Introduction
Brain tumors are characterized by the presence of
malignant tissues within the skull and central spinal canal.
Between 2008 and 2012 in the USA, the annual age-adjusted average
incidence rate for all primary brain and central nervous system
tumors was 21.98 per 100,000 individuals (7.23 malignant; 14.75
benign), and 356,858 brain tumors (117,023 malignant; 239,835
benign) were reported during this time (1). Gliomas are the most common primary brain
tumor, accounting for ~81% of malignant brain tumors (2). Between 2008 and 2012, the annual
age-adjusted average incidence rate of this type of brain tumor was
5.83 per 100,000 individuals (1). A
characteristic of glioma is that it spreads rapidly to normal brain
areas so that the boundary between normal tissue and the tumor
becomes indistinct (3). In
particular, it has the highest mortality rate due to its
specialized feature of rapid cell migration or invasion that cannot
be controlled by either surgery or irradiation (4,5). Thus,
patients with this brain disease have an average survival rate of 1
year from the time of tumor development.
Moreover, the diagnosis and treatment of glioma are
difficult, its pathology and pattern of invasion and migration are
poorly understood (6,7). Several studies have reported that the
pathological response of glioma, which is the malignant process of
infiltration into the extensive normal tissue, is due to the
activation of mitogen-activated protein kinase (MAPK), protein
kinase Cα (PKCα) and matrix metalloproteinases (MMPs) (8–11). The
MAPK family consists of three types of kinases, p38 MAPK, Jun
N-terminal kinase and extracellular signal-regulated kinase 1/2
(ERK1/2), which are involved in cell migration and the growth of
the majority of cancer cell types (12). Notably, ERK1/2 phosphorylation is
involved in the cell invasion, migration and motility coupled with
the progression of brain cancer (13,14).
Activation of PKCα is also implicated in the migration of glioma
cells (15). MMPs are extracellular
endopeptidases involved in motility and invasion (16,17).
Currently, numerous researchers are seeking novel
antioxidant and anticancer agents derived from plants. Thymol is a
component of a number of essential oils and is known for its
anti-inflammatory, anticancer and anti-bacterial effects (18). Studies on essential oils extracted
from a variety of plants have shown that they possess great
nutritional value, and significant biochemical and physiological
activities (18). These beneficial
properties have been put to use in the development of functional
and medicinal foods. The present study was performed to investigate
the effect of thymol on glioma cell migration to determine whether
it may have potential in glioma prevention and treatment.
Materials and methods
Reagents
Thymol, cell culture materials and the EZ-Cytox Cell
Viability Assay kit were purchased from Sigma-Aldrich (St. Louis,
MO, USA), Thermo Fisher Scientific (Gaithersburg, MD, USA) and
Daeil Lab Services Co., Ltd. (Seoul, Korea), respectively. Rabbit
polyclonal anti-rat anti-PKCα (catalog no., #2056), rabbit
polyclonal anti-rat anti-phosphorylated (P)-PKCα (catalog no.,
#9375) and rabbit polyclonal anti-rat anti-glyceraldehyde
3-phosphate dehydrogenase (catalog no., #2118) antibodies were
obtained from Cell Signaling Technology Inc. (Beverly, MA, USA).
Other antibodies, such as rabbit polyclonal anti-rat anti-ERK1/2
(catalog no., sc-94), mouse monoclonal anti-rat anti-P-ERK1/2
(catalog no., sc-7383), rabbit polyclonal anti-rat anti-MMP2
(catalog no., sc-10736) and goat polyclonal anti-rat anti-MMP9
(catalog no., sc-6840), were purchased from Santa Cruz
Biotechnology Inc. (Dallas, TX, USA). All other chemicals were
purchased from Sigma-Aldrich.
Cell culture and viability assay
Rat C6 glioma cells were obtained from the Korean
Cell Line Bank (Seoul, Korea) and were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(FBS) and 1% penicillin-streptomycin at 37°C in a 5% CO2
atmosphere. The C6 glioma cells were seeded at 5×104 cells/well in
a 96-well microplate containing DMEM and incubated for 24 h. The
cells were then incubated with different concentrations (0, 0.1,
0.3, 1, 3, 10, 30, 100 and 200 µM) of thymol in FBS-free medium for
24 h. Cell viability was then determined using an EZ-Cytox Cell
Viability Assay kit according to the manufacturer's protocol. The
cell viability of thymol-treated cells was determined relative to
that of control cells by measuring the absorbance at 450 nm.
Scratch wound healing assay
The C6 glioma cells were seeded at a density of
1×105 cells/ml in a six-well plate and incubated in 10%
FBS-containing medium for 24 h. These cells were then placed in
serum-free medium for 24 h. The scratch wound was made by
scratching the center of each well with a 200-µl sterile pipette
tip to form a cross. This was followed by incubation with or
without thymol (0, 3, 10 and 30 µm) in serum-containing medium for
an additional 24 h. Images of the cells that migrated into the
cell-free scratch wound area were acquired using an inverted
microscope (IX71; Olympus Corp., Tokyo, Japan) and analyzed using
Image J software (National Institutes of Health, Bethesda, MD,
USA).
