Introduction
Endometrial cancer (EC) is the most common malignant
neoplasm of the female reproductive tract in developed countries
(1). The disease is confined to the
uterus in >75% of cases and is typically characterized by a good
prognosis, with an overall 5-year survival rate of 75–80% (2,3).
The most commonly diagnosed histological subtype is
endometrioid endometrial cancer (EEC), which is characterized by a
lower aggressiveness and a higher long-term disease-free survival
following adequate primary and adjuvant treatment compared with
that of other histological types of EC (2).
The evaluation of tumor grade, depth of myometrial
invasion (MI), lymphovascular space involvement (LVSI), lymph node
status (LNS) and peritoneal cytology is considered mandatory in
order to assess the accuracy of the primary surgical staging and
the necessity of adjuvant treatment and to determine the
oncological prognosis (4,5). Upon diagnosis of EC, pathologists focus
on precisely evaluating the histological/morphological features of
the disease: This evaluation has led to interest in the role of
adenomyosis (AM) when occurring in association with EC.
AM is a benign condition defined as the presence of
endometrial glands and stroma located ≥1 intermediate power field
away from the native endomyometrial junction (6,7); notably,
AM has been documented to coexist with EEC in 16–34% of
hysterectomy specimens obtained for the treatment of EC (8–14).
The significance of the presence of AM in estimating
the prognosis of EEC is still debated, despite the fact that
studies have reported an excellent prognosis for EEC with
concomitant AM due to the lower histological grade and superficial
MI detected in such cases (8–12). Neoplastic areas close to the foci of
AM typically have a smooth contour and are frequently surrounded by
endometrial stroma with or without benign endometrial glands
(6). However, the predominant
disagreement with regard to the significance of AM in EEC concerns
whether it actively promotes or interferes with the MI of EC
(13).
More recent studies have hypothesized that, in cases
of EEC, the presence of AM may confer a poorer prognosis as it may
be considered a precursor of an EEC lesion and an enabling factor
allowing malignant cells to invade the myometrium by increasing the
contact area (13–15).
The present study aimed to detect the coexistence of
AM and EEC in order to evaluate whether AM may represent an
oncological marker of favorable prognosis, associated with an
earlier stage at diagnosis and biologically less aggressive
behaviour.
Materials and methods
Patients
The present retrospective observational cohort study
included patients referred to the Department of Surgical Sciences
of the University of Parma (Parma, Italy) for surgical treatment of
EC in the interval between January 1999 and January 2013. All
postmenopausal patients diagnosed with EEC who underwent total
hysterectomy (laparoscopic or laparotomic approach) with bilateral
salpingo-oophorectomy, pelvic lymphadenectomy and peritoneal
washing were considered eligible.
Patients with concurrent primary malignancies, EC of
non-endometrioid histological type, previous use of hormone
replacement therapy or raloxifene, levothyroxine treatment
(16,17), or a history of pelvic irradiation or
intrauterine hysteroscopic surgery for metrorrhagia (<1 year
prior to EEC diagnosis) (18) were
excluded from the study.
For all patients, demographic data were documented,
including age, body mass index (BMI), history of comorbid
conditions (hypertension and/or diabetes) and previous use of
tamoxifen (19,20). All eligible patients were adequately
informed at the time of admission regarding the possible use of
their data for further analysis and publication in accordance with
the Italian Privacy Law (675/96).
Uterine examination
All pathological reports were collected at the
Pathological Anatomy and Histology Unit of the University of Parma
following a specific uterine examination, conducted according to
the guidelines of Rosai and Ackerman's Surgical Pathology (10th
edition) (21), as follows. The
uterus was opened by cutting with scissors through both lateral
walls, from the cervix to the uterine cornua. The anterior half was
marked (e.g. by cutting a small wedge on one side; note that if the
tubes are attached, their insertion is anterior to that of the
round ligament). Additional cuts were made through any large mass
in the uterine wall. Parallel transverse sections were made through
each half of the specimen, ~1 cm apart, beginning at the upper
level of the endocervical canal and ending short of completion at
one side; each surface was carefully examined. Several sections of
the cervix were made along the endocervical canal. At least one
cross section was made for every myoma present and carefully
examined, with additional cuts made for larger myomas as
required.
The following sections were performed for
histological evaluation: i) Cervix, one section from the anterior
half and one from the posterior half; ii) corpus, ≥2 sections taken
close to the fundus and including the endometrium, a portion of
myometrium and, thickness permitting, serosa; additional sections
from any grossly abnormal areas; iii) myomas, 1–3 sections for
myomas; sections from any grossly abnormal area (e.g. soft, fleshy,
necrotic or cystic areas); iv) cervical or endometrial polyps,
submitted whole unless extremely large.
For all patients, the diagnosis of AM was confirmed
only when the distance between the lower border of the endometrium
and the foci of endometrial glands and stroma exceeded 2.5 mm
(22).
Other oncological features
In addition to the presence or absence of AM, the
tumor grade, depth of MI, tumor size, LVSI, LNS, peritoneal
cytology, concomitant detection of endometrial atypical hyperplasia
or polypoid endometrial features, and tumor stage according to the
International Federation of Gynecology and Obstetrics (FIGO) 2009
classification (5) were also
recorded.
