Introduction
Advanced gastric cancer may present with various
clinical manifestations. Visceral metastases, including those of
the liver and lung, arise with high incidence in numerous cases of
gastric cancer; however, bone metastasis occurs at a lower
frequency (<10%) (1,2). Advanced gastric cancer with the presence
of multiple bone metastases and/or disseminated intravascular
coagulation (DIC) is also rare. The frequency of comorbidities with
DIC in gastric cancer patients who have bone metastasis is as high
as 82–86% (3,4). The frequency of bone metastasis in
gastric cancer patients with DIC is also high at 87% (5). The existence of this gastric cancer
subgroup bearing bone metastasis with susceptibility to DIC has
been previously recognized (3,5). The
clinical features of this specific subgroup reportedly differ from
those presenting with typical gastric cancer, with the DIC subgroup
much less likely to develop visceral metastases to the liver and
lungs. However, the cancer is much more aggressive and patients
have a poorer prognosis. There are several reports of this subgroup
in the current literature (3,5). The median survival time ranges from 8 to
22 weeks (3,5), and there are currently no established
specific chemotherapies. It is believed that the subgroup of
gastric cancer with bone metastasis and DIC may have a different
underlying biological mechanism, and that chemotherapy regimens
against this may have different requirements. Methotrexate plus
5-fluorouracil is effective in controlling the gastric cancer with
metastasis and/or DIC subgroup (4,6); however,
there is limited information regarding the responses of recently
approved agents, including taxanes, cisplatin, S1 and capecitabine
(7–10).
