542.
2014. View
Article : Google Scholar : PubMed/NCBI
|
|
27
|
Morotti A, Cilloni D, Pautasso M, Messa F,
Arruga F, Defilippi I, Carturan S, Catalano R, Rosso V, Chiarenza
A, et al: NF-κB inhibition as a strategy to enhance
etoposide-induced apoptosis in K562 cell line. Am J Hematol.
81:938–945. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Rivas MA, Carnevale RP, Proietti CJ,
Rosemblit C, Beguelin W, Salatino M, Charreau EH, Frahm I, Sapia S,
Brouckaert P, et al: TNF alpha acting on TNFR1 promotes breast
cancer growth via p42/P44 MAPK, JNK, Akt and NF-kappa B-dependent
pathways. Exp Cell Res. 314:509–529. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Mueller T, Voigt W, Simon H, Fruehauf A,
Bulankin A, Grothey A and Schmoll HJ: Failure of activation of
caspase-9 induces a higher threshold for apoptosis and cisplatin
resistance in testicular cancer. Cancer Res. 63:513–521.
2003.PubMed/NCBI
|
|
30
|
Giuliani C, Napolitano G, Bucci I, Montani
V and Monaco F: Nf-κB transcription factor: Role in the
pathogenesis of inflammatory, autoimmune, and neoplastic diseases
and therapy implications. Clin Ter. 152:249–253. 2001.(In Italian).
PubMed/NCBI
|
|
31
|
Baud V and Jacque E: The alternative NF-κB
activation pathway and cancer: Friend or foe? Med Sci (Paris).
24:1083–1088. 2008.(In French). View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Moitreyee CK, Suraksha A and Swarup AS:
Potential role of NF-κB and RXR beta like proteins in interferon
induced HLA class I and beta globin gene transcription in K562
erythroleukaemia cells. Mol Cell Biochem. 178:103–112. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kamieńska E, Ociepa T, Wysocki M, Kurylak
A, Matysiak M, Urasiński T, Urasińska E and Domagała W: Activation
of NF-κB in leukemic cells in response to initial prednisone
therapy in children with acute lymphoblastic leukaemia: Relation to
other prognostic factors. Pol J Pathol. 62:5–11. 2011.PubMed/NCBI
|
|
34
|
Cosimo E, McCaig AM, Carter-Brzezinski LJ,
Wheadon H, Leach MT, Le Ster K, Berthou C, Durieu E, Oumata N,
Galons H, et al: Inhibition of NF-κB-mediated signaling by the
cyclin-dependent kinase inhibitor CR8 overcomes prosurvival stimuli
to induce apoptosis in chronic lymphocytic leukemia cells. Clin
Cancer Res. 19:2393–2405. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wu X, Xu W, Feng X, He Y, Liu X, Gao Y,
Yang S, Shao Z, Yang C and Ye Z: TNF-a mediated inflammatory
macrophage polarization contributes to the pathogenesis of
steroid-induced osteonecrosis in mice. Int J Immunopathol
Pharmacol. 28:351–361. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Schulz U, Munker R, Ertl B, Holler E and
Kolb HJ: Different types of human leukemias express the message for
TNF-alpha and interleukin-10. Eur J Med Res. 6:359–363.
2001.PubMed/NCBI
|
|
37
|
Aggarwal BB, Shishodia S, Ashikawa K and
Bharti AC: The role of TNF and its family members in inflammation
and cancer: lessons from gene deletion. Curr Drug Targets Inflamm
Allergy. 1:327–41. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Wang HQ, Li DL, Lu YJ, Cui XX, Zhou XF,
Lin WP, Conney AH, Zhang K, Du ZY and Zheng X: Anticancer activity
of Acanthopanax trifoliatus (L) Merr extracts is associated with
inhibition of NF-kB activity and decreased Erk1/2 and Akt
phosphorylation. Asian Pac J Cancer Prev. 15:9341–9346. 2014.
View Article : Google Scholar : PubMed/NCBI
|