Aims of the present study
The primary aim was to compare the cases of EEC with
concomitant AM (Group A) with the cases of EEC without AM (Group B)
in terms of FIGO stage at diagnosis. The secondary aim was to
compare Group A and Group B in terms of tumor size, LVSI,
peritoneal cytology and the presence of endometrial atypical
hyperplasia or polypoid endometrial features. Finally, the two
groups were compared in terms of BMI, history of hypertension,
diabetes and tamoxifen use.
Statistical analysis
Statistical analysis was performed using SPSS
version 19 software for Windows (IBM SPSS, Armonk, NY, USA). The
results were expressed as absolute numbers and percentages for
discrete variables and as the mean ± standard deviation for
continuous variables. Appropriate parametric and nonparametric
statistical tests were performed when possible, using the
Kolmogorov-Smirnov test of normal distribution of the sample.
Continuous variables were analyzed using a Student's t-test, and
categorical variables were analyzed by the χ2 test or
Fisher's exact test. P<0.05 was considered to indicate
statistical significance.
Results
General patient features
Among all patients who underwent surgery for EC
during the recruitment interval, 289 cases were determined to be
eligible for the current study. The general characteristics of all
included patients are reported in detail in Table I. AM was detected upon histological
investigation in only 37 patients (Group A), while the remaining
252 patients did not exhibit foci of AM (Group B).
 | Table I.General features of patients in the
two study groups. |
Table I.
General features of patients in the
two study groups.
| Variable | All patients
(n=289) | Group A (n=37) | Group B
(n=252) | P-value |
|---|
| Age at diagnosis,
years |
|
|
| >0.05 |
| Mean ±
SD | 64.8±9.9 | 66.6±11.7 | 64.5±9.6 |
|
|
Range | 33–85 |
|
|
|
| Body mass
index |
|
|
| <0.001 |
| Mean ±
SD | 27.9±4.6 | 31.9±3.8 | 27.3±4.4 |
|
|
Range | 17–44 |
|
|
|
| Diabetes, n
(%) |
|
|
| <0.001 |
|
Yes | 109 (37.7) | 28 (75.7) | 81
(32.1) |
|
| No | 180 (62.3) | 9
(24.3) | 171 (67.9) |
|
| Hypertension, n
(%) |
|
|
| <0.001 |
|
Yes | 164 (56.7) | 33 (89.2) | 131 (52.0) |
|
| No | 125 (43.3) | 4
(10.8) | 121 (48.0) |
|
| Tamoxifen use, n
(%) |
|
|
| <0.001 |
|
Yes | 19 (6.6) | 10 (27.0) | 9
(3.6) |
|
| No | 270 (93.4) | 27 (73.0) | 243 (96.4) |
|
Groups A and B were similar in terms of age at EEC
diagnosis (66.6±11.7 vs. 64.5±9.6 years, respectively); however,
the two groups (A vs. B) differed significantly with regard to mean
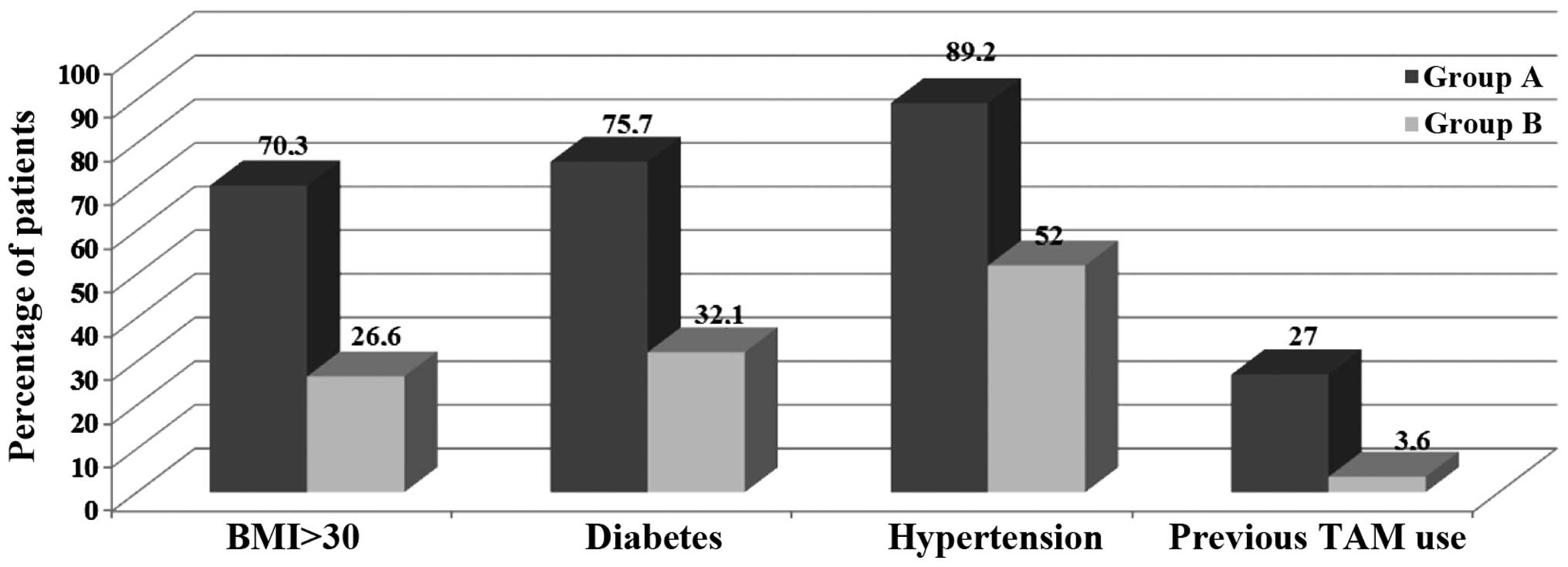
BMI (31.9±3.8 vs. 27.3±4.4), history of diabetes (75.7 vs. 32.1%),
hypertension (89.2 vs. 52%) and tamoxifen intake (27 vs. 3.6%)
(P<0.001; Fig. 1).