Boyden chamber assay
To determine the effect of thymol on the migration
of the C6 glioma cells, a Boyden chamber assay was performed in a
48-well chemotaxis chamber (Neuro-Probe, Gaithersburg, MD, USA), as
previously described (19). Briefly,
an absence or presence of thymol (3–30 µM) in DMEM containing 10%
FBS were loaded into the lower chamber. The lower chamber was
covered by a polycarbonate filter membrane (pore size, 8 µm) that
was coated with 0.1% collagen type-I (BD Biosciences, Franklin
Lakes, NJ, USA). C6 glioma cells (1×106 cells/ml) were
loaded into the upper chamber. Following incubation at 37°C in 5%
CO2 for 90 min, the cells on the lower surface of the
membrane were fixed and stained using Diff-Quick (Baxter
Healthcare, Deerfield, IL, USA). The cells that had migrated
through the membrane were imaged and counted under an inverted
microscope (IX71; Olympus Corporation, Tokyo, Japan).
Gelatin zymography
To determine the activity of gelatinases, such as
MMP2 and MMP9, in the thymol-treated C6 glioma cells, a gelatin
zymography assay was performed, as previously described (8). Briefly, to test the gelatin zymography,
samples of cultured media supernatant from C6 glioma cells were
collected. Samples (30 µl) were loaded onto a 8% sodium dodecyl
sulfate-polyacrylamide electrophoresis gel containing 0.2% gelatin
(WELGENE, Daegu, South Korea). Following electrophoresis, the gel
was incubated with 2.5% Triton X-100 (Sigma-Aldrich) and agitated.
Following incubation at 37°C for 24 h, the gel was stained using
Coomassie Brilliant Blue R 250 (Sigma-Aldrich). Stained bands were
visualized and quantified using Image J software.
Western blot analysis
To determine the expression of proteins associated
with the migration of C6 glioma cells, western blotting was
performed with specific antibodies. Briefly, 20 µg of protein was
prepared from each treatment group. Once the proteins had been
boiled at 100°C for 10 min, they were separated by electrophoresis
on 12% acrylamide gels and then transferred onto polyvinylidene
difluoride membranes (Amersham Pharmacia Biotech, Piscataway, NJ,
USA) in transfer buffer at 4°C for 2 h. The membranes were blocked
in 5% bovine serum albumin in Tris-buffered saline (TBS) at room
temperature for 1 h and then washed in TBS with 0.1% Tween 20
(TBS/T). Subsequently, the membranes were incubated overnight at
4°C with antibodies against P-ERK1/2 and P-PKCα, total ERK1/2
(T-ERK1/2) and PKC (T-PKC), MMP9 and MMP2 (all 1:1,000 dilution).
The membranes were washed with TBS/T, followed by incubation with
donkey anti-goat immunoglobulin (Ig) G (catalog no., sc-2033; Santa
Cruz Biotechnology, Inc.), horse anti-mouse IgG (catalog no., 7076;
Cell Signaling Technology, Inc.) or goat anti-rabbit IgG (catalog
no., 7074; Cell Signaling Technology, Inc.) horseradish
peroxidase-conjugated secondary antibodies (all 1:1,000 dilution).
The protein expression levels were analyzed via
electrochemiluminescence (ECL plus kit; Amersham Pharmacia
Biotech). The protein bands were visualized and quantified using
Image J Software.
Statistical analysis
The results are expressed as the mean ± standard
error of at least three independent experiments. The differences
between the test groups were examined using a one-way analysis of
variance followed by Tukey's test. P<0.05 was considered to
indicate a statistically significant difference. Statistical
analysis was performed with GraphPad Prism 4.0 software (GraphPad
Software Inc., San Diego, CA, USA).
