Introduction
Cancer stem/progenitor cells (CSCs) are a
subpopulation of cancer cells with the characteristics of tumor
initiation (1), resistance to therapy
(2) and metastasis (3). In breast cancer, breast CSCs (BCSCs)
have been identified as cells with cluster of differentiation
(CD)24−CD44+ surface markers (4) or with high intracellular aldehyde
dehydrogenase activity (5). BCSCs may
additionally be enriched by cultivation in a serum-free,
non-adherent environment, known as the mammosphere, which is a
floating clump composed of breast cancer cells (6,7). In
addition to tumor initiation and chemoresistant properties, BCSCs
have also been indicated to exhibit vasculogenic mimicry (VM)
activity (8), defined as the
formation of perfusable, matrix-rich and vasculogenic-like networks
by tumor cells, without involvement of endothelial cells (9). A previous study demonstrated that the VM
activity of BCSCs is regulated by epidermal growth factor receptor
(EGFR) signaling (8).
Hinokitiol, alternatively known as β-thujaplicin, is
a tropolone-associated natural compound isolated from heartwood
cupressaceous plants (10), and has
been widely used as an antimicrobial agent in toothpastes,
cosmetics and food (11). In addition
to antimicrobial activity, hinokitiol has been reported to possess
anti-inflammatory (12,13) and antitumor activity (14,15).
Previously, in prostate carcinoma cell lines, hinokitiol was
indicated to disrupt androgen receptor signaling and inhibit cell
growth (14). Hinokitiol may induce
G1 arrest in malignant melanoma cells through increased
cyclin-dependent kinase inhibitor 1B protein expression (15). Hinokitiol was also indicated to cause
caspase-dependent apoptosis (16) and
differentiation (17) in
teratocarcinoma F9 cells. A recent study indicated that hinokitiol
induced autophagy in murine breast and colorectal cancer cells via
downregulation of the v-akt murine thymoma viral oncogene homolog
1/mechanistic target of rapamycin (serine/threonine kinase)
signaling pathway, which led to cell death (18). Although the anti-tumor activity of
hinokitiol has been reported, its effect on CSCs remains to be
elucidated.
The present study indicated that hinokitiol may
inhibit the VM activity of BCSCs enriched by mammosphere
cultivation at a concentration below the half maximal inhibitory
concentration (IC50). Hinokitiol was also indicated to
decrease the protein expression of EGFR without affecting the
expression of messenger (m)RNA. In addition, downregulation of EGFR
protein by hinokitiol was mediated through proteasome degradation.
Inhibited proteasome activity by MG132 abolished the anti-VM
activity of hinokitiol. The results of the present study suggest
that hinokitiol may act as an anti-VM agent, and may be useful for
the development of novel breast cancer therapeutic agents
Materials and methods
Cell culture and reagents
The MDA-MB-231 human breast cancer cell line was
obtained from American Type Culture Collection (ATCC; Manassas, VA,
USA) and maintained according to ATCC's recommendations. The
AS-B244 human breast cancer cell line was established from the
tissue of a female patient (Academia Sinica, Taipei, Taiwan) with
triple negative breast cancer and maintained as previously
described (19). Hinokitiol was
purchased from Sigma-Aldrich (St. Louis, MO, USA), dissolved in
ethanol (EtOH; Avantor Performance Materials, Center Valley, PA,
USA) as a 100 mM stock solution and stored at −20°C. MG132 was
purchased from Tocris Bioscience (Bristol, United Kingdom),
dissolved in dimethyl sulfoxide (Sigma-Aldrich) as a 100 mM stock
solution and stored at −20°C.
Enrichment of BCSCs by mammosphere
cultivation
Cells were resuspended in Dulbecco's Modified
Eagle's Medium: Nutrient Mixture F-12 (Thermo Fisher Scientific
Inc., Waltham, MA, USA) containing 1% methyl cellulose
(Sigma-Aldrich) to avoid cell aggregation, fibroblast growth
factor-basic (20 ng/ml; PeproTech, Inc., Rocky Hill, NJ, USA),
human epidermal growth factor (20 ng/ml; PeproTech, Inc.,), insulin
(5 µg/ml; Sigma-Aldrich) and B27 supplement (dilution, 1:50;
Gibco®; Thermo Fisher Scientific, Inc.). Cells were seeded at
104 cells per dish into ultralow-attachment 10 cm dishes
(Corning Life Sciences BV, Amsterdam, Netherlands). Following 7
days of incubation, the mammospheres were collected using a 100 mm
cell strainer (BD Biosciences, San Jose, CA, USA) and dissociated
by HyQTase (Hyclone; GE Healthcare, Logan, UT, USA) to achieve a
single cell suspension for additional experiments.
Cytotoxicity analysis
For the cytotoxicity assay, 1×104
mammosphere cells/well were seeded in 96-well plates (Corning Life
Sciences BV) and cultured for 48 h with or without hinokitiol or
0.1% EtOH. Cell viability was determined by WST-1 reagent
(BioVision, Inc., Milpitas, CA, USA) using a microplate reader
(SpectraMax Plus 384; Molecular Devices, LLC, Sunnyvale, CA, USA),
according to the manufacturer's protocol. The IC50 value
was calculated by GraFit version 7 software (Erithacus Software
Ltd., East Grinstead, UK).