The aggressive nature and progression of this
subgroup often leaves patients without the chance to undergo a
second treatment option. Furthermore, it is difficult to measure
the response to specific therapeutics due to the discrepancy of
tumor marker levels and the time-consuming nature of imaging
analyses. Among the associated biomarkers, circulating tumor cell
(CTC) count is a direct indicator of tumor growth.
Despite gastric cancer not generally being
associated with high CTC counts (median, 2 cells/7.5 ml) (11), this particular subgroup of gastric
cancer with multiple bone metastases and/or DIC is believed to
exhibit high CTC counts. However, there are currently no reports
confirming this. The present study describes the CTC count in two
patients that fall within the advanced gastric cancer with
metastasis and/or DIC subgroup, and investigate its possible
function as a clinical biomarker. The present observational study
was approved by the Ethics Committee of the School of Medicine of
Akita University (Akita, Japan). Written informed consent and an
agreement to publish were obtained from each patient.
Case report
Case 1
In January 2014, a 51-year-old male patient
presented to the Department of Clinical Oncology, Akita University
Hospital (Akita, Japan) and was diagnosed with scirrhous-type
gastric cancer, with bone metastases to the thoracic and lumbar
vertebrates and multiple costae, in addition to malignant ascites
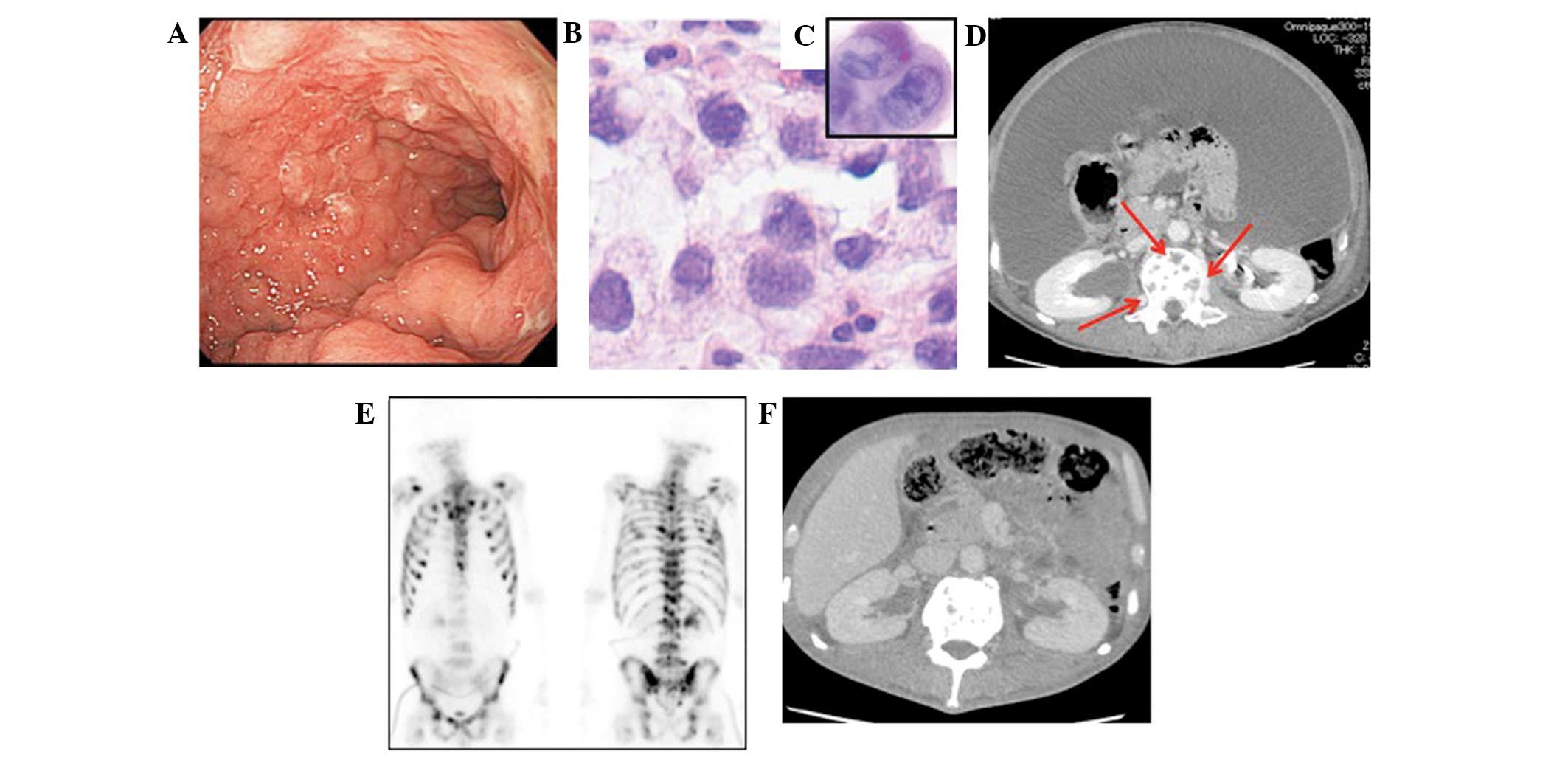
(Fig. 1). Poorly-differentiated
adenocarcinoma and signet ring cell carcinoma were detected in the
stomach, and ascites was noted (Fig. 1B
and C). A left nephrostomy was performed due to the occurrence
of hydronephrosis and enabled the administration of chemotherapy.