Results
Effect of thymol on C6 glioma cell
viability
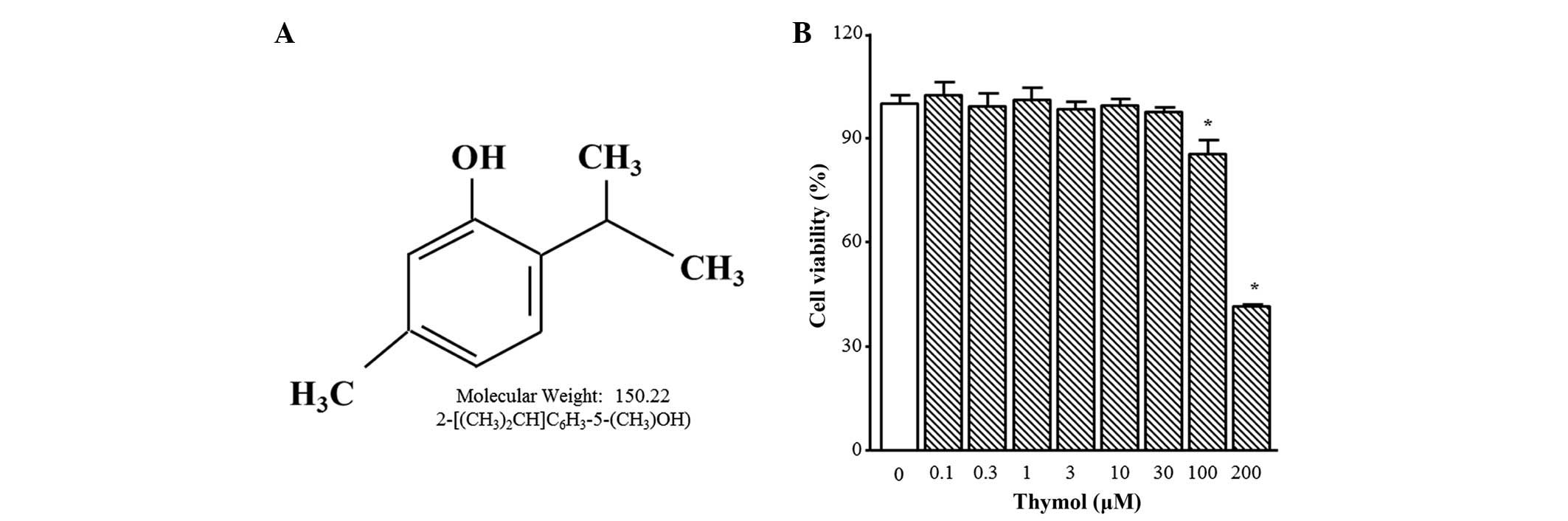
The chemical structure of thymol is presented in
Fig. 1A. Thymol cytotoxicity was
tested by treating C6 glioma cells with different concentrations
(0.1–200 µM) of thymol for 24 h. Cell viability was not altered by
thymol up to 30 µM, but 100 µM and 200 µM of thymol induced
significant decreases in cell viability of 24.0±6.5 and 54.2±3.5%,
respectively (Fig. 1B; P=0.0361 and
P<0.0001, respectively). Therefore, all of the following
experiments were performed using 30 µM thymol or less.
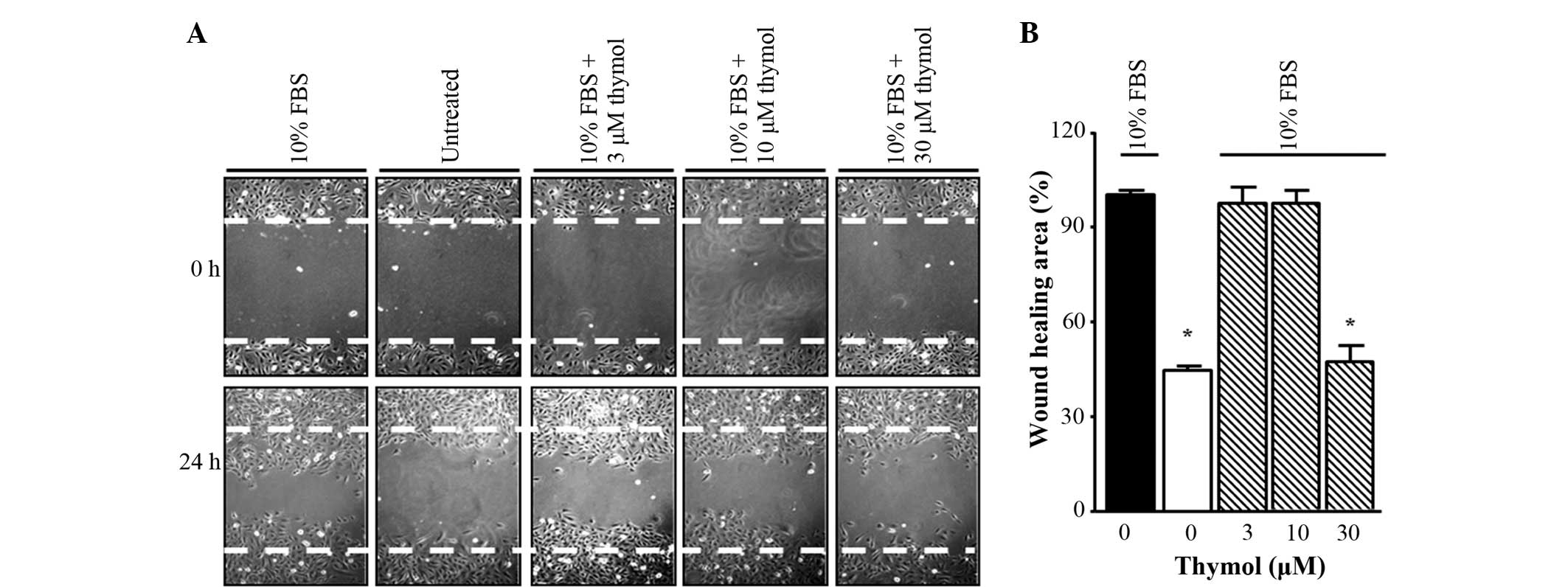
Thymol suppresses C6 glioma cell
migration
To determine whether thymol could inhibit the
migration of FBS-stimulated glioma cells, a scratch wound healing
assay was first performed. The cells were incubated in FBS-free
medium for 24 h and then stimulated with 10% FBS in the presence of
different concentrations of thymol (3–30 µM) for 24 h. As shown in
Fig. 2, 30 µM thymol treatment caused
a decrease in scratch wound healing compared with FBS treatment
alone, whereas for other concentrations of thymol (3–10 µM), the
inhibition ratios indicated similar results to the untreated group.