In vitro VM activity assay
Wells of µ-microslide (ibidi GmbH, Martinsried,
Germany) were coated with 10 µl of Matrigel (BD Biosciences) at a
concentration of 8 mg/ml, and incubated at 37°C overnight.
Mammosphere cells were suspended at 2×104 cells/50 µl in
M200 medium (Gibco; Thermo Fisher Scientific, Inc.) containing 1X
low serum growth supplement (Gibco; Thermo Fisher Scientific, Inc.)
and loaded into one Matrigel-coated well. The slide was then
incubated at 37°C in a 5% CO2 incubator and the VM
structures were recorded by inverted microscopy (AE30; Motic
Electric Group Co., Ltd., Xiamen, China) at 6 h post-seeding.
Images of the wells were analyzed with the Tubeness function on
Image J version 1.50e software (National Institutes of Health,
Bethesda, MA, USA), and VM scores were calculated according to a
formula described by Aranda et al (20) as follows: VM = [(no.spouting cells ×
1) + (no. connected cells × 2) + (no. polygons × 3)] / total no.
cells.
Western blot analysis
Cells were harvested using trypsin (Gibco; Thermo
Fisher Scientific, Inc.), lysed in mammalian protein extraction
reagent (Pierce; Thermo Fisher Scientific Inc.) and the protein
concentration was determined by bicinchoninic acid reagent (Pierce;
Thermo Fisher Scientific Inc.). In total, 25 µg of extracted
protein was separated by 10% sodium dodecyl sulfate-polyacrylamide
gel electrophoresis and transferred to a polyvinylidene difluoride
membrane (Immobilon-P; Merck Millipore, Darmstadt, Germany). The
membranes were then blocked with 5% skimmed milk (Sigma-Aldrich),
dissolved in Tris-buffered saline with 0.05% Tween-20 (TBST;
Sigma-Aldrich) at room temperature for 1 h, followed by incubation
with primary antibodies [mouse anti-human EGFR monoclonal antibody
was purchased from Santa Cruz Biotechnology Inc. (Dallas, TX, USA;
catalog no. sc-377229); mouse anti-human tubulin monoclonal
antibody was purchased from Novus Biologicals, LLC (Littleton, CO,
USA; catalog no. NB100-690)] at 4°C overnight. Hydrogen
peroxidase-conjugated anti-rabbit or anti-mouse immunoglobulin G
polyclonal antibody (catalog no 7076; Cell Signaling Technology,
Inc., Danvers, MA, USA) were used as secondary antibodies. The
membranes were washed 3 times for 5 min each time with TBST
following blocking, primary antibody incubation and secondary
antibody incubation. Developed chemiluminescence signals from
catalyzed ECL substrate (PerkinElmer Inc., Waltham, MA, USA) were
detected by a Luminescence-Image Analyzer (ImageQuant LAS 4000
mini; GE Healthcare Bio-sciences, Pittsburgh, PA, USA).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was extracted from AS-B244 and MDA-MB-231
cells and purified using an RNA extraction kit (Quick-RNA™ MiniPrep
kit; Zymo Research Corporation, Irvine, CA, USA) and complementary
DNA (cDNA) was synthesized with a first strand cDNA synthesis kit
(RevertAid First Strand cDNA Synthesis kit; Fermentas; Thermo
Fisher Scientific, Inc.). The expression of genes was detected with
specific primers and the KAPA SYBR™ fast qPCR kit (Kapa Biosystems,
Inc., Wilmington, MA, USA) with the ABI StepOne™ Real-Time PCR
System (Applied Biosystems; Thermo Fisher Scientific, Inc.). The
primer sequences used in the present study were synthesized by
Integrated DNA Technologies Pte., Ltd. (Singapore, China) and were
as follows: EGFR, forward 5′-CAGCGCTACCTTGTCATTCA-3′ and reverse
5′-TGCACTCAGAGAGCTCAGGA-3′; mitochondrial ribosomal protein L19,
forward 5′-GGGATTTGCATTCAGAGATCAG-3′ and reverse
5′-GGAAGGGCATCTCGTAAG-3′. The cycling conditions were as follows:
50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 95°C for
10 sec and 60°C for 1 min. The end-point used in quantification was
calculated by the StepOne™ software (v2.2.2; Applied Biosystems;
Thermo Fisher Scientific, Inc.), and the quantification cycle
number (Cq value) for each analyzed sample was calculated. EGFR
expression was normalized to MRPL19, which has been reported as one
of the most stable internal control genes (21–23), to
derive the change in Cq value (∆Cq). The primer sequence for MRPL19
was as follows: Forward, 5′-GGGATTTGCATTCAGAGATCAG-3′; and reverse,
5′-GGAAGGGCATCTCGTAAG-3′. The relative gene expression differences
between groups was calculated using 2-∆∆Cq (24). The PCR experiments were repeated three
times.