AM is associated with lower FIGO
stage
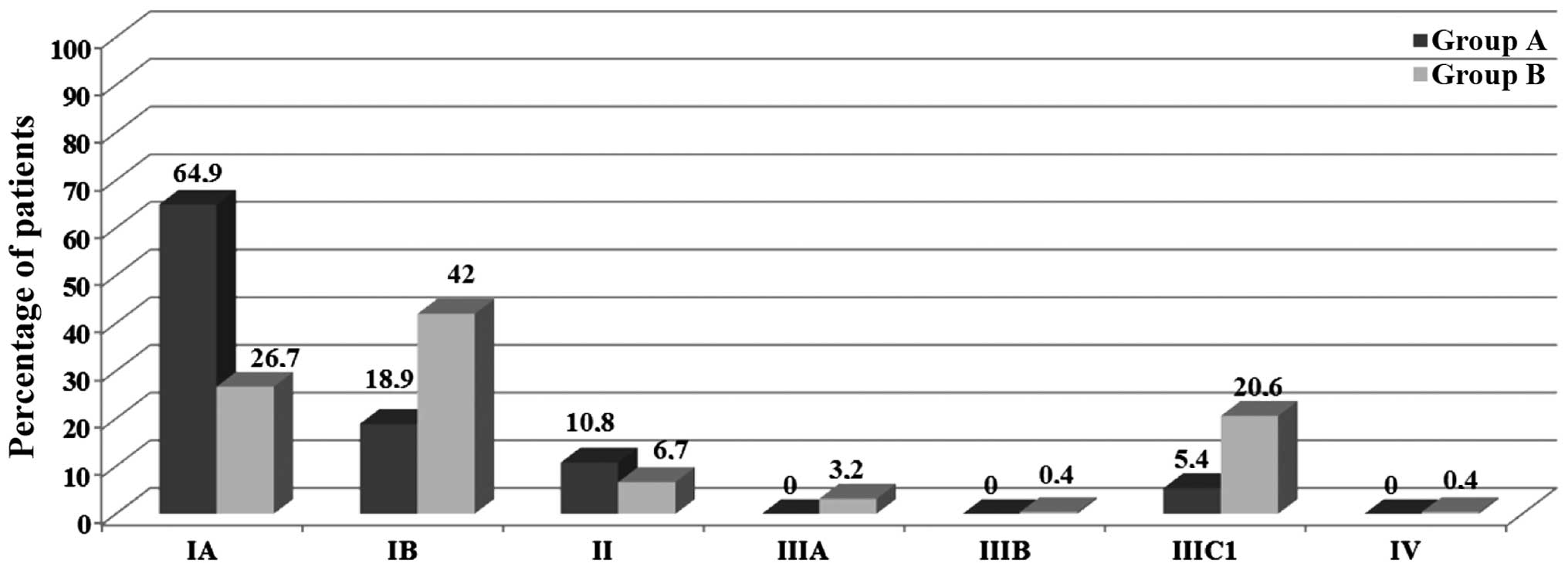
Surgical FIGO stage was determined to differ
significantly between the two study groups: 83.8% of Group A
patients were assigned to FIGO stage I, vs. 68.7% of Group B
patients (P<0.01). Notably, within FIGO stage I, 64.9% of Group
A patients were categorized as stage IA, vs. 26.6% of Group B
patients (P<0.001; Fig. 2). A
significant difference in FIGO stage between the two Groups was
also confirmed in stage III: Stage IIIC1 was documented in 5.4% of
Group A patients vs. 20.6% of Group B patients (P<0.001;
Fig. 2).
Surgical outcome
No significant difference was identified between the
two groups in terms of the outcome of the surgical techniques used:
The laparoscopic approach was performed in 21.6% of Group A vs.
19.8% of Group B. Similarly, the mean numbers of lymph nodes
removed were 29.5±6.6 and 28.7±7.4, respectively (P>0.05;
Table II).
 | Table II.Detailed surgical and histological
features of patients in the two study groups. |
Table II.