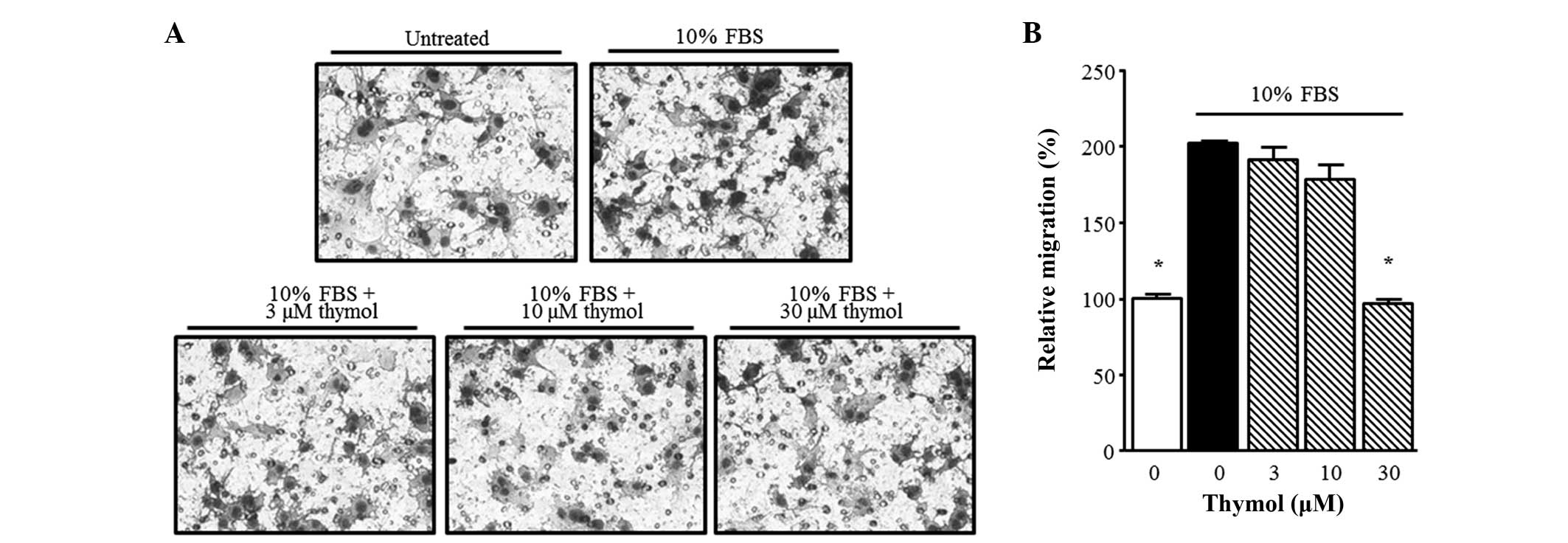
Next, in order to confirm the inhibitory effect of thymol on C6
glioma cell migration, a Boyden chamber assay was performed. Thymol
(30 µM) suppressed the FBS-stimulated migration of glioma cells
over 90 min. As shown in Fig. 3,
FBS-stimulated C6 glioma cell migration was significantly inhibited
by 30 µM thymol (P=0.0001).