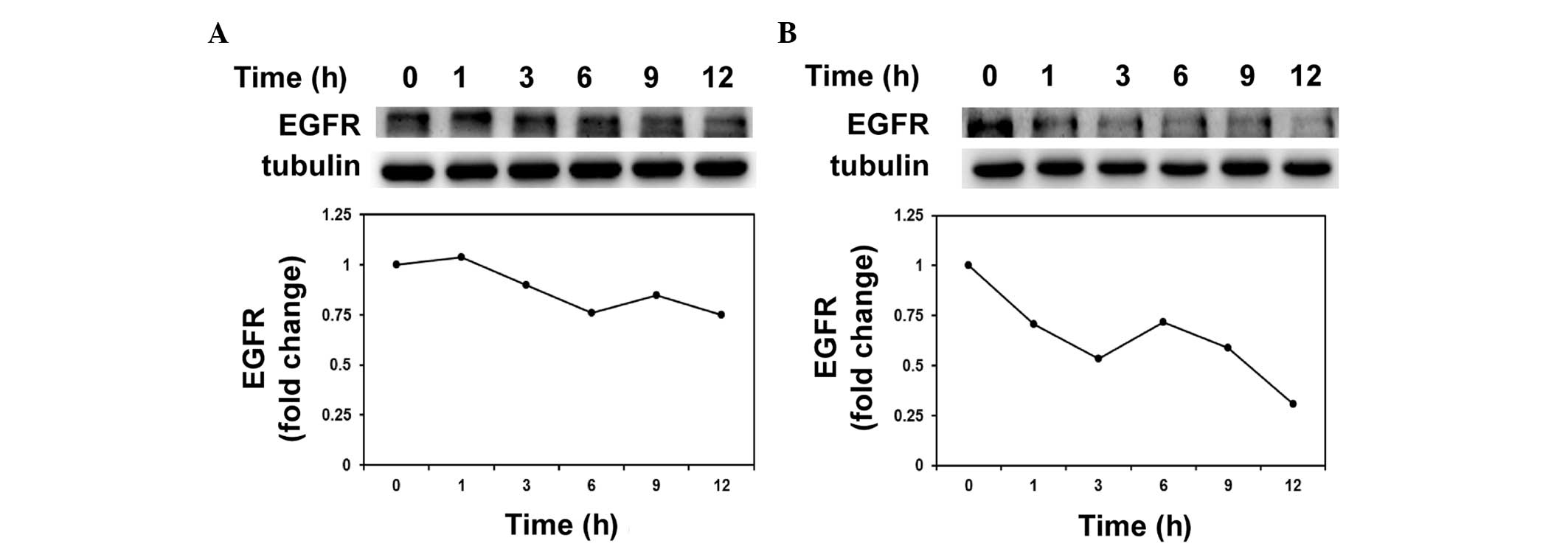
Protein stability assay
Cells were seeded into a 12-well plate (Corning Life
Sciences BV) at a density of 1×105 cells/well and
incubated with 50 µg/ml cycloheximide (Sigma-Aldrich) for 1, 3, 6,
9 or 12 h with 0.1% EtOH or 10 µM hinokitiol. Cells were harvested
with trypsin at 1, 3, 6, 9 or 12 h post-seeding and the protein
expression was detected by western blot analysis.
Statistical analysis
Quantitative data are presented as the mean ±
standard deviation. Comparisons between two groups were analyzed
with the Student's t-test. Comparisons among multiple groups
(>3) were analyzed with one-way analysis of variance, and
post-hoc tests were performed with Tukey Multiple Comparison
analysis. P<0.05 was considered to indicate a statistically
significant difference.
Results
Hinokitiol dose-dependently inhibits
VM activity of mammosphere cells derived from human breast cancer
cell lines
Hinokitiol, alternatively known as β-thujaplicin, is
a tropolone-associated natural compound with antimicrobial,
anti-inflammatory and antitumor activity. The present study
initially examined the cytotoxic effect of hinokitiol on
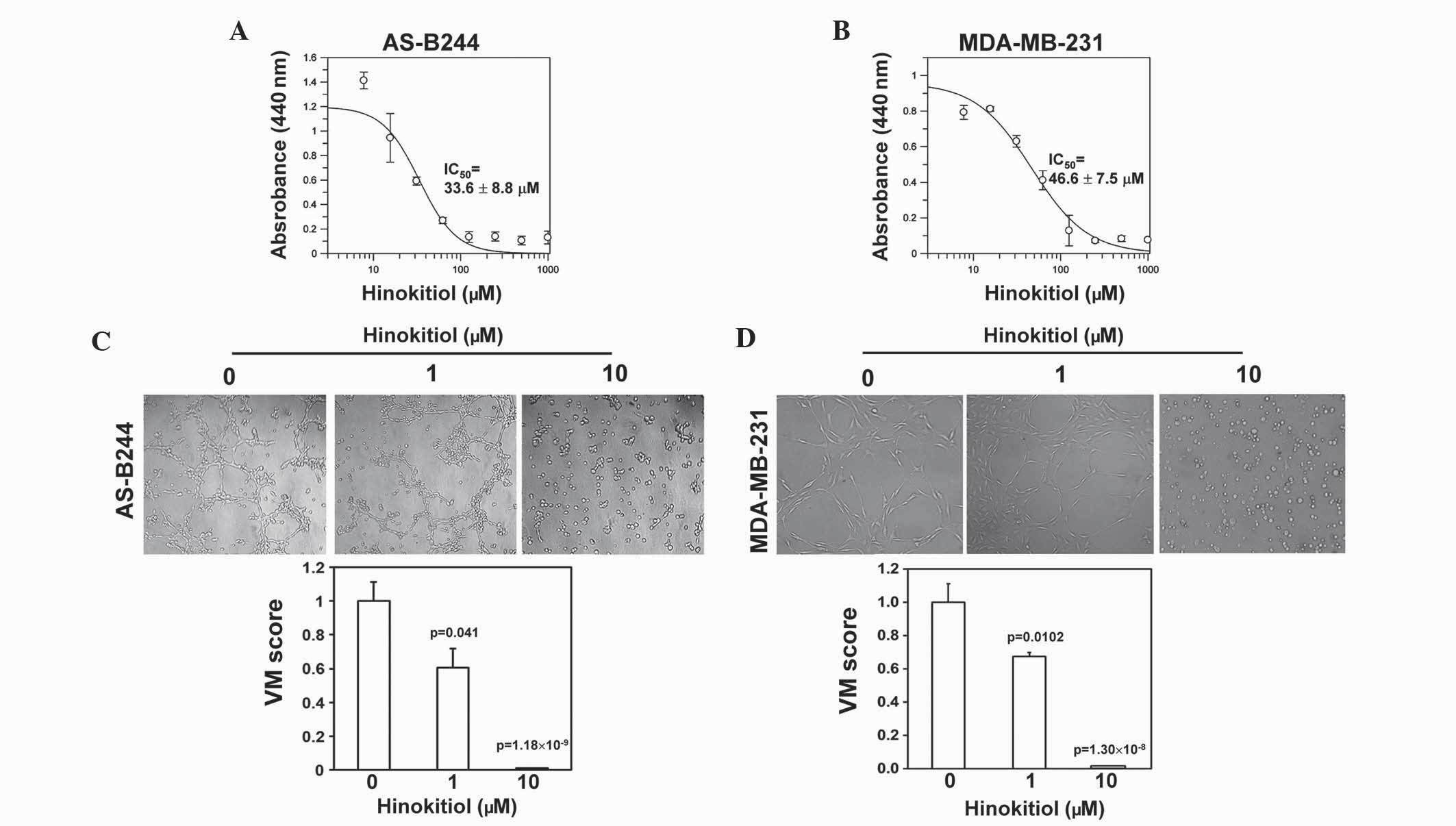
mammosphere cells derived from AS-B244 or MDA-MB-231 human breast
cancer cells. The results revealed that the IC50 values
of hinokitiol for AS-B244 or MDA-MB-231 cells were 33.6±8.8 and
46.6±7.5 µM, respectively (Fig. 1A and
B). Furthermore, the present study determined whether
hinokitiol exhibited an inhibitory effect on the VM activity of
BCSCs at concentrations below the IC50. The mammosphere
cells derived from AS-B244 or MDA-MB-231 cells were treated with 1
or 10 µM hinokitiol for 24 h and seeded into Matrigel-coated
microwells for the analysis of in vitro VM activity in the
presence of hinokitiol. Treatment with hinokitiol dose-dependently
inhibited the VM activity of AS-B244 (Fig. 1C; 1 µM, P=0.041; 10 µM,
