Introduction
Angiogenesis is important in tumor progression, and
has been one of the targets of cancer treatment (1). Angiogenesis inhibitors have been
demonstrated to confer survival benefits in patients with
colorectal, non-small cell lung, breast and renal cell carcinoma
(2). Several angiogenesis inhibitors
are currently available worldwide, and unique adverse events
associated with their use, which do not occur with traditional
chemotherapy regimens, have been suspected (3). These include modest-to-mild
hypertension, proteinuria, and epistaxis, which are manageable,
although serious adverse events such as gastrointestinal
hemorrhage, gastrointestinal tract perforation, and arterial and
venous thromboembolisms have also been reported (2).
The oral tyrosine kinase inhibitor pazopanib was the
first molecular-targeted agent approved for the treatment of
advanced soft tissue sarcoma (4).
Pazopanib is a novel multi-kinase inhibitor that inhibits
angiogenesis, and has been demonstrated to improve progression-free
survival, compared with placebo, in the PALETTE trial (4). The majority of the adverse events
reported for this drug, including hypertension, fatigue and
diarrhea, were mild-to-modest, although severe hemorrhage has also
been reported (5–8).
Thus far, bleeding events associated with the use of
bevacizumab, sorafenib, sunitinib and pazopanib have been reported
(9,10). To avoid them, care must be taken when
selecting patients for angiogenesis treatments (10).
In the present study, a case of mortality due to
massive hemorrhage following remarkable tumor shrinkage induced by
radiation therapy and subsequent pazopanib treatment is
reported.
Case report
A 71-year-old Japanese woman was referred to the
Department of Neurosurgery of Keio University School of Medicine
(Tokyo, Japan) in September 2007 for a left buccal palsy. The
patient had never smoked and did not drink alcohol. Her medical
history revealed hypertension, which was well-controlled with
candesartan (8 mg/day), and chronic hepatitis C, which was
inactive. Her performance status was evaluated as 1, due to hearing
impairment. Written informed consent was obtained from the
patient's guardian for the publication of the study.
An infratemporal fossa tumor was diagnosed and
removed surgically. The pathological diagnosis was leiomyosarcoma.
In total, the patient underwent six attempts of surgical removal
and three courses of radiotherapy (64 Gy/32 fractions, 54 Gy/27
fractions and 60 Gy/30 fractions; Clinac® iX System Linear
Accelerator; Varian Medical Systems, Palo Alto, CA, USA) for local
recurrence. Metastasis to the right lung was later identified, with
rapidly progressing local invasion from the left infratemporal
fossa into the maxillary sinus and oral cavity. In consequence, the
patient underwent four cycles of systemic chemotherapy over three
months with mesna (1500 mg; intravenous), adriamycin (60
mg/m2; intravenous), ifosfamide (2.5 g/m2;
intravenous) and dacarbazine (300 mg/m2; intravenous)
(MAID) regimen. Remarkable responses were achieved in the locally
advanced primary tumors and pulmonary lesions. Following 2 years of
treatment, although the primary lesion had stabilized, the lung
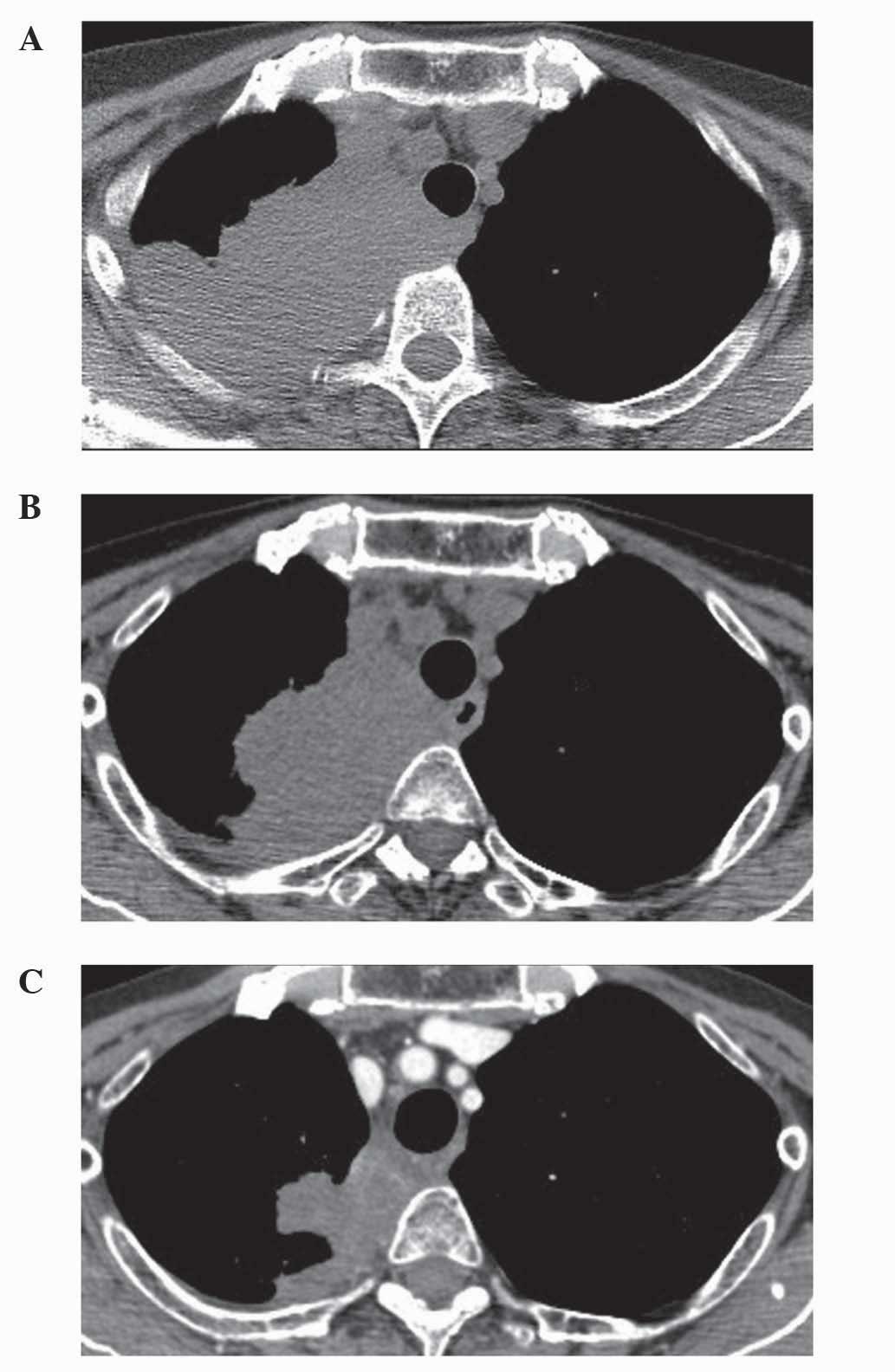
metastasis had progressed as revealed by a computed tomography scan
(Fig. 1A; Aquilion™; Toshiba Medical
