Introduction
Lipomatous tumors constitute the largest subgroup of
mesenchymal neoplasms and are commonly encountered in clinical
practice. The diagnosis of lipomatous tumors is primarily based on
clinical features and histological patterns (1). Previously, immunostaining for murine
double-minute 2 (MDM2), cyclin-dependent kinase 4 (CDK4), and p16
has been demonstrated to be a highly sensitive and specific method
for distinguishing between atypical lipomatous tumors (ALTs) and
other lipomatous tumors (2).
Cytogenetic and molecular genetic studies have
provided insight into the pathogenesis of a variety of lipomatous
tumors. Overall, ~75% of ordinary lipomas harbor chromosomal
rearrangements of 12q13-15, resulting in deregulation of the high
mobility group AT-hook 2 (HMGA2) gene (3). By contrast, the cytogenetic hallmark of
ALT is the presence of one or more supernumerary ring or giant
marker chromosomes (4). The rings and
giant markers invariably contain amplified sequences derived from
chromosome 12q13-15 (5), including
the MDM2 and CDK4 genes.
Several cases of lipomatous tumors with minimal
nuclear atypia and low-level gain of the 12q13-15 region have been
reported (6–8). The present study describes the
cytogenetic and molecular cytogenetic findings of a lipomatous
tumor with minimal nuclear atypia arising in the shoulder of a
49-year-old woman. Written informed consent for this study was
obtained from the patient.
Case report
A 49-year-old woman presented to Fukuoka University
Hospital (Fukuoka, Japan) with a slow-growing, painless mass in the
right shoulder that had been developing over the previous ten
years. Physical examination revealed a 13-cm, soft, mobile,