Recombinant thrombomodulin α (380 U/kg) was administered for 4 days
to improve DIC, and concomitant chemotherapy with paclitaxel (PTX;
45–80 mg/m2, weekly) was administered for 3 weeks. The
platelet count immediately increased, but the ascites did not
improve. Following one cycle of PTX, the carcinoembryonic antigen
(CEA) level decreased from 288.7 to 160.2 ng/ml (normal range, ~4.9
ng/ml), but the carbohydrate antigen 19-9 (CA19-9) level increased
from 158.3 to 690.5 U/ml (normal range, ~37.0 U/ml). Additionally,
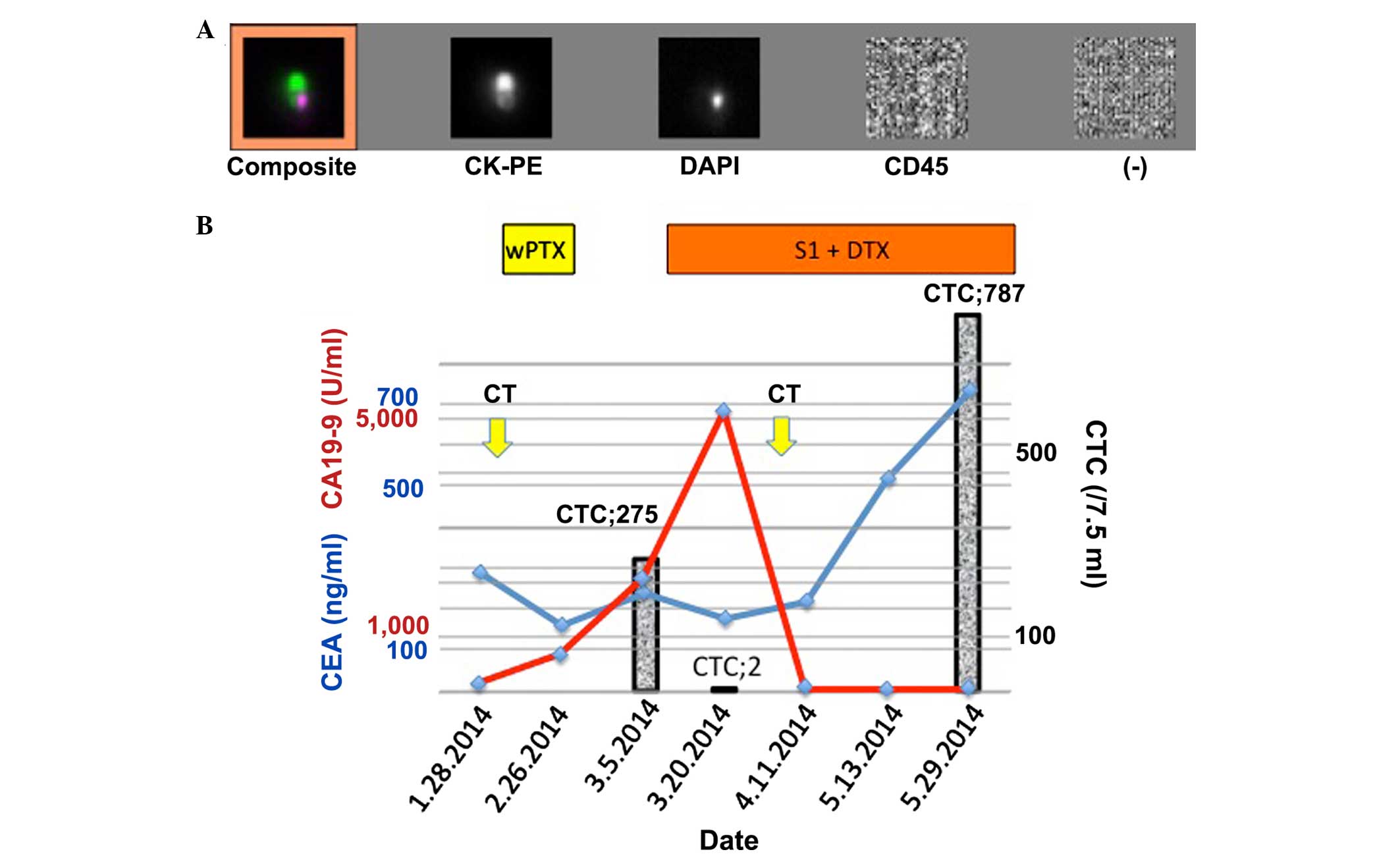
the CTC count was calculated as 275 cells/7.5 ml during the same
time period (Fig. 2). A previously
described method was used to isolate the CTCs (12,13). In
brief, CTCs were isolated from 20 ml of peripheral venous blood
drawn using a CellSearch® Circulating Tumor Cell kit and a
CellTracks® AutoPrep® system (Janssen Diagnostics; Johnson &
Johnson, New Brunswick, NJ, USA). This procedure was outsourced to
Special Reference Laboratories, Inc. (Tokyo, Japan). The drugs were
changed and a regimen of S1 (40 mg/m2, twice daily, for
14 days) plus docetaxel (DTX; 33 mg/m2) were
administered. Following this, on the 17th day, the CTC count
decreased to 2 cells/7.5 ml, but the CA19-9 level remained elevated
at 5,150.8 U/ml (Fig. 2B). On day 22,
the CA19-9 level decreased to 1,808.8 U/ml, and finally, it reached
58.0 U/ml on day 35. Computed tomography (CT) imaging confirmed the
concomitant disappearance of ascites (Fig. 1F). This state was consistent for 1.5
months, during which the patient was treated as an outpatient. Two
months after the initiation of the second chemotherapy course, the
CA19-9 level remained suppressed, although the CEA level increased
to 733.7 ng/ml, and the CTC count increased to 787 cells/7.5 ml.