P=1.18×10−9) or MDA-MB-231 (Fig. 1D; 1 µM, P=0.0102; 10 µM,
P=1.30×10−8) mammosphere cells. These results indicate
that hinokitiol may inhibit VM activity of BCSCs.
Hinokitiol inhibits EGFR expression
without changing mRNA expression
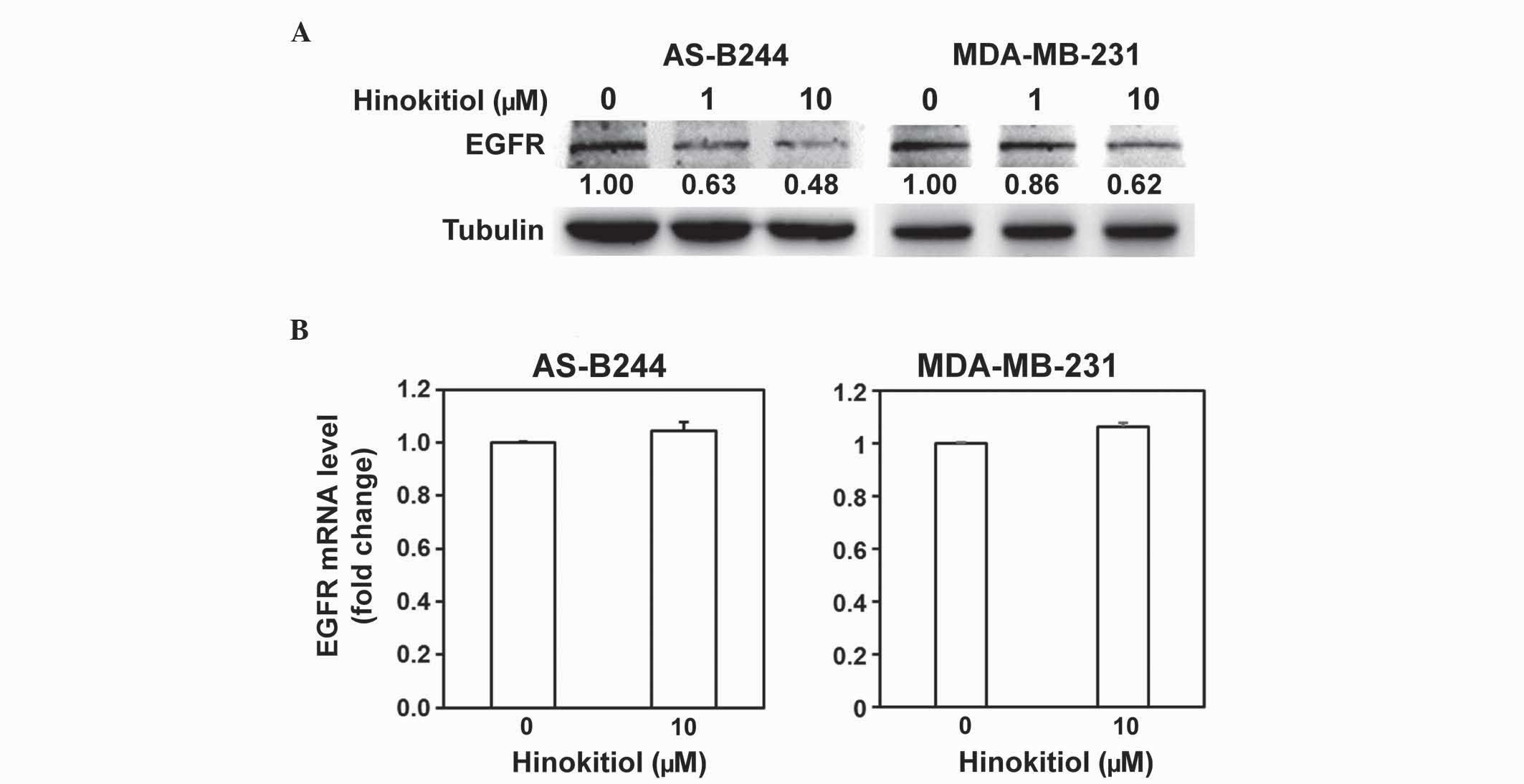
A previous study demonstrated that the VM activity
of BCSCs is mediated by the activation of EGF/EGFR (8). The present study examined the effect of
hinokitiol on EGFR expression. In the western blot analysis, the
protein level of EGFR in AS-B244 or MDA-MB-231 mammosphere cells
was downregulated in a dose-dependent manner by hinokitiol
(Fig. 2A). The present study examined
whether hinokitiol affected the mRNA expression of EGFR. In the
RT-qPCR analysis, the mRNA level of EGFR in AS-B244 or MDA-MB-231
mammosphere cells did not change with hinokitiol treatment
(Fig. 2B; AS-B244, P=0.1088;
MDA-MB-231, P=0.0502). Previous studies have reported that surface
EGFR expression may be regulated by the proteasomal protein
degradation pathway (25,26). The present study examined the protein
stability of EGFR following hinokitiol treatment, and the results
indicated that hinokitiol decreased EGFR protein stability in
AS-B244 mammosphere cells (Fig.
3).
Hinokitiol inhibits EGFR expression
via proteasome-mediated degradation
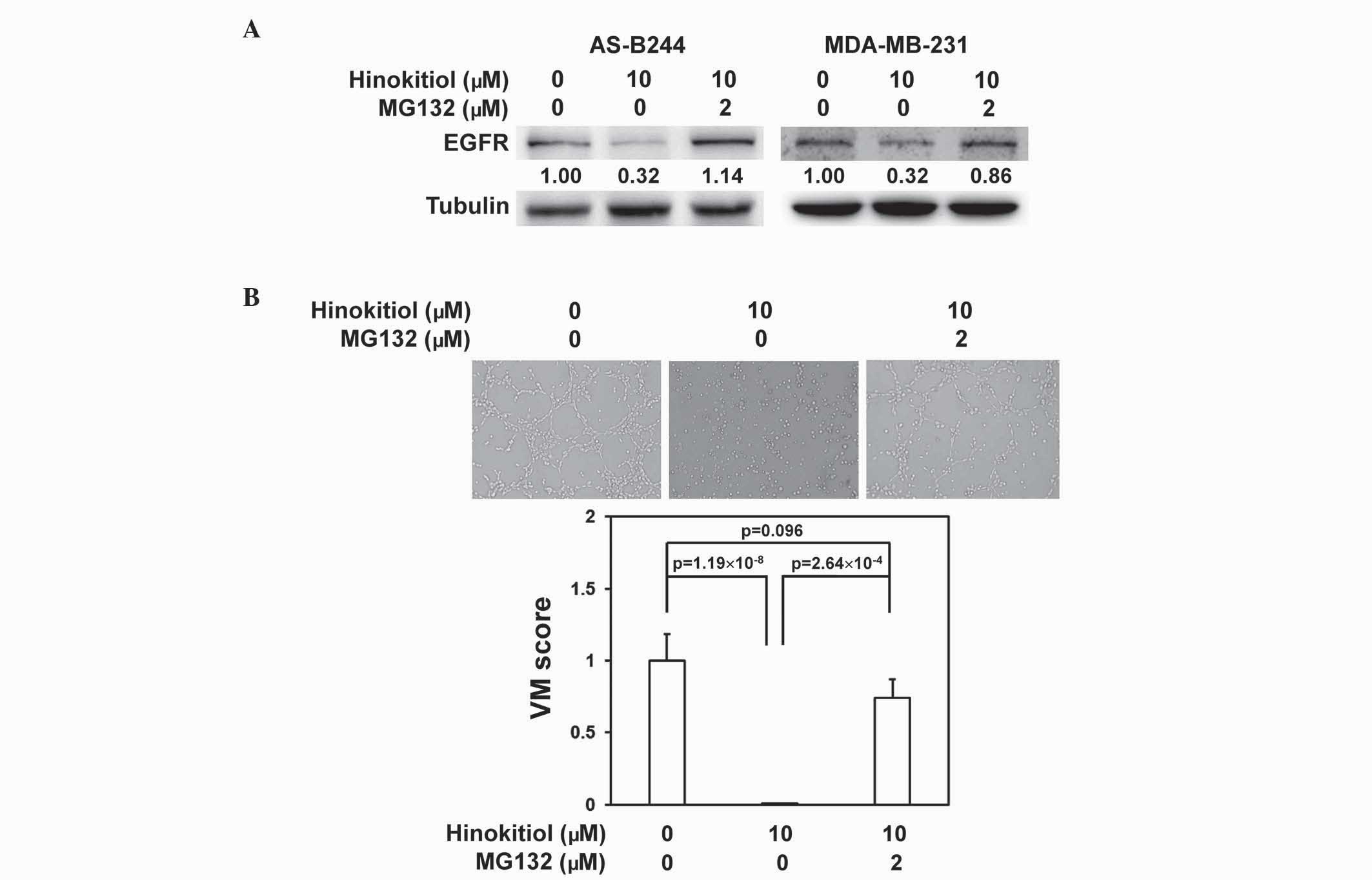
The present study determined whether the
downregulation of EGFR protein expression by hinokitiol in BCSCs
was mediated by the proteasomal degradation pathway. Following
co-treatment in combination with MG132, a proteasome inhibitor,
hinokitiol lost the ability to inhibit EGFR protein expression in
AS-B244 or MDA-MB-231 mammosphere cells (Fig. 4A). The VM inhibition activity of
hinokitiol in AS-B244 mammosphere cells was additionally reversed
by MG132 treatment (Fig. 4B;
P=2.64×10−4). These results suggest that the VM
inhibition activity of hinokitiol in breast cancer stem/progenitor