non-tender mass. The neurovascular examinations were unremarkable.
The laboratory values were within normal ranges. Magnetic resonance
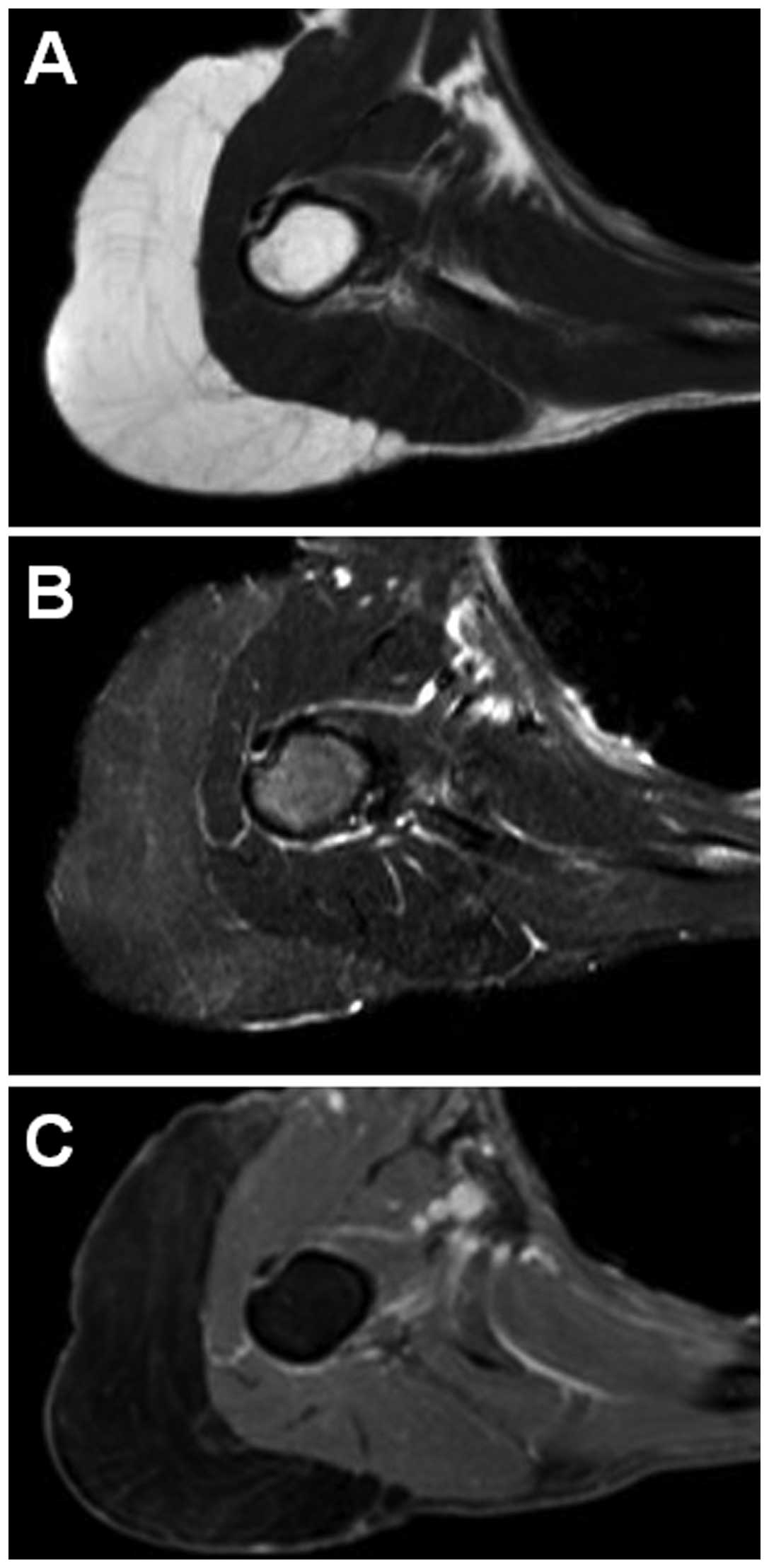
imaging (MRI) revealed a well-defined subcutaneous mass. The mass
exhibited a signal intensity identical to that of subcutaneous fat
on T1-weighted sequences (Fig. 1A)
and was completely suppressed on T2-weighted short tau inversion
recovery (STIR) sequences (Fig. 1B).
Faint hyperintensity within the mass was also observed on
T2-weighted STIR sequences. Contrast-enhanced fat-suppressed
T1-weighted sequences demonstrated faint enhancement (Fig. 1C). A marginal excision was therefore
performed.
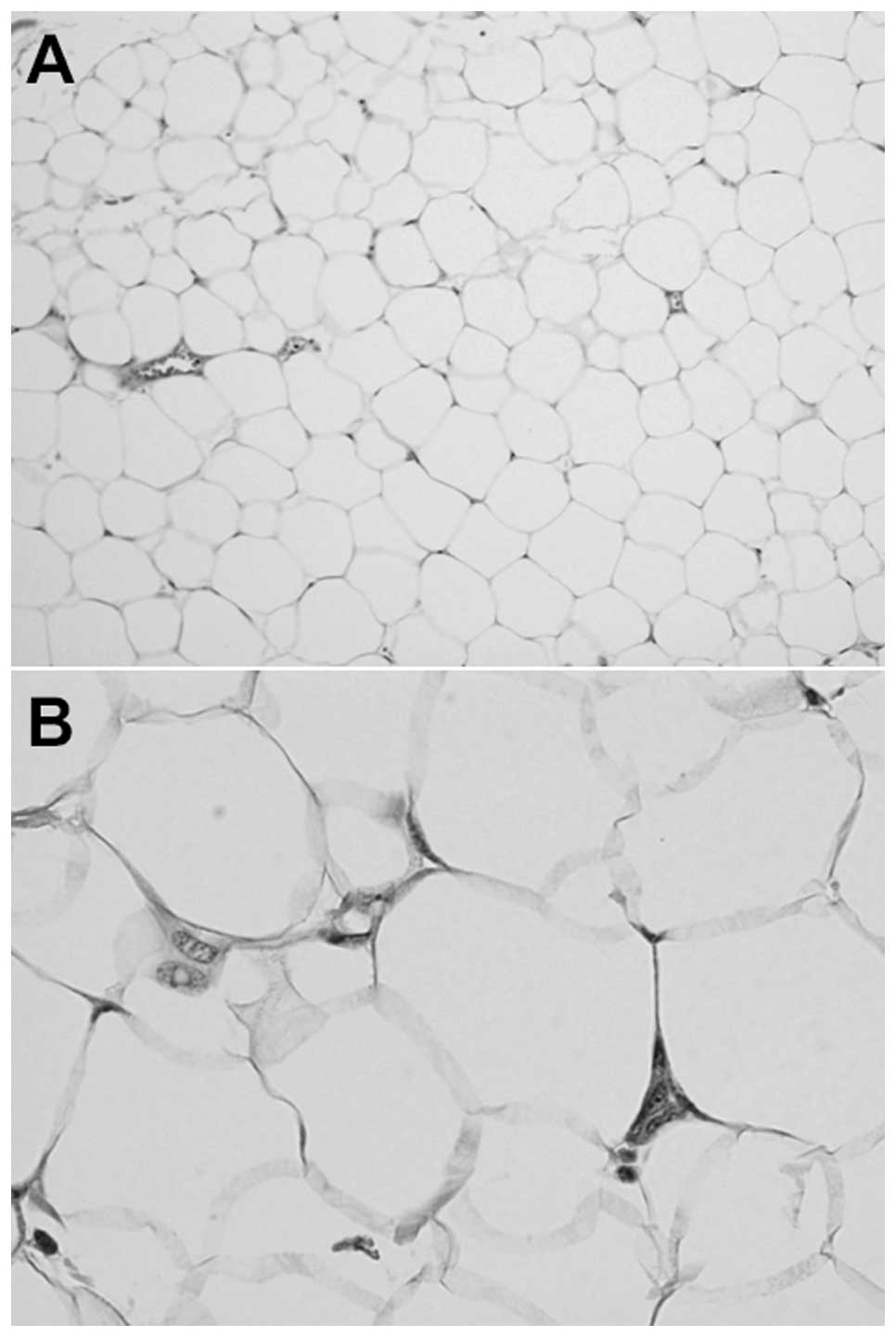
Microscopically, the tumor was composed of lobules
of mature adipocytes separated by delicate thin fibrous septa
(Fig. 2A). There was minimal nuclear
atypia in certain cells (Fig. 2B),