Detailed surgical and histological
features of patients in the two study groups.
| Variable | All patients
(n=289) | Group A (n=37) | Group B
(n=252) | P-value |
|---|
| Lymph nodes
removed, n |
|
|
| >0.05 |
| Mean ±
SD | 28.8±7.3 | 29.5±6.6 | 28.7±7.4 |
|
|
Range | 16.1–41.9 |
|
|
|
| Tumor size, mm |
|
|
| <0.001 |
| Mean ±
SD | 4.0±0.7 | 3.3±0.7 | 4.1±0.6 |
|
|
Range | 2.1–5.8 |
|
|
|
| Endometrial
hyperplasia, n (%) |
|
|
| <0.01 |
|
Yes | 40
(13.8) | 11 (29.7) | 29
(11.5) |
|
| No | 249 (86.2) | 26 (70.3) | 223 (88.5) |
|
| Endometrial polyps,
n (%) |
|
|
| <0.001 |
|
Yes | 42
(14.5) | 14 (37.8) | 28
(11.1) |
|
| No | 247 (85.5) | 23 (62.2) | 224 (88.9) |
|
| Lymphovascular
space involvement, n (%) |
|
|
| <0.001 |
|
Yes | 105 (36.3) | 4
(10.8) | 101 (40.1) |
|
| No | 184 (63.7) | 33 (89.2) | 151 (59.9) |
|
| Lymph node
involvement, n (%) |
|
|
| <0.05 |
|
Yes | 55
(19.0) | 2 (5.4) | 53
(21.0) |
|
| No | 234 (81.0) | 35 (94.6) | 199 (79.0) |
|
| Myometrial
involvement <50%, n (%) |
|
|
| <0.001 |
|
Yes | 106 (36.7) | 26 (70.3) | 80
(31.7) |
|
| No | 183 (63.3) | 11 (29.7) | 172 (68.3) |
|
| Positive peritoneal
cytology, n (%) |
|
|
| >0.05 |
|
Yes | 9
(3.1) | 1 (2.7) | 8
(3.2) |
|
| No | 280 (96.9) | 36 (97.3) | 244 (96.8) |
|
| Tumor grading, n
(%) |
|
|
| =0.05 |
| G1 | 116 (40.1) | 19 (51.4) | 97
(38.5) |
|
| G2 | 130 (45.0) | 16 (43.2) | 114 (45.2) |
|
| G3 | 43
(14.9) | 2 (5.4) | 41
(16.3) |
|
| Surgical approach,
n (%) |
|
|
| >0.05 |
|
Laparoscopy | 58
(20.1) | 8
(21.6) | 50
(19.8) |
|
|
Laparotomy | 231 (79.9) | 29 (78.4) | 202 (80.2) |
|
Other oncological features
Although no difference was identified between Groups
A and B in terms of positive peritoneal cytology rate (3.2 vs.
2.7%, respectively), a borderline statistically significant
difference was found in tumor grade (G1, 51.4 vs. 38.5%; G2, 43.2%
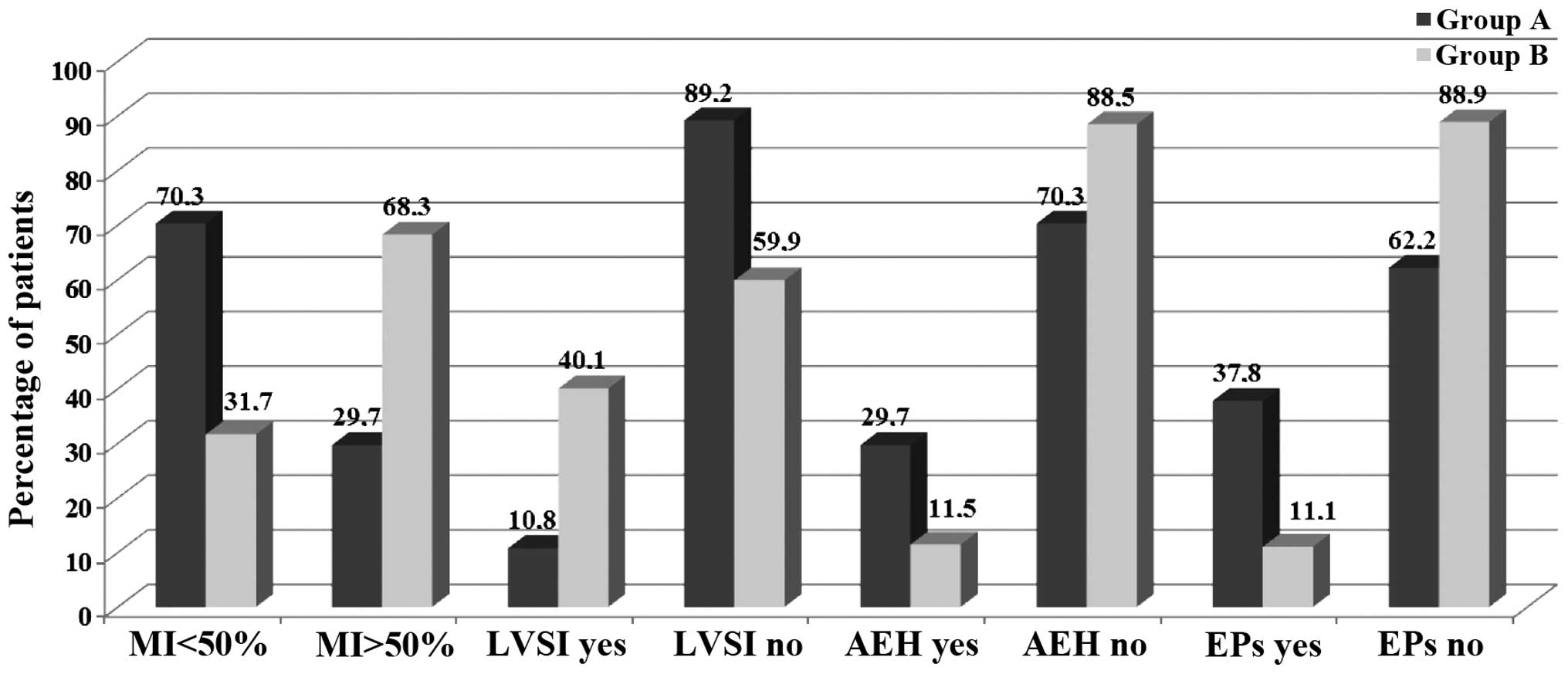
vs. 45.2%; G3, 5.4% vs. 16.3%, respectively; P=0.05). Furthermore,
the two groups differed markedly in terms of MI: <50% invasion
of the uterus was detected in 70.3% of Group A vs. 31.7% of Group B
patients (P<0.001); LVSI was positive in 10.8% of Group A vs.