Subsequently, the chemotherapy regimen was changed to cisplatin (23
mg/m2) plus irinotecan (47 mg/m2), but
failed. The patient survived for a total of 160 days, but finally
succumbed to renal failure due to cancerous peritonitis.
Case 2
In February 2014, a 59-year-old female presented to
the Department of Clinical Oncology, Akita University Hospital, and
was diagnosed with scirrhous-type gastric cancer, with bone
metastases to the thoracic and lumbar vertebrates, the left
shoulder joint, the bilateral iliac bones and the right hip joint,
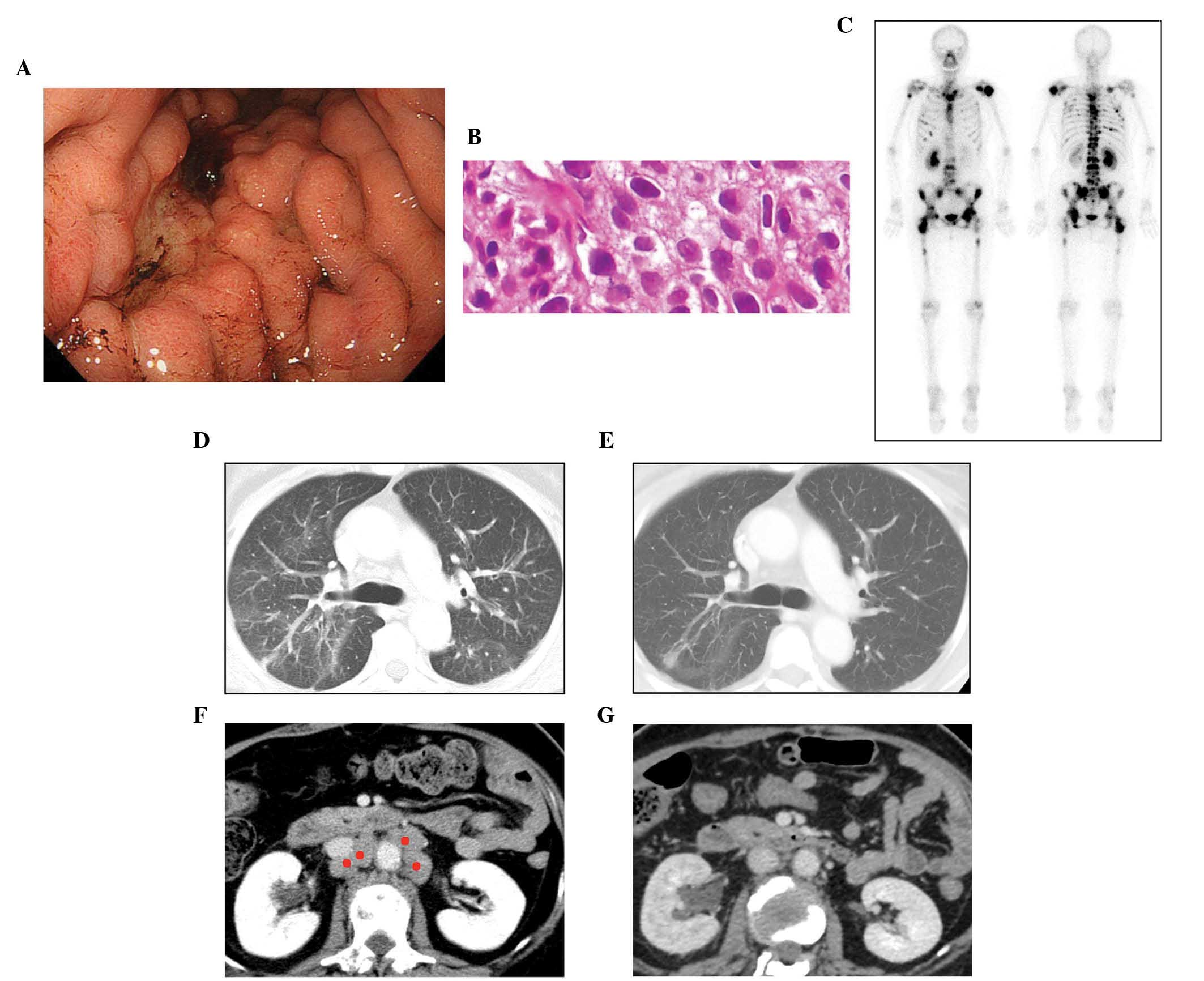
in addition to multiple lymph node metastases (Fig. 3). A poorly-differentiated
adenocarcinoma was detected at an enlarged gastric fold using a
gastrointestinal fibroscope biopsy (Fig.
3B). During the first examination, the patient suffered from
dyspnea caused by pulmonary lymphangitis carcinomatosa (Fig. 3D). Two days prior to initiating the
first course of chemotherapy, the CTC count was noted at 235
cells/7.5 ml. The CEA and CA19-9 levels were also elevated, at
120.0 ng/ml and 5,201.5 U/ml, respectively. Chemotherapy with S1
(40 mg/m2, twice daily, for 14 days) plus DTX (40
mg/m2) was then initiated. In order to efficiently
evaluate the therapeutic effect, CTC counts and tumor marker