and a small number of binucleated cells were also observed.
Immunohistochemically, the tumor cells were revealed to not express
MDM2. The pathological diagnosis was lipomatous tumor with minimal
nuclear atypia.
A representative fresh tissue sample was obtained
for cytogenetic analysis. Standard culture and harvest procedures
were performed, as previously described (9). The karyotypes were expressed according
to the International System for Human Cytogenetic Nomenclature 2009
(10). In total, 20 metaphase cells
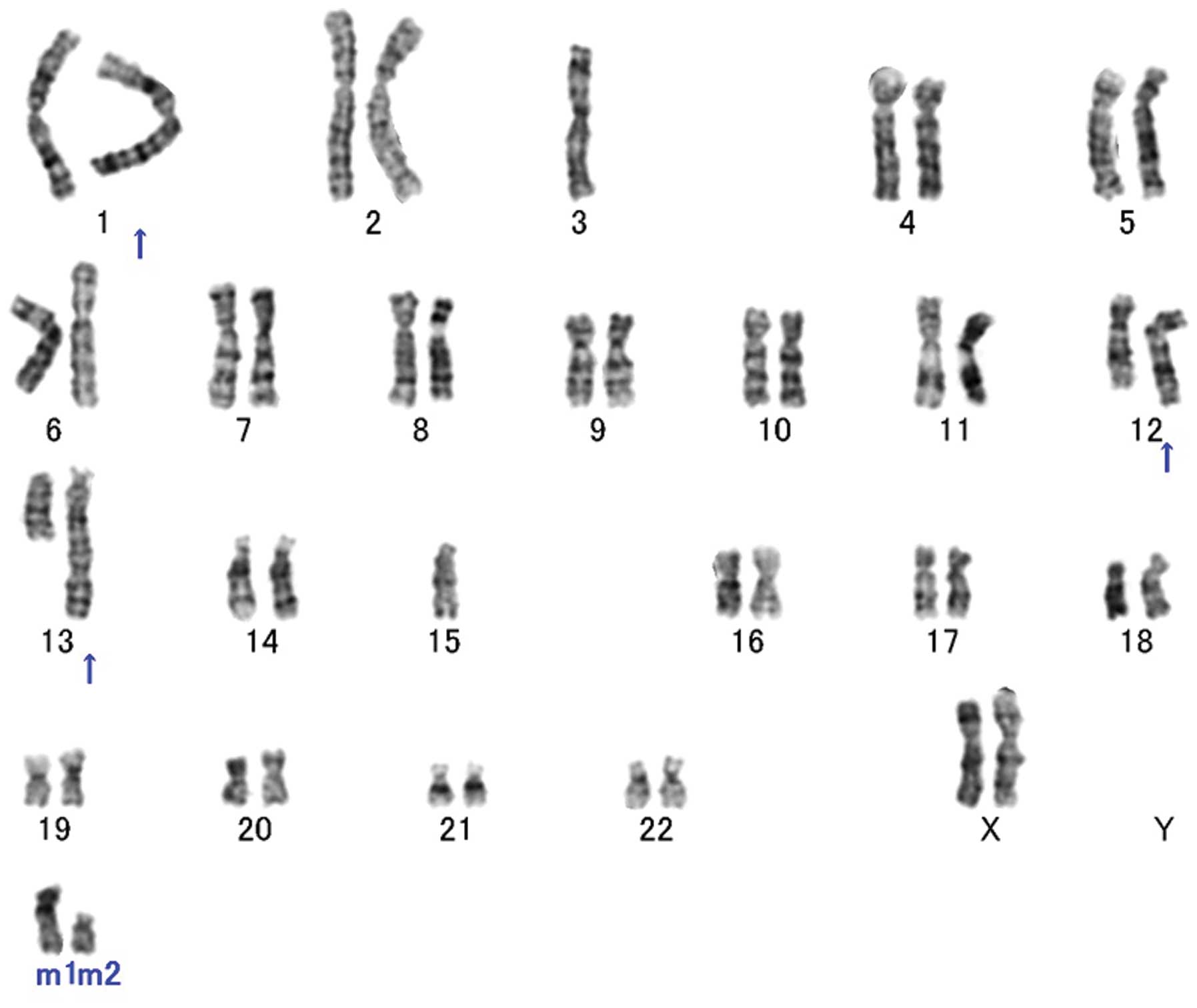
were routinely analyzed. Conventional cytogenetic analysis revealed
a complex karyotype with several numerical and structural
alterations, including 12q rearrangements (Fig. 3). The karyotype was identified as
46,XX, add(1)(q42),-3,
ins(12;?)(q15;?),ins(13;?)(q14;?),-15,+mar1,+mar2(20).