40.1% of Group B (P<0.001), whilst a positive LNS was detected
in 5.4% of Group A vs. 21% of Group B patients (P<0.05)
(Table II; Fig. 3).
Histological features
The histological detection of atypical hyperplasia
associated with EEC was reported in 29.7% of cases in Group A vs.
11.5% in Group B (P<0.01), whilst the detection of concomitant
endometrial polyps occurred in 37.8% of cases in Group A vs. 11.1%
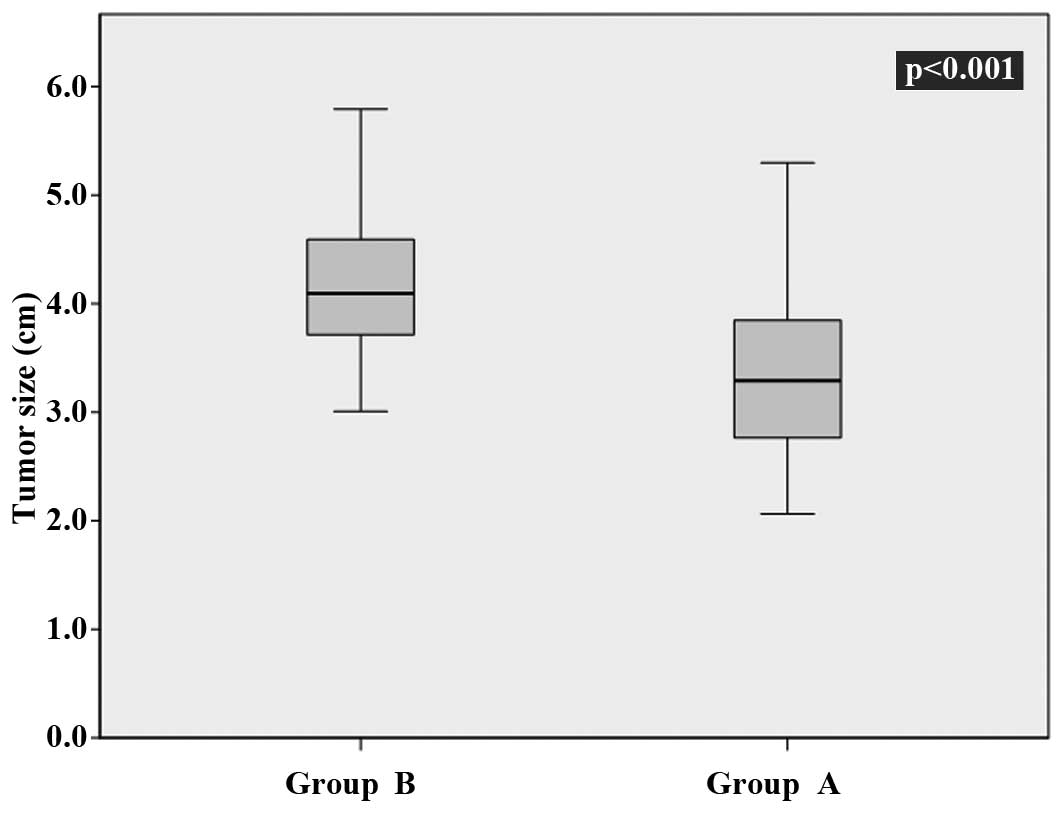
in Group B (P<0.001). The mean tumor sizes detected were 3.3±0.7
mm (Group A) vs. 4.1±0.6 mm (Group B; P<0.001) (Table II; Fig.
4).
Discussion
EC is the most frequent gynaecological neoplasm in
developed countries (23), with EEC
the most frequent histological type. EEC is typically associated
with a lower aggressiveness and an improved long-term disease-free
survival following adequate treatment compared with that of other
ECs (24).
Histology, depth of MI, tumor grade, presence of
LVSI, positive peritoneal cytology, tumor size and LNS have been
universally recognized as prognostic factors for determining
long-term clinical course of the disease (25,26).
Although certain studies have evaluated the association between AM
and the clinical course of EEC, the mechanism by which AM
interferes with tumoral progression remains unresolved (9,12,27,28).