cells may be mediated by proteasomal degradation of EGFR.
Discussion
Malignant tissues remain small in size (a few
millimeters) when there is a lack of novel vasculature (27). One of the mechanisms of metastasis is
the shedding of tumor cells into newly synthesized vessels
(28). In breast cancer, the
overexpression of vascular endothelial growth factor (VEGF), which
is a key molecule for regulating angiogenesis (29), is associated with disease progression
(30). Targeting VEGF signaling by
antibodies or tyrosine kinase receptor inhibitors has been
developed as a therapeutic strategy for several types of cancer
(31). The monoclonal anti-VEGF
antibody, bevacizumab, was approved for the treatment of certain
types of colon, lung, kidney and brain cancer, but was subsequently
announced to be unsafe and ineffective in breast cancer patients by
the Food and Drug Administration of the USA in 2011 (32). One of the potential mechanisms for the
poor effectiveness of bevacizumab in breast cancer was hypothesized
to be due to VM (33). The
development of novel agents that target VM may be important in the
future of breast cancer therapy.
CSCs have been reported to be important in tumor
vasculatures (34). CD44+
type I epithelial ovarian cancer cells have been demonstrated to
form vascular structures when cultured in Matrigel conditions in
vitro and to transdifferentiate into CD34+
endothelial progenitor cells (35).
The progeny of glioma CSCs cultured in endothelial conditions have
previously exhibited features of functional endothelial cells
(36). In another study, blocking
VEGF signaling suppressed the maturation of tumor endothelial
progenitors into endothelium, and the inhibition of Notch signaling
abolished the transition of glioma CSCs into endothelial
progenitors (37). These previous
studies suggest that CSCs may support tumor vascularization via
direct transdifferentiation into endothelial cells or progenitors.