Spectral karyotyping (SKY) analysis was performed on
unstained cytogenetic preparations, according to the manufacturer's
instructions (Applied Spectral Imaging, Carlsbad, CA, USA) and
previous descriptions (11). Spectral
images were acquired using an SD200 spectral bio-imaging system
(Applied Spectral Imaging) and analyzed using the SkyView software
(Applied Spectral Imaging). In total, five metaphase cells were
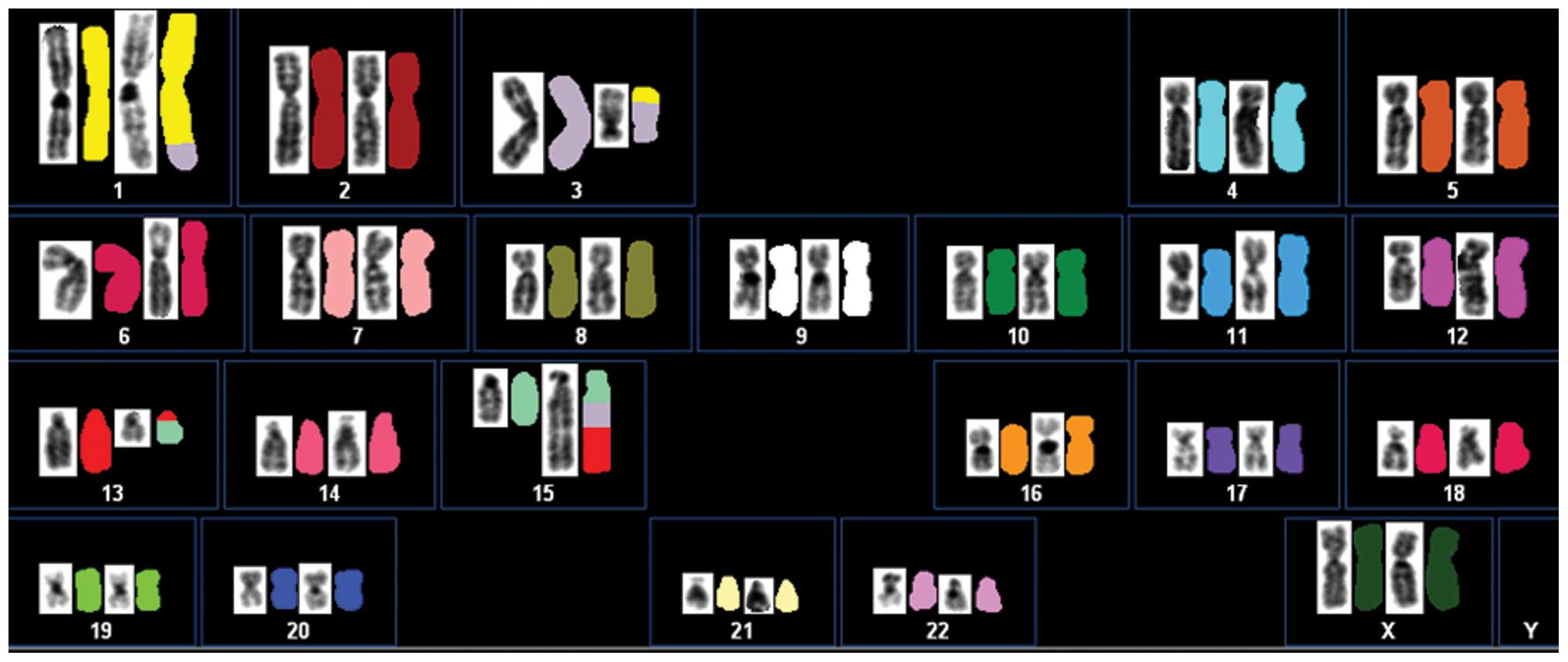
analyzed. SKY analysis revealed that the aberrations were more
complex than was demonstrated by G-banding analysis. Based on the
combined results, the karyotype was reinterpreted as follows: 46,
XX, der(1)t(1;3)(q42;p21),
der(3)t(1;3)del(3)(q13),dup(12)(q15q13),der(13)t(13;15)(q12;q24), der(15)t(3;15)(?;q21)t(3;13)(?;q12) (Fig. 4).
Fluorescence in situ hybridization (FISH) was
performed on paraffin-embedded tissue sections using a Poseidon
Repeat-Free MDM2 (12q15)/SE12 control probe (Kreatech
Diagnostics, Amsterdam, Netherlands). Briefly, the 4-µm thick
paraffin-embedded tissue sections were deparaffinized, dehydrated
and incubated with pepsin, according to the manufacturer's
instructions. The probe and slides were co-denatured at 80°C for 5
min and incubated overnight at 37°C in a humidified chamber.