AM is a common benign condition that is frequently
detected in hysterectomy specimens containing EEC. However
comprehending the relationship between EEC and AM remains an
important challenge for gynaecologists and oncologists, as the role
of AM in the pathogenesis and consequent clinical behaviour of EEC
is unclear. Despite evidence of a frequent association between AM
and EEC, which typically presents with a lower grade and lower
degree of MI and lymph node involvement (4), no studies have been able to elucidate
the relationship between these events (9,12,27,28).
The frequent coexistence of the two diseases may be
explained by a possible common risk factor or by a mutual
pathogenic mechanism. Three theories have been proposed to describe
the aetiology of AM: The first theory assumes that AM may originate
from an invagination of the endometrial mucosa between bundles of
uterine smooth muscle cells; the second theory suggests that
endometrial tissue may enter the myometrium via the intramyometrial
lymphatic system; and the third theory attributes AM to a de
novo metaplasia arising from ectopic intramyometrial
endometrial tissue (4).
In the present study, the prevalence of AM in
specimens from hysterectomies performed for the treatment of EEC
was determined to be 12.8%, which was lower compared with the
percentages reported by previous studies (8).
According to the current data, AM was strongly
associated with the following risk factors: Personal history of
diabetes, hypertension, high BMI and tamoxifen intake. Notably,
these represent the same factors contributing to atypical
hyperplasia and endometrial polyps through the creation of a
hyper-estrogenic status. An overexpression of estrogen receptors in
AM foci has been suggested as evidence of this possible association
(8,29)
supported by the fact that the concomitant EEC diagnosed was
frequently of type I, typical of a hyper-estrogenic status and
endometrial hyperplasia (3,30).
In 2007, Ismiil et al (13) suggested that the probability of deep
MI is greater when AM is coexistent with grade 1 EEC, contrary to
what may be expected (typically grade 1 EEC has a lower
aggressiveness). The findings of the current study, in accordance
with those of Musa et al (4),
indicated that concurrent AM and EEC was associated with a lower
tumor grade, MI of <50%, and the absence of LVSI and lymph node
metastasis. This evidence explains and supports the strong inverse
correlation between presence of AM and FIGO stage identified in the
study: FIGO stage IA was detected in 64.9% of the cases with
concomitant AM and in only 26.6% of cases without AM. Further
confirmation of this trend is provided by the comparison of cases
diagnosed at an advanced FIGO stage, as only 4% of all stage IIIC1
cases were positive for AM detection.
A strength of the present data is the homogeneity
between the two groups in terms of lymph node removal, as all
patients underwent lymphadenectomy and no significant difference
was found in the mean number of lymph nodes removed in each group.
Additionally, the current study confirms the importance of LVSI in
predicting lymph node involvement (~50% of patients with LVSI were
of stage IIIC1), as well as demonstrating that the presence of AM
represents a protective factor for LVSI (positive LVSI was detected
at a rate of ~10% in the presence of AM vs. ~40% in the absence of
AM).
Although this evidence has been reported by Musa
et al (4), further studies are
necessary in order to validate the process of detection of AM in
intraoperative frozen sections and to assess its possible role as
indicator of whether lymphadenectomy should be performed (19,31–33).
The rationale for a surgical evaluation of the
coexistence of AM and EEC is comparable to that of intraoperatively
defining tumor stage. Both could be used as a guideline for
estimating the risk of lymph node involvement.
Numerous studies have demonstrated that, with an
increase of tumor size, the probability of MI and detection of a
higher tumor grade increases (34,35). Based
on this evidence, the current study analyzed the possible
association between the presence of AM and the mean tumor size
detected. Notably, a significant correlation between presence of AM
and smaller tumor size was identified. To the best of our
knowledge, this finding is the first confirmation that concurrent
EEC and AM is associated with a higher differentiation grade and a
lower tumor size. Lower tumor size is also often associated with a
reduced MI, and the current findings confirm this: EEC concomitant
with AM was associated with a statistically significant percentage
of cases with MI <50%. If confirmed by future studies, this
evidence could clarify the mechanisms involved in LVSI and MI, as
tumor size and grade are both independent risk factors for a poor
oncological prognosis.
The inverse correlation between MI and AM may be
explained by a possible altered adhesion mechanism between AM foci
and cancer cells (with a reduced ability to go deeper into the
myometrium) or by a lower cancer aggressiveness when AM
coexists.
On the basis of these data, it may be speculated
that the only disadvantage linked to coexistence of AM with EEC may
be the increased difficulty in preoperative sonographic evaluation
of the endometrium-myometrium junction and estimation of MI
(36–38). The impossibility to evaluate the
aforementioned feature due to the retrospective design represents a
limitation of the current study. Additional study limitations
include the absence of a preoperative Human Epididymis Protein 4
serum assay (39) and a long-term
follow-up able to evaluate the impact of AM and disease recurrence
rate/disease-free survival rate. Finally, the reason associated
with a possible underestimation of AM detection rate (inferior to
that reported in the literature) may be related to the exclusion of
EC types other than EEC. We believe that a possible overestimation
of LVSI was related to the fact that the pathologist was not
blinded to the aim of the study.
In conclusion, in patients with EEC, the presence of
associated AM may allow early detection with a good probability of
diagnosing a disease with a higher grade of differentiation, a
lower MI, an absence of LVSI, a small tumor size and a negative
lymph node status. While an attempt at preoperative staging through
ultrasound estimation of MI may be impaired by the presence of AM,
the intraoperative evaluation of AM (in coexistence with EEC) may
potentially aid the surgeon in estimating the oncological risk of
the patient and selecting the most appropriate surgical treatment.