measurements were conducted on day 11 following the initiation of
chemotherapy. Discrepant results for CEA (82.5 ng/ml) and CA19-9
(6,543.2 U/ml) levels were obtained. Conversely, the CTC count had
decreased to 7 cells/7.5 ml (Fig. 4).
The S1 plus DTX treatment was continued, and subsequently, the
CA19-9 level decreased to 1,393.1 U/ml on day 31. By day 68, the
CA19-9 and CEA levels had decreased to 835.2 U/ml and 5.9 ng/ml,
respectively. On day 95, CT confirmed that the abdominal lymph node
swelling had resolved and the lymphangitis had improved (Fig. 3E and G). However, following day 110,
levels of the two markers began to increase, and on day 159, the
levels of CA19-9 and CEA reached 2,858.4 U/ml and 81.7 ng/ml,
respectively. Despite the Response Evaluation Criteria in Solid
Tumors (14) by CT indicating that
the therapeutic response was a stable disease, by day 159, the CTC
count had increased to 513 cells/7.5 ml. The chemotherapeutic
regimen was then changed to cisplatin (23 mg/m2) plus
irinotecan (50 mg/m2), but this subsequently failed due
to the progression of lumbar spine metastasis, as identified by
magnetic resonance imaging. The patient survived for a total of 246
days, but finally succumbed to respiratory failure due to cancerous
lymphangitis.