A recent study indicated that BCSCs display VM activity in
vivo and in vitro (8).
Other studies have also demonstrated that the VM activity in
mammosphere cells is derived from melanoma cells (38) or CD133+ glioblastoma
stem-like cells (39). These studies
indicated that CSC-derived VM structures may be an important blood
supply system in cancer. The present study demonstrated that
hinokitiol, a tropolone-associated natural compound, may suppress
the VM activity of BCSCs via the proteasomal degradation of EGFR.
Previous studies have suggested that enhancing the degradation of
EGFR may provide a promising approach in cancer treatment (25). It has additionally been demonstrated
that hinokitiol is able to cause death of breast cancer cells via
induction of autophagy (18). Huang
et al (40) demonstrated that
hinokitiol may inhibit the expression of matrix metalloprotease-1
through the reduction of nuclear factor-κB (NF-κB) activation,
resulting in the suppression of metastasis in a mouse melanoma
model. Zhang et al (41)
demonstrated that the anti-VM activity of thalidomide in melanoma
was associated with the NF-κB signaling pathway. The anti-VM
activity of hinokitiol may also be mediated through an NF-κB
associated signaling pathway, which requires additional
investigation. The study conducted by Itzhaki et al
(42) indicated that the simultaneous
anti-cell growth and anti-VM activity of nicotinamide resulted in
efficient anti-neoplastic effects in aggressive melanomas.
Therefore, similar agents with anti-cell growth and anti-VM
activity may act as potential anticancer compounds in the future
development of breast cancer therapy.
In conclusion, the present study demonstrates that
hinokitiol exhibits an inhibitory effect in VM activity of BCSCs
through proteasomal degradation of EGFR. The anti-CSC activity of
hinokitiol and the underling molecular mechanisms will be worthy of
investigation in the future.