Certainly, prospective large-scale studies are necessary to
validate AM as an independent favorable oncological prognostic
factor for EEC patients and to evaluate its usefulness, alone or in
combination with other known prognostic factors, so as to define
the most adequate personalized primary and adjuvant therapy.
References
|
1
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Prat J: Prognostic parameters of
endometrial carcinoma. Hum Pathol. 35:649–462. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Giordano G, D'Adda T, Bottarelli L,
Lombardi M, Brigati F, Berretta R and Merisio C: Two cases of
low-grade endometriod carcinoma associated with undifferentiated
carcinoma of the uterus (dedifferentiated carcinoma): A molecular
study. Pathol Oncol Res. 18:523–528. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Musa F, Frey MK, Im HB, Chekmareva M,
Ellenson LH and Holcomb K: Does the presence of adenomyosis and
lymphovascular space invasion affect lymph node status in patients
with endometrioid adenocarcinoma of the endometrium? Am J Obstet
Gynecol. 207:417e1–e6. 2012. View Article : Google Scholar
|
|
5
|
Pecorelli S: Revised FIGO staging for
carcinoma of the vulva cervix and endometrium. Int J Gynaecol
Obstet. 105:103–104. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hanley KZ, Dustin SM, Stoler MH and Atkins
KA: The significance of tumor involved adenomyosis in otherwise
low-stage endometrioid adenocarcinoma. Int J Gynecol Pathol.
29:445–451. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Garcia L and Isaacson K: Adenomyosis:
Review of the literature. J Minim Invasive Gynecol. 18:428–437.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bergeron C, Amant F and Ferenczy A:
Pathology and physiopathology of adenomyosis. Best Pract Res Clin
Obstet Gynaecol. 20:511–21. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mittal KR and Barwick KW: Endometrial
adenocarcinoma involving adenomyosis without true myometrial
invasion is characterized by frequent preceding estrogen therapy
low histologic grades and excellent prognosis. Gynecol Oncol.
49:197–201. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jacques SM and Lawrence WD: Endometrial
adenocarcinoma with variable-level myometrial involvement limited
to adenomyosis: A clinicopathologic study of 23 cases. Gynecol
Oncol. 37:401–407. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hernandez E and Woodruff JD: Endometrial
adenocarcinoma arising in adenomyosis. Am J Obstet Gynecol.
138:827–832. 1980.PubMed/NCBI
|
|
12
|
Kucera E, Hejda V, Dankovcik R, Valha P,
Dudas M and Feyereisl J: Malignant changes in adenomyosis in
patients with endometrioid adenocarcinoma. Eur J Gynaecol Oncol.
32:182–184. 2011.PubMed/NCBI
|
|
13
|
Ismiil N, Rasty G, Ghorab Z, Nofech-Mozes
S, Bernardini M, Ackerman I, Thomas G, Covens A and Khalifa MA:
Adenomyosis involved by endometrial adenocarcinoma is a significant
risk factor for deep myometrial invasion. Ann Diagn Pathol.
11:252–257. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Seidman JD and Kjerulff KH: Pathologic
findings from the Maryland women's health study: Practice patterns
in the diagnosis of adenomyosis. Int J Gynecol Pathol. 15:217–221.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Ismiil ND, Rasty G, Ghorab Z, Nofech-Mozes
S, Bernardini M, Thomas G, Ackerman I, Covens A and Khalifa MA:
Adenomyosis is associated with myometrial invasion by FIGO 1
endometrial adenocarcinoma. Int J Gynecol Pathol. 26:278–283. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Saccardi C, Gizzo S, Ludwig K, Guido M,
Scarton M, Gangemi M, Tinelli R and Litta PS: Endometrial polyps in
women affected by levothyroxine-treated hypothyroidism -
histological features, immunohistochemical findings and possible
explanation of etiopathogenic mechanism, A pilot study. Biomed Res
Int. 2013:5034192013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Gizzo S, Saccardi C, Patrelli TS, et al:
Update on raloxifene: Mechanism of action, clinical efficacy,
adverse effects and contraindications. Obstet Gynecol Surv.
68:467–481. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Saccardi C, Conte L, Fabris A, et al:
Hysteroscopic enucleation in toto of submucous type 2 myomas:
Long-term follow-up in women affected by menorrhagia. J Minim
Invasive Gynecol. 21:426–430. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gizzo S, Di Gangi S, Bertocco A, Noventa
M, Fagherazzi S, Ancona E, Saccardi C, Patrelli TS, D'Antona D and
Nardelli GB: Levonorgestrel intrauterine system in adjuvant
tamoxifen treatment, Balance of breast risks and endometrial
benefits - systematic review of literature. Reprod Sci. 21:423–431.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Saccardi C, Gizzo S, Patrelli TS, Ancona
E, Anis O, Di Gangi S, Vacilotto A, D'Antona D and Nardelli GB:
Endometrial surveillance in tamoxifen users: Role, timing and
accuracy of hysteroscopic investigation, Observational longitudinal
cohort study. Endocr Relat Cancer. 20:455–462. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rosai J: Female reproductive system. Rosai
and Ackerman's Surgical Pathology. 2:(10th). Mosby Elsevier.