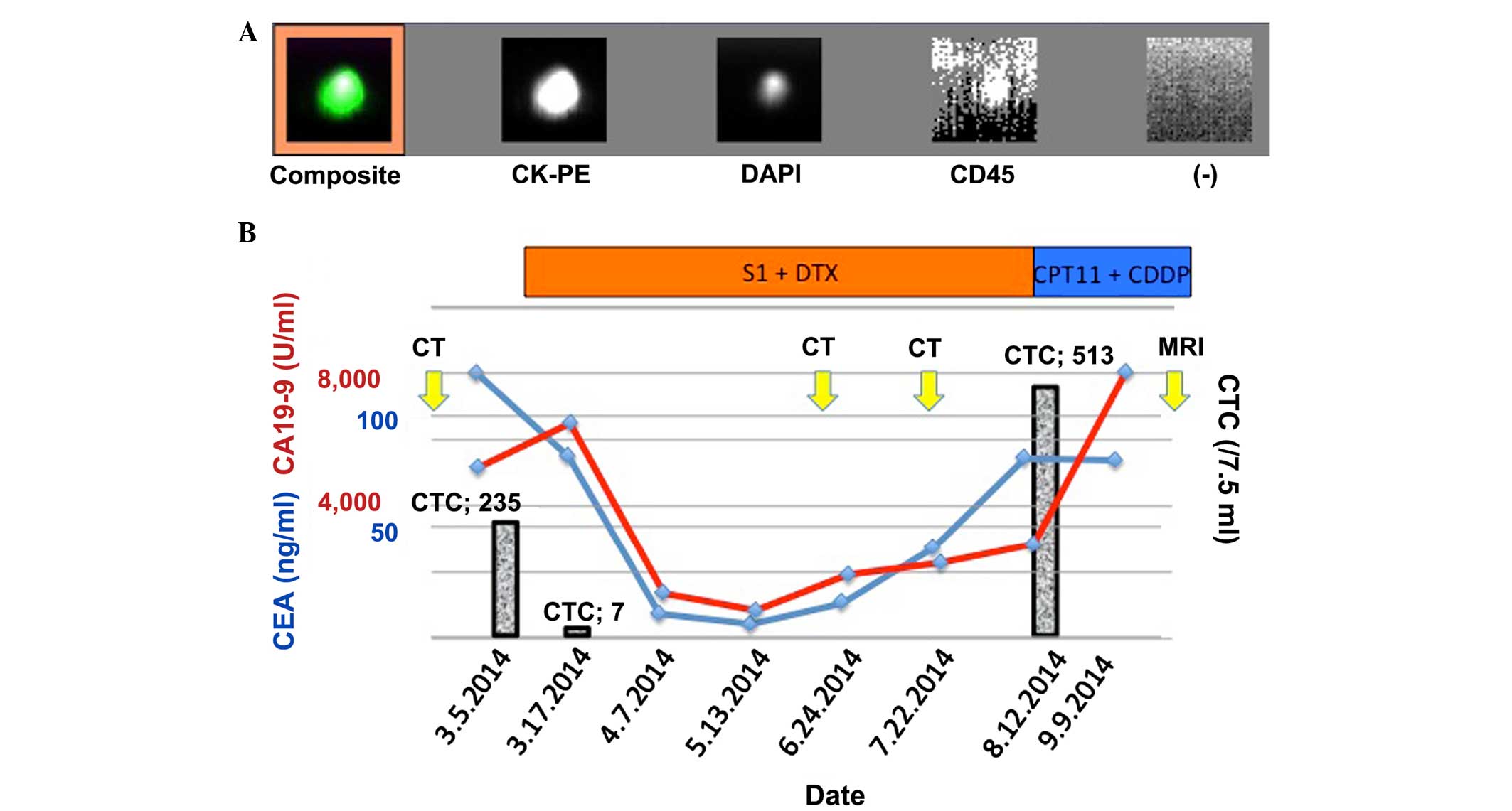 | Figure 4.Clinical course of case 2. (A)
Appearance of the captured CTC. The composite image of CTC stained
with CK-PE and DAPI is presented representatively. Cluster of
differentiation 45 (leukocyte common antigen) is negative in the
CTC. (−) indicates the background. (B) Clinical course of case 2.