Post-hybridization washing was performed following standard
procedures. The slides were counterstained with DAPI. Overall, ≥200
interphase nuclei were scrutinized. An MDM2/SE12 ratio
>2.0 was considered amplified, whereas a ratio ≤2.0 was
considered non-amplified. The interphase FISH analysis revealed no
MDM2 gene amplification (data not shown).
The post-operative course was uneventful, and the
patient continues to experience good health without evidence of
local recurrence at four months post-surgery.
Discussion
Cytogenetic analysis of lipomatous tumors has
revealed that the various histological subtypes are characterized
by distinctive clonal chromosomal abnormalities (1). Ordinary lipomas harbor translocations of
12q13-15, loss of 13q or rearrangements of 6p21 (3). The most frequent translocation is
t(3;12)(q27-28;q13-15), observed in ~25% of cases with 12q13-15
alterations (12). HMGA2
(12q14) is the target gene of copy number alterations and
rearrangements/gene fusions in ordinary lipomas with 12q13-15
aberrations (3). By contrast, ALTs
possess supernumerary ring or giant marker chromosomes, typically
as the sole anomaly or concomitant with a small number of other
numerical or structural aberrations (4). These abnormal chromosomes are mainly
composed of amplified sequences of 12q13-15 (5). MDM2 (12q15) is consistently
amplified and overexpressed in ALTs, but not in lipomas and is
thought to be the main driver gene of the 12q amplicon (4). The present case demonstrated only a
minor degree of nuclear atypia and failed to reveal amplification
and overexpression of the MDM2 gene.
In the present study, a duplication of chromosome
segment 12q13-15 was identified by SKY. There are extremely few
studies reporting lipomatous tumor with this chromosomal aberration
(6,8).
Mandahl et al (6) suggested
that duplication of 12q may be sufficient for the development of
minimal nuclear atypia and formation of ALTs. Italiano et al
(8) revealed the existence of a
genetic and morphological continuum between ordinary lipomas and
ALTs. Storlazzi et al (7)
described the case of a patient with atypical lipomatous tumor that
demonstrated minimal nuclear atypia and was cytogenetically
characterized by the consistent presence of 1–3 supernumerary rings
and low-level amplification of the MDM2 gene. Overall, these
observations indicate the existence of a subset of lipomatous
tumors that are characterized by minimal nuclear atypia and gain or
low-level amplification of 12q sequences.
Deletions or structural rearrangements of the long
arm of chromosome 13 have been observed in benign and intermediate
lipomatous tumors (13). In the
present study, complex rearrangements of 13q12 were identified. The
HMG family gene high mobility group box 1 (HMGB1) has been
identified as located at this chromosomal region. Kazmierczak et
al (14) revealed that there are
no intragenic rearrangements of HMGB1 in lipomas with 13q12
aberrations. By contrast, Petit et al (15) revealed a novel HMGA2-lipoma
HMGIC fusion partner fusion gene in a lipoma that possessed a
karyotype of t(12;13) (q13-15;12). Additional studies are required
to elucidate the significance of this cytogenetic alteration in the
development of lipomatous tumors.
In summary, the present study described a unique
case of lipomatous tumor with minimal nuclear atypia and
duplication of 12q13-15. The present and previously reported
studies suggest that the degree of nuclear atypia may be associated
with the level of chromosome 12q amplification in lipomatous
tumors.
Acknowledgements
This study was supported in part by the Foundation
for the Promotion of Medical Science and JSPS KAKENHI (grant no.
25462355).