Systems Ltd., Otawara, Japan). A further course of MAID
chemotherapy was administered. However, the tumor was refractory to
this treatment, and the patient was referred to the Keio Cancer
Center of Keio University School of Medicine (Tokyo, Japan) in
January 2013. Since her pulmonary lesion was increasing in size,
thus increasing the risk of airway obstruction, radiation therapy
(50 Gy/20 fractions; Clinac® iX System Linear Accelerator) was
administered to the patient, which resulted in 13% tumor shrinkage
(Fig. 1B). Subsequently, the patient
was administered oral pazopanib daily (800 mg/day; GlaxoSmithKline,
Brentford, UK) as palliative chemotherapy in the outpatient
clinic.
Upon initiation of pazopanib, grade 2 hypertension
and grade 2 mucositis [according to the National Cancer Institute
Common Terminology Criteria for Adverse Events (11)] were observed on day 14. Due to the
development of corns on both feet on day 55 (known as grade 3
palmar-plantar erythrodysesthesia syndrome), pazopanib was ceased
for 2 weeks, and recommenced when this symptom had resolved. At
this stage, 38% shrinkage of the tumor in the right lung was
evident (Fig. 1C). However, following
3 months of treatment, the patient suddenly vomited blood at home
and succumbed to the hemorrhage.
At autopsy, the primary leiomyosarcoma in the brain
and the metastasis in the right lung were confirmed. However, no
ulcer formation in the gastrointestinal tract, ruptured blood
vessel or cavity formation were observed.
Discussion
The present study reports a case of
hemorrhage-associated mortality and tumor shrinkage induced by
radiation therapy, followed by pazopanib treatment. There are three
main possible explanations for the fatal hemorrhage.
First, the possibility that the hemorrhage was an
adverse reaction to pazopanib must be considered, since vascular
endothelial growth factor (VEGF) and multi-kinase inhibitors may
cause hemorrhage, and pazopanib has been reported to cause
gastrointestinal and intracranial hemorrhage (5–8).
Second, the effects of radiation continue for few
months post-treatment and include severe fibrosis (3). A previous study reported one patient who
succumbed to hemorrhage upon receiving >50 Gy of radiation to
the pulmonary artery and bronchus (12). Furthermore, another report warned
against using stereotactic body radiation therapy in patients with
tumors located near the central airways due to various adverse
effects, including hemorrhagic mortality (13). In the present case, although the
patient was exposed to <50 Gy dose, the radiation may still have
been responsible for the hemorrhage.
Third, there is a risk of severe bleeding in
patients with remarkable shrinkage of tumor following irradiation,
which is generally associated with fibrosis in the surrounding
tissues (3). As reported by Hui et
al (3), tumor necrosis and
cavitation are associated with bleeding events. Thus, tumor
shrinkage following treatment with anti-angiogenic therapy is
associated with an increased risk of bleeding (3). In the present case, although there was
no cavity formation, remarkable tumor shrinkage had occurred.
Considering that the lung metastasis in the present patient was
adjacent to the trachea, shrinkage of the tumor may have caused
perforation of the trachea and subsequent hemorrhage from the
tumor.