CTC is indicated by the bar graph. DTX, docetaxel; CK-PE,
pan-cytokeratin antibody; CTC, circulating tumor cell; DAPI,
4′,6-diamidino-2-phenylindole; MRI, magnetic resonance imaging; CT,
computed tomography; CD45, cluster of differentiation 45; CDDP,
cisplatin; CPT11, irinotecan. |
Discussion
CTCs may not be detected in a number of advanced
gastric cancer cases, in contrast to breast and prostate cancers
(15,16). When the markers to capture CTC are
changed from epithelial cell adhesion molecules to cytokeratins,
circulating gastric cancer cells are only detected in 11.6–15.5% of
cases (17). A recent study revealed
that gastric CTCs were detected in 28/42 cases using the
CellSearch® system (18). In
particular, a high number of CTCs (30–18,015 cells/7.5 ml) were
detected in all 5/5 cases with bone marrow metastases. The bone
marrow is considered a reservoir for disseminated tumor cells
(19). Thus, gastric cancer subtypes
that are characterized by multiple bone metastases and/or DIC may
be associated with high numbers of CTC, as observed in the present
two cases. The high CTC count exhibited in such patients may be
useful to monitor therapeutic effect. Regarding the cases described
in the present study, the change in the CTC count was considered to
be the earliest marker applicable in current practice. In case 1,
an effective chemotherapy regimen was conducted, however, the
CA19-9 level was elevated for the first 16 days then decreased. By
contrast, the CTC count had decreased to <1% of the
pre-treatment level by the 17th day. The CTC count served as a
beneficial marker, and functioned as a determining factor aiding
the decision on whether treatment should be continued or not.
Furthermore, once the CTC count had recovered to the pre-treatment
level, CA19-9 remained at a low level. Clinical symptoms, including
bone pain, worsened gradually and later CT imaging indicated
disease progression. The other tumor marker, CEA, was also
ineffective in this case. In case 2, the CTC count had decreased to
<3% of the pre-treatment level on the 11th day after treatment.
Simultaneously, the values of CEA and CA19-9 were measured,
however, they demonstrated differential readings. Additionally, on
the 159th day, when the CTC count reached 218% of the initial
level, the values of CEA and CA19-9 remained below the initial
levels. Such evidence demonstrates that the CTC count may function
as a definitive and instant marker without a time lag, as shown
previously (15,16).
In conclusion, the CTC count may serve as a reliable
predictive biomarker of therapeutic response for gastric cancer
subgroups, characterized by multiple bone metastases and/or DIC.
CTC count may also present a useful tool for molecular biological
analysis. Further confirmatory studies are warranted to investigate
the detection rate of CTCs in this particular subgroup of advanced
gastric cancer with bone metastasis and/or DIC.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for reviewing the English language of
the original manuscript.
Glossary
Abbreviations
Abbreviations:
|
CTC
|
circulating tumor cell
|
|
DIC
|
disseminated intravascular
coagulation
|
|
PTX
|
paclitaxel
|
|
CEA
|
carcinoembryonic antigen
|
|
CA19-9
|
carbohydrate antigen 19-9
|
|
DTX
|
docetaxel
|
|
CT
|
computed tomography
|
References
|
1
|
Yoshikawa K and Kitaoka H: Bone metastasis
of gastric cancer. Jpn J Surg. 13:173–176. 1983. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Silvestris N, Pantano F, Ibrahim T,
Gamucci T, De Vita F, Di Palma T, Pedrazzoli P, Barni S, Bernardo
A, Febbraro A, et al: Natural history of malignant bone disease in
gastric cancer: Final results of a multicenter bone metastasis
survey. PLoS One. 8:e744022013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Rhee J, Han SW, Oh DY, Im SA, Kim TY and
Bang YJ: Clinicopathologic features and clinical outcomes of
gastric cancer that initially presents with disseminated
intravascular coagulation: A retrospective study. J Gastroenterol
Hepatol. 25:1537–1542. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Takashima A, Shirao K, Hirashima Y,
Takahari D, Okita NT, Nakajima TE, Kato K, Hamaguchi T, Yamada Y