1399–1422. 2011.
|
|
22
|
Kurman RJ, Ellenson LH and Ronnett BM:
Blaustein's Pathology of the Female Genital Tract (6th). New York:
Springer. 2011. View Article : Google Scholar
|
|
23
|
Wright JD, Medel Barrena NI, Sehouli J,
Fujiwara K and Herzog TJ: Contemporary management of endometrial
cancer. Lancet. 379:1352–1360. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Berretta R, Patrelli TS, Faioli R, Mautone
D, Gizzo S, Mezzogiorno A, Giordano G and Modena AB:
Dedifferentiated endometrial cancer: An atypical case diagnosed
from cerebellar and adrenal metastasis, Case presentation and
review of literature. Int J Clin Exp Pathol. 6:1652–1657.
2013.PubMed/NCBI
|
|
25
|
Gizzo S, Fabris A, Litta P and Saccardi C:
Estimated intermediate risk endometrial cancer, Debate and new
perspectives on therapy individualization and prognosis
establishment starting from a peculiar case. Int J Clin Exp Pathol.
7:2664–2669. 2014.PubMed/NCBI
|
|
26
|
Berretta R, Patrelli TS, Migliavacca C,
Rolla M, Franchi L, Monica M, Modena AB and Gizzo S: Assessment of
tumor size as a useful marker for the surgical staging of
endometrial cancer. Oncol Rep. 31:2407–2412. 2014.PubMed/NCBI
|
|
27
|
Chrysostomou M, Akalestos G, Kallistros S,
Papadimitriou V, Nazar S and Chronis G: Incidence of adenomyosis
uteri in a Greek population. Acta Obstet Gynecol Scand. 70:441–444.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Gün I, Oner O, Bodur S, Ozdamar O and Atay
V: Is adenomyosis associated with the risk of endometrial cancer?
Med Glas (Zenica). 9:268–272. 2012.PubMed/NCBI
|
|
29
|
Ueki K, Kumagai K, Yamashita H, Li ZL,
Ueki M and Otsuki Y: Expression of apoptosis-related proteins in
adenomyotic uteri treated with danazol and GnRH agonists. Int J
Gynecol Pathol. 23:248–258. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Merisio C, Berretta R, De Ioris A,
Pultrone DC, Rolla M, Giordano G, Tateo S and Melpignano M:
Endometrial cancer in patients with preoperative diagnosis of
atypical endometrial hyperplasia. Eur J Obstet Gynecol Reprod Biol.
122:107–111. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kim YB and Niloff JM: Endometrial
carcinoma: Analysis of recurrence in patients treated with a
strategy minimizing lymph node sampling and radiation therapy.
Obstet Gynecol. 82:175–180. 1993.PubMed/NCBI
|
|
32
|
Todo Y, Kato H, Kaneuchi M, Watari H,
Takeda M and Sakuragi N: Survival effect of para-aortic
lymphadenectomy in endometrial cancer (SEPAL study): A
retrospective cohort analysis. Lancet. 375:1165–1172. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Patrelli TS, Berretta R, Rolla M, Vandi F,
Capobianco G and Gramellini D: BacchiM odena A and Nardelli GB:
Pelvic lymphadenectomy in endometrial cancer: Our current
experience. Eur J Gynaecol Oncol. 30:536–538. 2009.PubMed/NCBI
|
|
34
|
Mariani A, Webb MJ, Keeney GL and Podratz
KC: Routes of lymphatic spread, A study of 112 consecutive patients
with endometrial cancer. Gynecol Oncol. 81:100–104. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Schink JC, Rademaker AW, Miller DS and
Lurain JR: Tumor size in endometrial cancer. Cancer. 67:2791–2794.
1991. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Boes AS, Tousseyn T, Vandenput I,
Timmerman D, Vergote I, Moerman P and Amant F: Pitfall in the
diagnosis of endometrial cancer, Case report of an endometrioid
adenocarcinoma arising from uterine adenomyosis. Eur J Gynaecol
Oncol. 32:431–434. 2011.PubMed/NCBI
|
|
37
|
Berretta R, Merisio C, Piantelli G, Rolla
M, Giordano G, Melpignano M and Nardelli GB: Preoperative
transvaginal ultrasonography and intraoperative gross examination
for assessing myometrial invasion by endometrial cancer. J
Ultrasound Med. 27:349–355. 2008.PubMed/NCBI
|
|
38
|
Savelli L, Ceccarini M, Ludovisi M,
Fruscella E, De Iaco PA, Salizzoni E, Mabrouk M, Manfredi R, Testa
AC and Ferrandina G: Preoperative local staging of endometrial
cancer, Transvaginal sonography vs. Histopathology. Ultrasound
Obstet Gynecol. 31:560–566. 2008. View
Article : Google Scholar : PubMed/NCBI
|
|
39
|
Gizzo S, Ancona E, Saccardi C, D'Antona D,
Nardelli GB and Plebani M: Could kidney glomerular filtration
impairment represent the ‘Achilles heel’ of HE4 serum marker? A
possible further implication. Clin Chem Lab Med. 52:e45–e46. 2014.
View Article : Google Scholar : PubMed/NCBI
|