Acknowledgements
The present study was supported by an
inter-institutional cooperation project between Chung Shan Medical
University and Ditmanson Medical Foundation Chia-Yi Christian
Hospital (Taichung, Taiwan; grant no. CSMU-CYC-102-01) and the
Ministry of Science and Technology in Taiwan (grant nos.
NSC-101-2314-040-015-MY2 and MOST-103-2314-B-040-015-MY3).
Glossary
Abbreviations
Abbreviations:
|
VM
|
vasculogenic mimicry
|
|
BCSCs
|
breast cancer stem/progenitor
cells
|
|
EGFR
|
epidermal growth factor receptor
|
References
|
1
|
White AC and Lowry WE: Refining the role
for adult stem cells as cancer cells of origin. Trends Cell Biol.
25:11–20. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
O'Connor ML, Xiang D, Shigdar S, Macdonald
J, Li Y, Wang T, Pu C, Wang Z, Qiao L and Duan W: Cancer stem
cells: A contentious hypothesis now moving forward. Cancer Lett.
344:180–187. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Geng SQ, Alexandrou AT and Li JJ: Breast
cancer stem cells: Multiple capacities in tumor metastasis. Cancer
Lett. 349:1–7. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Al-Hajj M, Wicha MS, Benito-Hernandez A,
Morrison SJ and Clarke MF: Prospective identification of
tumorigenic breast cancer cells. Proc Natl Acad Sci USA.
100:3983–3988. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ginestier C, Hur MH, Charafe-Jauffret E,
Monville F, Dutcher J, Brown M, Jacquemier J, Viens P, Kleer CG,
Liu S, et al: ALDH1 is a marker of normal and malignant human
mammary stem cells and a predictor of poor clinical outcome. Cell
Stem Cell. 1:555–567. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Saadin K and White IM: Breast cancer stem
cell enrichment and isolation by mammosphere culture and its
potential diagnostic applications. Expert Rev Mol Diagn. 13:49–60.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ponti D, Costa A, Zaffaroni N, Pratesi G,
Petrangolini G, Coradini D, Pilotti S, Pierotti MA and Daidone MG:
Isolation and in vitro propagation of tumorigenic breast cancer
cells with stem/progenitor cell properties. Cancer Res.
65:5506–5511. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lee CH, Wu YT, Hsieh HC, Yu Y, Yu AL and
Chang WW: Epidermal growth factor/heat shock protein 27 pathway
regulates vasculogenic mimicry activity of breast cancer
stem/progenitor cells. Biochimie. 104:117–126. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Seftor RE, Hess AR, Seftor EA, Kirschmann
DA, Hardy KM, Margaryan NV and Hendrix MJ: Tumor cell vasculogenic
mimicry: From controversy to therapeutic promise. Am J Pathol.
181:1115–1125. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Jayakumar T, Hsu WH, Yen TL, Luo JY, Kuo
YC, Fong TH and Sheu JR: Hinokitiol, a natural tropolone
derivative, offers neuroprotection from thromboembolic stroke in
vivo. Evid Based Complement Alternat Med. 2013:8404872013.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Saeki Y, Ito Y, Shibata M, Sato Y, Okuda K
and Takazoe I: Antimicrobial action of natural substances on oral
bacteria. Bull Tokyo Dent Coll. 30:129–135. 1989.PubMed/NCBI
|
|
12
|
Shih YH, Lin DJ, Chang KW, Hsia SM, Ko SY,
Lee SY, Hsue SS, Wang TH, Chen YL and Shieh TM: Evaluation physical
characteristics and comparison antimicrobial and anti-inflammation
potentials of dental root canal sealers containing hinokitiol in
vitro. PLoS One. 9:e949412014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Shih MF, Chen LY, Tsai PJ and Cherng JY:
In vitro and in vivo therapeutics of β-thujaplicin on LPS-induced
inflammation in macrophages and septic shock in mice. Int J
Immunopathol Pharmacol. 25:39–48. 2012.PubMed/NCBI
|
|
14
|
Liu S and Yamauchi H: Hinokitiol, a metal
chelator derived from natural plants, suppresses cell growth and
disrupts androgen receptor signaling in prostate carcinoma cell
lines. Biochem Biophys Res Commun. 351:26–32. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Liu S and Yamauchi H: P27-Associated G1
arrest induced by hinokitiol in human malignant melanoma cells is
mediated via down-regulation of pRb, Skp2 ubiquitin ligase and
impairment of Cdk2 function. Cancer Lett. 286:240–249. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ido Y, Muto N, Inada A, Kohroki J, Mano M,
Odani T, Itoh N, Yamamoto K and Tanaka K: Induction of apoptosis by
hinokitiol, a potent iron chelator, in teratocarcinoma F9 cells is
mediated through the activation of caspase-3. Cell Prolif.
32:63–73. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Muto N, Dota A, Tanaka T, Itoh N, Okabe M,
Inada A, Nakanishi T and Tanaka K: Hinokitiol induces
differentiation of teratocarcinoma F9 cells. Biol Pharm Bull.