and Shimada Y: Sequential chemotherapy with methotrexate and
5-fluorouracil for chemotherapy-naive advanced gastric cancer with
disseminated intravascular coagulation at initial diagnosis. J
Cancer Res Clin Oncol. 136:243–248. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Etoh T, Baba H, Taketomi A, Nakashima H,
Kohnoe S, Seo Y, Fukuda T and Tomoda H: Diffuse bone metastasis
with hematologic disorders from gastric cancer: Clinicopathological
features and prognosis. Oncol Rep. 6:601–605. 1999.PubMed/NCBI
|
|
6
|
Etoh T, Baba H, Taketomi A, Nakashima H,
Kohnoe S, Seo Y, Saito T and Tomoda H: Sequential methothrextate
and 5-fuororacil therapy for diffuse bone metastasis from gastric
cancer. Anticancer Res. 18:2085–2088. 1998.PubMed/NCBI
|
|
7
|
Kang BW, Kim JG, Kwon OK, Chung HY and Yu
W: Non-platinum-based chemotherapy for treatment of advanced
gastric cancer: 5-fluorouracil, taxanes, and irinotecan. World J
Gastroenterol. 20:5396–5402. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Boku N, Yamamoto S, Fukuda H, Shirao K,
Doi T, Sawaki A, Koizumi W, Saito H, Yamaguchi K, Takiuchi H, et
al: Gastrointestinal Oncology Study Group of the Japan Clinical
Oncology Group: Fluorouracil versus combination of irinotecan plus
cisplatin versus S-1 in metastatic gastric cancer: A randomised
phase 3 study. Lancet Oncol. 10:1063–1069. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Cao C, Zhang X, Kuang M, Gu D, He M, Chen
J and Tang C: Survival benefit from S-1 as compared to Fluorouracil
in Asian patients with advanced gastrointestinal cancer: A
meta-analysis. Cancer Sci. 105:1008–1014. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bang YJ, Van Cutsem E, Feyereislova A,
Chung HC, Shen L, Sawaki A, Lordick F, Ohtsu A, Omuro Y, Satoh T,
et al: ToGA Trial Investigators: Trastuzumab in combination with
chemotherapy versus chemotherapy alone for treatment of
HER2-positive advanced gastric or gastro-oesophageal junction
cancer (ToGA): A phase 3, open-label, randomised controlled trial.
Lancet. 376:687–697. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hiraiwa K, Takeuchi H, Hasegawa H, Saikawa
Y, Suda K, Ando T, Kumagai K, Irino T, Yoshikawa T, Matsuda S, et
al: Clinical significance of circulating tumor cells in blood from
patients with gastrointestinal cancers. Ann Surg Oncol.
15:3092–3100. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Otsuka K, Imai H, Soeda H, Komine K,
Ishioka C and Shibata H: Practical utility of circulating tumour
cells as biomarkers in cancer chemotherapy for advanced colorectal
cancer. Anticancer Res. 33:625–629. 2013.PubMed/NCBI
|
|
13
|
Komine K, Inoue M, Otsuka K, Fukuda K,
Nanjo H and Shibata H: Utility of measuring circulating tumor cell
counts to assess the efficacy of treatment for carcinomas of
unknown primary origin. Anticancer Res. 34:3165–3168.
2014.PubMed/NCBI
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cristofanilli M, Budd GT, Ellis MJ,
Stopeck A, Matera J, Miller MC, Reuben JM, Doyle GV, Allard WJ,
Terstappen LW and Hayes DF: Circulating tumor cells, disease
progression, and survival in metastatic breast cancer. N Engl J
Med. 351:781–971. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Moreno JG, O'Hara SM, Gross S, Doyle G,
Fritsche H, Gomella LG and Terstappen LW: Changes in circulating
carcinoma cells in patients with metastatic prostate cancer
correlate with disease status. Urology. 58:386–392. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Koga T, Tokunaga E, Sumiyoshi Y, Oki E,
Oda S, Takahashi I, Kakeji Y, Baba H and Maehara Y: Detection of
circulating gastric cancer cells in peripheral blood using real
time quantitative RT-PCR. Hepatogastroenterology. 55:1131–1135.
2008.PubMed/NCBI
|
|
18
|
Toyoshima K, Hayashi A, Kashiwagi M,
Hayashi N, Iwatsuki M, Ishimoto T, Baba Y, Baba H and Ohta Y:
Analysis of circulating tumor cells derived from advanced gastric
cancer. Int J Cancer. 137:991–998. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Pantel K and Alix-Panabières C: Bone
marrow as a reservoir for disseminated tumor cells: A special
source for liquid biopsy in cancer patients. Bonekey Rep.
3:5842014. View Article : Google Scholar : PubMed/NCBI
|