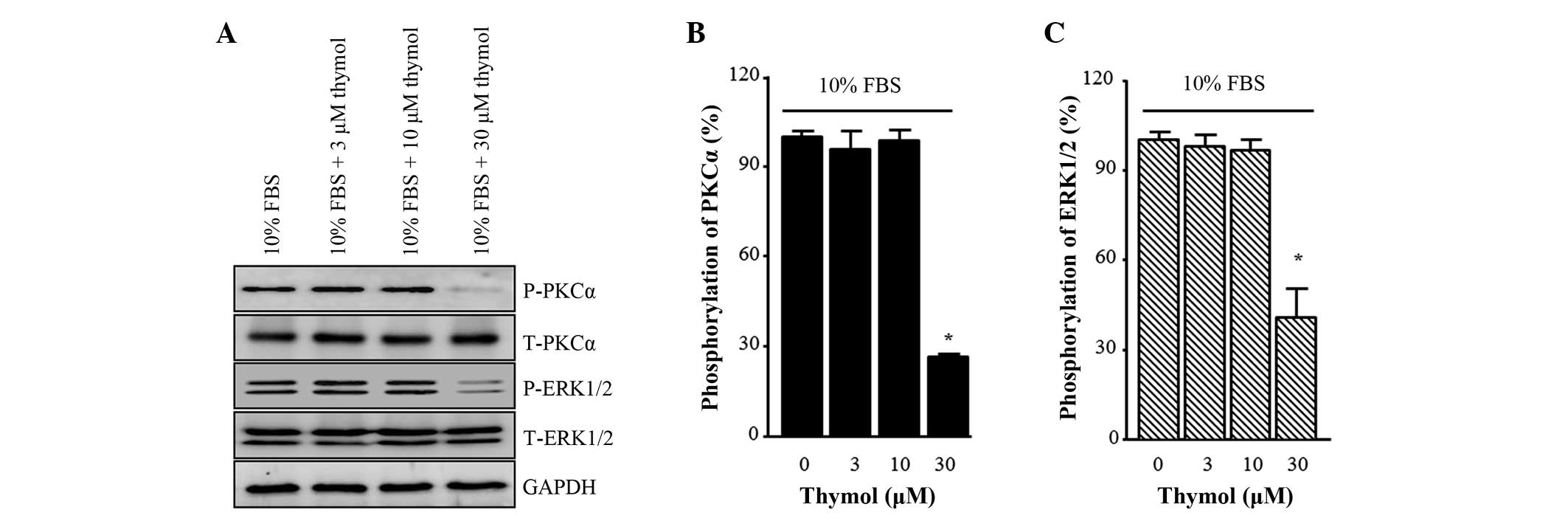
Effect of thymol on the
phosphorylation of PKCα and ERK1/2
To investigate the expression of protein markers for
C6 glioma cell migration, the phosphorylation levels of PKCα and
ERK1/2 were measured. As shown in Fig.
4, FBS-induced PKCα phosphorylation was not affected by 3 and
10 µM thymol compared with the control. However, it decreased to
26.5±1.2%, when the cells were treated with 30 µM thymol (Fig. 4B; P<0.0001). FBS
induced-phosphorylation of ERK1/2 also showed a similar pattern,
with no change observed in response to 3 and 10 µM thymol and a
significant reduction observed in response to 30 µM thymol
(Fig. 4C; P=0.0044).
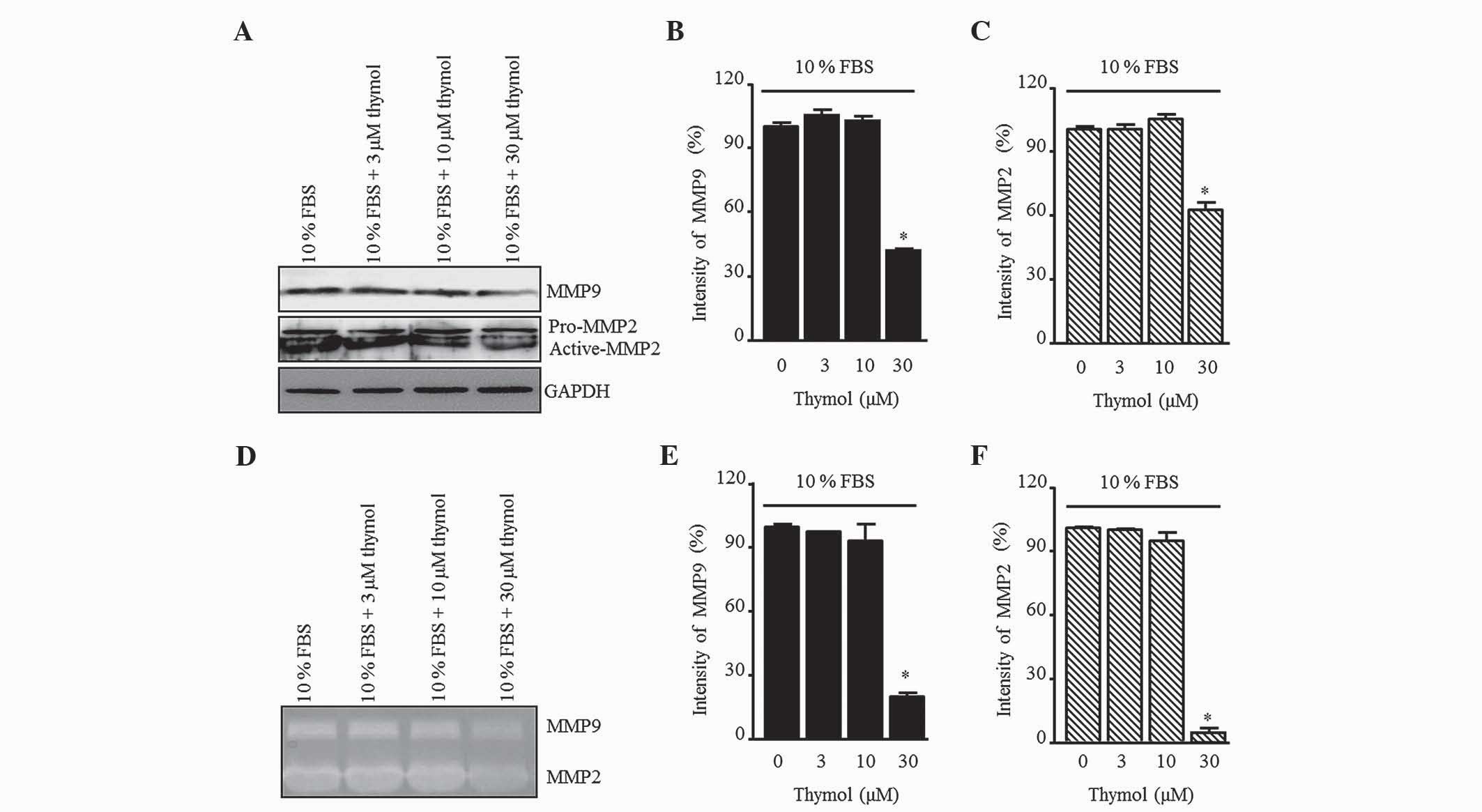
Effect of thymol on MMP9 and MMP2
To elucidate the inhibitory effect of thymol on the
production of gelatinases in FBS-stimulated C6 glioma cells, the
MMP9 and MMP2 levels were measured by western blotting (Fig. 5A). Thymol (30 µM) decreased the
expression of MMP9 and MMP2, whereas there was no change relative
to the control when 3 and 10 µM thymol was used (Fig. 5B and C; P=0.0012 and P=0.0001,
respectively). Next, MMP activation was confirmed by gelatin
zymography (Fig. 5D). The pattern of
MMP secretion in the zymography assay was similar to that of the
control group in response to 3 and 10 µM thymol, but a significant
reduction was observed in response to 30 µM thymol (Fig. 5E and F; P=0.0001).
Discussion
The present study demonstrated that a natural
monoterpenic phenol, thymol, inhibited the phosphorylation of
ERK1/2 and PKCα, reduced the expression of MMP2 and MMP9, and
decreased the migration of the C6 glioma cells. The anticancer
effect of thymol on various cancers has been reported (20–24).
However, the effect of thymol on the motility of glioma cells has
not yet been established. Malignant glioma has been reported as an
incurable tumor and represents ~50% of all brain tumors. Glioma is