References
|
1
|
Nishio J: Contributions of cytogenetics
and molecular cytogenetics to the diagnosis of adipocytic tumors. J
Biomed Biotechnol. 2011:5240672011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Thway K, Flora R, Shah C, Olmos D and
Fisher C: Diagnostic utility of p16, CDK4, and MDM2 as an
immunohistochemical panel in distinguishing well-differentiated and
dedifferentiated liposarcomas from other adipocytic tumors. Am J
Surg Pathol. 36:462–469. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bartuma H, Hallor KH, Panagopoulos I,
Collin A, Rydholm A, Gustafson P, Bauer HC, Brosjö O, Domanski HA,
Mandahl N and Mertens F: Assessment of the clinical and molecular
impact of different cytogenetic subgroups in a series of 272
lipomas with abnormal karyotype. Genes Chromosomes Cancer.
46:594–606. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Dei Tos AP and Pedeutour F: Atypical
lipomatous tumour. World Health Organization Classification of
Tumours of Soft Tissue and Bone. Fletcher CDM, Bridge JA,
Hogendoorn PCW and Mertens F: IARC Press. (Lyon). 33–36. 2013.
|
|
5
|
Pedeutour F, Forus A, Coindre JM, et al:
Structure of the supernumerary ring and giant rod chromosomes in
adipose tissue tumors. Genes Chromosomes Cancer. 24:30–41. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mandahl N, Akerman M, Aman P, et al:
Duplication of chromosome segment 12q15-24 is associated with
atypical lipomatous tumors: A report of the CHAMP collaborative
study group. CHromosomes And MorPhology. Int J Cancer. 67:632–635.
1996. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Storlazzi CT, Mertens F, Domanski H,
Fletcher CDM, Wiegant J and Mandahl N: Ring chromosomes and
low-grade gene amplification in an atypical lipomatous tumor with
minimal nuclear atypia. Int J Oncol. 23:67–71. 2003.PubMed/NCBI
|
|
8
|
Italiano A, Cardot N, Dupré F, Monticelli
I, Keslair F, Piche M, Mainguené C, Coindre JM and Pedeutour F:
Gains and complex rearrangements of the 12q13-15 chromosomal region
in ordinary lipomas: The “missing link” between lipomas and
liposarcomas? Int J Cancer. 121:308–315. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Nishio J, Aoki M, Nabeshima K, Iwasaki H
and Naito M: Cytogenetic and molecular cytogenetic findings in
giant dedifferentiated liposarcoma of the thigh. Oncol Rep.
27:764–768. 2012.PubMed/NCBI
|
|
10
|
Shaffer LG, Slovak ML and Campbell LJ:
ISCN (2009): An International System for Human Cytogenetic
Nomenclature. S Karger. Basel: 2009.
|
|
11
|
Nishio J, Aoki M, Nabeshima K, Iwasaki H
and Naito M: Characterization of giant marker and ring chromosomes
in a pleomorphic leiomyosarcoma of soft tissue by spectral
karyotyping. Oncol Rep. 28:533–538. 2012.PubMed/NCBI
|
|
12
|
Sandberg AA: Updates on the cytogenetics
and molecular genetics of bone and soft tissue tumors: Lipoma.
Cancer Genet Cytogenet. 150:93–115. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Dahlén A, Debiec-Rychter M, Pedeutour F,
Domanski HA, Höglund M, Bauer HC, Rydholm A, Sciot R, Mandahl N and
Mertens F: Clustering of deletions on chromosome 13 in benign and
low-malignant lipomatous tumors. Int J Cancer. 103:616–623. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kazmierczak B, Dal Cin P, Meyer-Bolte K,
Van den Berghe H and Bullerdiek J: HMG1 is not rearranged by 13q12
aberrations in lipomas. Genes Chromosomes Cancer. 24:290–292. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Petit MM, Schoenmakers EF, Huysmans C,
Geurts JM, Mandahl N and Van de Ven WJ: LHFP, a novel translocation
partner gene of HMGIC in a lipoma, is a member of a new family of
LHFP-like genes. Genomics. 57:438–441. 1999. View Article : Google Scholar : PubMed/NCBI
|