18:1576–1579. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang WK, Lin ST, Chang WW, Liu LW, Li TY,
Kuo CY, Hsieh JL and Lee CH: Hinokitiol induces autophagy in murine
breast and colorectal cancer cells. Environ Toxicol. 2014.
|
|
19
|
Chang WW, Lin RJ, Yu J, Chang WY, Fu CH,
Lai A, Yu JC and Yu AL: The expression and significance of
insulin-like growth factor-1 receptor and its pathway on breast
cancer stem/progenitors. Breast Cancer Res. 15:R392013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Aranda E and Owen GI: A semi-quantitative
assay to screen for angiogenic compounds and compounds with
angiogenic potential using the EA. hy926 endothelial cell line.
Biol Res. 42:377–389. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ayakannu T, Taylor AH and Willets JM:
Validation of endogenous control reference genes for normalizing
gene expression studies in endometrial carcinoma. Mol Hum Reprod.
21:723–735. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Mohelnikova-Duchonova B, Oliverius M,
Honsova E and Soucek P: Evaluation of reference genes and
normalization strategy for quantitative real-time PCR in human
pancreatic carcinoma. Dis Markers. 32:203–210. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McNeill RE, Miller N and Kerin MJ:
Evaluation and validation of candidate endogenous control genes for
real-time quantitative PCR studies of breast cancer. BMC Mol Biol.
8:1072007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lipkowitz S: The role of the
ubiquitination-proteasome pathway in breast cancer: Ubiquitin
mediated degradation of growth factor receptors in the pathogenesis
and treatment of cancer. Breast Cancer Res. 5:8–15. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Authier F, Métioui M, Bell AW and Mort JS:
Negative regulation of epidermal growth factor signaling by
selective proteolytic mechanisms in the endosome mediated by
cathepsin B. J Biol Chem. 274:33723–33731. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Folkman J: Tumor angiogenesis: Therapeutic
implications. N Engl J Med. 285:1182–1186. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Schneider BP and Miller KD: Angiogenesis
of breast cancer. J Clin Oncol. 23:1782–1790. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hicklin DJ and Ellis LM: Role of the
vascular endothelial growth factor pathway in tumor growth and
angiogenesis. J Clin Oncol. 23:1011–1027. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Foekens JA, Peters HA, Grebenchtchikov N,
Look MP, Meijer-van Gelder ME, Geurts-Moespot A, van der Kwast TH,
Sweep CG and Klijn JG: High tumor levels of vascular endothelial
growth factor predict poor response to systemic therapy in advanced
breast cancer. Cancer Res. 61:5407–5414. 2001.PubMed/NCBI
|
|
31
|
Hoeben A, Landuyt B, Highley MS, Wildiers
H, Van Oosterom AT and De Bruijn EA: Vascular endothelial growth
factor and angiogenesis. Pharmacol Rev. 56:549–580. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Sasich LD and Sukkari SR: The US FDAs
withdrawal of the breast cancer indication for Avastin
(bevacizumab). Saudi Pharm J. 20:381–385. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Kirschmann DA, Seftor EA, Hardy KM, Seftor
RE and Hendrix MJ: Molecular pathways: Vasculogenic mimicry in
tumor cells: Diagnostic and therapeutic implications. Clin Cancer
Res. 18:2726–2732. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Silvan U, Diez-Torre A, Bonilla Z, Moreno
P, Diaz-Nunez M and Arechaga J: Vasculogenesis and angiogenesis in
nonseminomatous testicular germ cell tumors. Urol Oncol. 33:268
e217–228. 2015. View Article : Google Scholar
|
|
35
|
Alvero AB, Fu HH, Holmberg J, Visintin I,
Mor L, Marquina CC, Oidtman J, Silasi DA and Mor G: Stem-like
ovarian cancer cells can serve as tumor vascular progenitors. Stem
Cells. 27:2405–2413. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ricci-Vitiani L, Pallini R, Biffoni M,
Todaro M, Invernici G, Cenci T, Maira G, Parati EA, Stassi G,
Larocca LM and De Maria R: Tumour vascularization via endothelial
differentiation of glioblastoma stem-like cells. Nature.
468:824–828. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Wang R, Chadalavada K, Wilshire J, Kowalik
U, Hovinga KE, Geber A, Fligelman B, Leversha M, Brennan C and
Tabar V: Glioblastoma stem-like cells give rise to tumour
endothelium. Nature. 468:829–833. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Monzani E and La Porta CA: Targeting
cancer stem cells to modulate alternative vascularization
mechanisms. Stem Cell Rev. 4:51–56. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Chiao MT, Yang YC, Cheng WY, Shen CC and
Ko JL: CD133+ glioblastoma stem-like cells induce vascular mimicry
in vivo. Curr Neurovasc Res. 8:210–219. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang CH, Lu SH, Chang CC, Thomas PA,
Jayakumar T and Sheu JR: Hinokitiol, a tropolone derivative,
inhibits mouse melanoma (B16-F10) cell migration and in vivo tumor
formation. Eur J Pharmacol. 746:148–157. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Zhang S, Li M, Gu Y, Liu Z, Xu S, Cui Y
and Sun B: Thalidomide influences growth and vasculogenic mimicry
channel formation in melanoma. J Exp Clin Cancer Res. 27:602008.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Itzhaki O, Greenberg E, Shalmon B, Kubi A,
Treves AJ, Shapira-Frommer R, Avivi C, Ortenberg R, Ben-Ami E,
Schachter J, et al: Nicotinamide inhibits vasculogenic mimicry, an
alternative vascularization pathway observed in highly aggressive
melanoma. PLoS One. 8:e571602013. View Article : Google Scholar : PubMed/NCBI
|