a highly invasive and lethal form of brain cancer, which is
extremely difficult to treat via surgery or combined radiotherapy
and chemotherapy (3). Consequently,
the median patient survival time is limited to 12 months according
to World Health Organization research (8). The relapse rate is extremely high and
may lead to mortality, even with aggressive therapy. Although the
strategy used in the present study is not a direct clinical method,
this approach, in addition to those focusing on apoptosis or cell
death, may prove useful for improving patient survival and slowing
down cancer progression. Hence, we speculated that the inhibition
of glioma cell migration could be a therapeutic strategy. Thus, a
component from plant essential oils that could be effective for the
treatment and, more importantly, the prevention of glioma was
searched for. As a result, the present study was focused on thymol,
which has been widely reported as a component of plant essential
oils (18).
In the present study, the scratch wound healing and
Boyden chamber assays were used to assess glioma migration. The
results indicated that thymol inhibits FBS-induced C6 glioma cell
migration. Furthermore, thymol significantly inhibited the
phosphorylation of PKCα and ERK1/2, as well as the activation and
expression of MMP9 and MMP2. PKC is an intracellular signaling
protein of the protein kinase family that regulates cell survival,
proliferation and differentiation, and the cell cycle (25–27). PKC
phosphorylation is involved in malignant transformation and tumor
promotion, as well as in invasion and metastasis. The high motility
of glioma cells is key to the inability to manage the disease,
preventing complete tumor removal and leading to therapeutic
failure and recurrence (15).
Moreover, elevated MMP2, which is regulated by PKC, is tightly
involved in glioma invasion (9).