Therefore, it may be hypothesized that the synergic
effects of pazopanib, radiotherapy and tumor shrinkage may have
been responsible for the bleeding in the present case.
In conclusion, remarkable tumor response associated
with radiation therapy may be a risk factor for severe hemorrhage
when combined with pazopanib. Thus, clinicians should be alert to
the possibility of this adverse event. In this situation, delaying
the introduction of pazopanib therapy may be an option. Further
investigation of the possible mechanisms leading to fatal
hemorrhage, and analysis of the risk factors associated with the
hemorrhagic adverse events caused by VEGF and multi-kinase
inhibitors, are required.
References
|
1
|
Ferrara N: Role of vascular endothelial
growth factor in physiologic and pathologic angiogenesis:
Therapeutic implications. Semin Oncol. 29(Suppl 16): S10–S14. 2002.
View Article : Google Scholar
|
|
2
|
Hapani S, Sher A, Chu D and Wu S:
Increased risk of serious hemorrhage with bevacizumab in cancer
patients: A meta-analysis. Oncology. 79:27–38. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hui EP, Ma BB, King AD, Mo F, Chan SL, Kam
MK, Loong HH, Ahuja AT, Zee BC and Chan AT: Hemorrhagic
complications in a phase II study of sunitinib in patients of
nasopharyngeal carcinoma who has previously received high-dose
radiation. Ann Oncol. 22:1280–1287. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
van der Graaf WT, Blay JY, Chawla SP, Kim
DW, Bui-Nguyen B, Casali PG, Schöffski P, Aglietta M, Staddon AP,
Beppu Y, et al: EORTC Soft Tissue and Bone Sarcoma Group; PALETTE
study group: Pazopanib for metastatic soft-tissue sarcoma
(PALETTE): A randomised, double-blind, placebo-controlled phase 3
trial. Lancet. 379:1879–1886. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Iwamoto FM, Lamborn KR, Robins HI, Mehta
MP, Chang SM, Butowski NA, Deangelis LM, Abrey LE, Zhang WT, Prados
MD and Fine HA: Phase II trial of pazopanib (GW786034), an oral
multi-targeted angiogenesis inhibitor, for adults with recurrent
glioblastoma (North American Brain Tumor Consortium Study 06–02).
Neuro Oncol. 12:855–861. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Taylor SK, Chia S, Dent S, Clemons M,
Agulnik M, Grenci P, Wang L, Oza AM, Ivy P, Pritchard KI and Leighl
NB: A phase II study of pazopanib in patients with recurrent or
metastatic invasive breast carcinoma: A trial of the Princess
Margaret Hospital phase II consortium. Oncologist. 15:810–818.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Bible KC, Suman VJ, Menefee ME, Smallridge
RC, Molina JR, Maples WJ, Karlin NJ, Traynor AM, Kumar P, Goh BC,
et al: Mayo Phase 2 Consortium; Mayo Clinic Endocrine Malignances
Disease Oriented Group: A multiinstitutional phase 2 trial of
pazopanib monotherapy in advanced anaplastic thyroid cancer. J Clin
Endocrinol Metab. 97:3179–3184. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Bender Glade JL, Lee A, Reid JM, Baruchel
S, Roberts T, Voss SD, Wu B, Ahern CH, Ingle AM, Harris P, et al:
Phase I pharmacokinetic and pharmacodynamic study of pazopanib in
children with soft tissue sarcoma and other refractory solid
tumors: A childrens oncology group phase I consortium report. J
Clin Oncol. 31:3034–3043. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hang XF, Xu WS, Wang JX, Wang L, Xin HG,
Zhang RQ and Ni W: Risk of high-grade bleeding in patients with
cancer treated with bevacizumab: A meta-analysis of randomized
controlled trials. Eur J Clin Pharmacol. 67:613–623. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Schutz FA, Je Y, Richards CJ and Choueiri
TK: Meta-analysis of randomized controlled trials for the incidence
and risk of treatment-related mortality in patients with cancer
treated with vascular endothelial growth factor tyrosine kinase
inhibitors. J Clin Oncol. 30:871–877. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
National Cancer Institute: Common
terminology criteria for adverse events. version 4.0.3. simpleevs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdfAccessed.
Feb 06–2016
|
|
12
|
Nishimura S, Takeda A, Sanuki N, Ishikura
S, Oku Y, Aoki Y, Kunieda E and Shigematsu N: Toxicities of organs
at risk in the mediastinal and hilar regions following stereotactic
body radiotherapy for centrally located lung tumors. J Thorac
Oncol. 9:1370–1376. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Timmerman R, McGarry R, Yiannoutsos C,
Papiez L, Tudor K, DeLuca J, Ewing M, Abdulrahman R, DesRosiers C,
Williams M and Fletcher J: Excessive toxicity when treating central
tumors in a phase II study of stereotactic body radiation therapy
for medically inoperable early-stage lung cancer. J Clin Oncol.
24:4833–4839. 2006. View Article : Google Scholar : PubMed/NCBI
|