Notably, Tam et al studied the role of PKCα in cancer
treatment and reported that it has been a major focus for various
types of cancer and even cancer stem cells (28). In addition, Hu et al reported
that activation of PKCα is also implicated in glioma cell migration
(15). ERK1/2 also contributes to
cell migration and proliferation in cancer cells (29,30).
Hence, the expression and phosphorylation of ERK1/2 was measured in
the C6 glioma cells of the present study. The results showed that
30 µM thymol attenuated ERK1/2 phosphorylation, but did not alter
T-ERK1/2 expression. Recently, the role of ERK1/2 in the expression
of the MMP family (MMP2 and MMP9) has been reported. MMP2 and MMP9
are also involved in tumor invasiveness and migration (10).
Taken together, the present results suggest that
thymol inhibits glioma cell migration through PKCα and ERK1/2
phosphorylation, which consequently results in a decrease in MMP9
and MMP2 expression. The study indicates that thymol is a potential
candidate for the treatment of malignant gliomas, as it reduces the
FBS-induced motility of C6 glioma cells. The results of the present
study shed light on the mechanism underlying the inhibitory effects
of thymol on C6 glioma cell migration. Further studies are
warranted to address whether the inhibitory effect of thymol on
PKCα and ERK1/2 phosphorylation is associated with neuroprotective
effects in normal cells.
Acknowledgements
The present study was supported by Konkuk University
(Seoul, Republic of Korea) in 2015.
References
|
1
|
Ostrom QT, Gittleman H, Fulop J, Liu M,
Blanda R, Kromer C, Wolinsky Y, Kruchko C and Barnholtz-Sloan JS:
CBTRUS statistical report: Primary brain and central nervous system
tumors diagnosed in the United States in 2008–2012. Neuro Oncol.
17(Suppl 4): iv1–iv622015. View Article : Google Scholar
|
|
2
|
Ostrom QT, Bauchet L, Davis FG, Deltour I,
Fisher JL, Langer CE, Pekmezci M, Schwartzbaum JA, Turner MC, Walsh
KM, et al: The epidemiology of glioma in adults: A “state of the
science” review. Neuro Oncol. 16:896–913. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Belda-Iniesta C, de Castro Carpeño J,
Casado Sáenz E, Cejas Guerrero P, Perona R and González Barón M:
Molecular biology of malignant gliomas. Clin Transl Oncol.
8:635–641. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Coniglio SJ and Segall JE: Review:
Molecular mechanism of microglia stimulated glioblastoma invasion.
Matrix Biol. 32:372–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Qi ST, Liu Y, Pan J, Chotai S and Fang LX:
A radiopathological classification of dural tail sign of
meningiomas. J Neurosurg. 117:645–653. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Hess KR, Broglio KR and Bondy ML: Adult
glioma incidence trends in the United States, 1977–2000. Cancer.
101:2293–2299. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chinot OL, Macdonald DR, Abrey LE,
Zahlmann G, Kerloëguen Y and Cloughesy TF: Response assessment
criteria for glioblastoma: Practical adaptation and implementation
in clinical trials of antiangiogenic therapy. Curr Neurol Neurosci
Rep. 13:3472013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Le DM, Besson A, Fogg DK, Choi KS, Waisman
DM, Goodyer CG, Rewcastle B and Yong VW: Exploitation of astrocytes
by glioma cells to facilitate invasiveness: A mechanism involving
matrix metalloproteinase-2 and the urokinase-type plasminogen
activator-plasmin cascade. J Neurosci. 23:4034–4043.
2003.PubMed/NCBI
|
|
9
|
Uhm JH, Dooley NP, Villemure JG and Yong
VW: Glioma invasion in vitro: Regulation by matrix
metalloprotease-2 and protein kinase C. Clin Exp Metastasis.
14:421–433. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Glassmann A, Reichmann K, Scheffler B,
Glas M, Veit N and Probstmeier R: Pharmacological targeting of the
constitutively activated MEK/MAPK-dependent signaling pathway in
glioma cells inhibits cell proliferation and migration. Int J
Oncol. 39:1567–1575. 2011.PubMed/NCBI
|
|
11
|
Soletti RC, Alves T, Vernal J, Terenzi H,
Anderluh G, Borges HL, Gabilan NH and Moura-Neto V: Inhibition of
MAPK/ERK, PKC and CaMKII signaling blocks cytolysin-induced human
glioma cell death. Anticancer Res. 30:1209–1215. 2010.PubMed/NCBI
|
|
12
|
Strnisková M, Barancík M and Ravingerová
T: Mitogen-activated protein kinases and their role in regulation
of cellular processes. Gen Physiol Biophys. 21:231–255.
2002.PubMed/NCBI
|
|
13
|
Chen CM, Hsieh YH, Hwang JM, Jan HJ, Hsieh
SC, Lin SH and Lai CY: Fisetin suppresses ADAM9 expression and
inhibits invasion of glioma cancer cells through increased
phosphorylation of ERK1/2. Tumour Biol. 36:3407–3415. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mendes O, Kim HT, Lungu G and Stoica G:
MMP2 role in breast cancer brain metastasis development and its
regulation by TIMP2 and ERK1/2. Clin Exp Metastasis. 24:341–351.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Hu JG, Wang XF, Zhou JS, Wang FC, Li XW
and Lü HZ: Activation of PKC-alpha is required for migration of C6
glioma cells. Acta Neurobiol Exp (Wars). 70:239–245.
2010.PubMed/NCBI
|
|
16
|
Köhrmann A, Kammerer U, Kapp M, Dietl J
and Anacker J: Expression of matrix metalloproteinases (MMPs) in
primary human breast cancer and breast cancer cell lines: New
findings and review of the literature. BMC Cancer. 9:1882009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Khasigov PZ, Podobed OV, Gracheva TS,
Salbiev KD, Grachev SV and Berezov TT: Role of matrix
metalloproteinases and their inhibitors in tumor invasion and
metastasis. Biochemistry (Mosc). 68:711–717. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Undeğer U, Başaran A, Degen GH and Başaran
N: Antioxidant activities of major thyme ingredients and lack of
(oxidative) DNA damage in V79 Chinese hamster lung fibroblast cells
at low levels of carvacrol and thymol. Food Chem Toxicol.
47:2037–2043. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Grommes C, Landreth GE, Sastre M, et al:
Inhibition of in vivo glioma growth and invasion by peroxisome
proliferator-activated receptor gamma agonist treatment. Mol
Pharmacol. 70:1524–1533. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Deb DD, Parimala G, Saravana Devi S and
Chakraborty T: Effect of thymol on peripheral blood mononuclear
cell PBMC and acute promyelotic cancer cell line HL-60. Chem Biol
Interact. 193:97–106. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Chang HT, Hsu SS, Chou CT, Cheng JS, Wang
JL, Lin KL, Fang YC, Chen WC, Chien JM, Lu T, et al: Effect of
thymol on Ca2+ homeostasis and viability in MG63 human
osteosarcoma cells. Pharmacology. 88:201–212. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Hsu SS, Lin KL, Chou CT, Chiang AJ, Liang
WZ, Chang HT, Tsai JY, Liao WC, Huang FD, Huang JK, et al: Effect
of thymol on Ca2+ homeostasis and viability in human glioblastoma
cells. Eur J Pharmacol. 670:85–91. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kang SH, Kim YS, Kim EK, et al: Anticancer
Effect of Thymol on AGS Human Gastric Carcinoma Cells. J Microbiol
Biotechnol. 26:28–37. 2016.PubMed/NCBI
|
|
24
|
Archana PR, Rao Nageshwar B and Rao Satish
BS: Modulation of gamma ray-induced genotoxic effect by thymol, a
monoterpene phenol derivative of cymene. Integr Cancer Ther.
10:374–383. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Carduner L, Picot CR, Leroy-Dudal J, Blay
L, Kellouche S and Carreiras F: Cell cycle arrest or survival
signaling through αv integrins, activation of PKC and ERK1/2 lead
to anoikis resistance of ovarian cancer spheroids. Exp Cell Res.
320:329–342. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kim H, Zamel R, Bai XH and Liu M: PKC
activation induces inflammatory response and cell death in human
bronchial epithelial cells. PLoS One. 8:e641822013. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hartleben B, Widmeier E, Suhm M, Worthmann
K, Schell C, Helmstädter M, Wiech T, Walz G, Leitges M, Schiffer M
and Huber TB: APKCλ/ι and aPKCζ contribute to podocyte
differentiation and glomerular maturation. J Am Soc Nephrol.
24:253–267. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tam WL, Lu H, Buikhuisen J, Soh BS, Lim E,
Reinhardt F, Wu ZJ, Krall JA, Bierie B, Guo W, et al: Protein
Kinase C α is a central signaling node and therapeutic target for
breast cancer stem cells. Cancer Cell. 24:347–364. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li Q, Fu GB, Zheng JT, He J, Niu XB, Chen
QD, Yin Y, Qian X, Xu Q, Wang M, et al: NADPH oxidase subunit p22
(phox)-mediated reactive oxygen species contribute to angiogenesis
and tumor growth through AKT and ERK1/2 signaling pathways in
prostate cancer. Biochim Biophys Acta. 1833:3375–3385. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Sun J, Liu SZ, Lin Y, Cao XP and Liu JM:
TGF-β promotes glioma cell growth via activating Nodal expression
through Smad and ERK1/2 pathways. Biochem Biophys Res Commun.
443:1066–1072. 2014. View Article : Google Scholar : PubMed/NCBI
|