Introduction
Acute myeloid leukaemia (AML) is a haematological
malignancy with a heterogeneous clinical course. Currently, gene
expression profiling and various mutations (NPM1,
CEBPA, FLT3-ITD) are used to stratify AML patients
and distinguish prognostic subgroups (1). The standard of care for fit AML patients
remains intensive chemotherapy based on anthracycline and
cytarabine regimens, while in elderly populations, new therapeutic
strategies are being tested (2–6).
Azacitidine, a chemical nucleoside analogue, appears to be a
promising option for elderly patients (7–9).
Unfortunately, a large proportion of patients do not respond to AML
therapy, while others will relapse in a short period of time. In
the context of these data, new diagnostic and prognostic biomarkers
are required for the appropriate selection of patients suitable for
chemotherapy regimens and novel target therapies.
MicroRNAs (miRs) are a large family of non-coding
small RNAs that are involved in many crucial processes, including
cell differentiation, proliferation and apoptosis (10–12).
Dysregulation of numerous miRs has been reported in solid tumours
and haematological malignancies. Certain miRs have been identified
to act as a tumour suppressors or oncogenes (12,13).
Furthermore, miRs are able to regulate the expression of many
target genes through mRNA interference.
The miR-181 family has been previously studied in
haematological malignancies (14–19). Human
miR-181 genes are located on chromosomes 1, 9 and 19 (14). Members of miR-181 group (miR-181a,
miR-181b, miR-181c and miR-181d) have been conserved during
evolution, which may suggest that they are functionally important.
Previous evidence has suggested an association between miR-181a
expression and French-American-British (FAB) AML subtypes (20).
The purpose of the current study was to evaluate
miR-181a expression in AML patients prior to chemotherapy compared
with healthy controls, and miR-181 expression after chemotherapy
was completed. The results were analysed with regard to the
clinical features of AML patients and the type of induction
chemotherapy (intensive vs. low-dose).
Materials and methods
Patient characteristics
This study included 95 patients [mean age ± standard
deviation (SD), 60.2±15.0 years; age range 22–90 years; male, 61%]
with newly diagnosed AML. Samples of the bone marrow for miR-181
expression analysis were collected prior to the start of
chemotherapy and repeated following the completion of induction
chemotherapy (40 patients). Patients were treated in the Department
of Haematology, Blood Neoplasms and Bone Marrow Transplantation of
Wroclaw Medical University (Wroclaw, Poland). A control group of 20
healthy subjects was also assessed [mean age ± SD, 64.2±10.5 years;
age range, 39–80 years; male, 65%). According to the AML FAB
classification, 7 patients had AML M0, 34 had M1, 29 had M2, 14 had
M4 and 11 had M5. There were 73 patients with primary leukaemia and
22 patients with leukaemia secondary to myelodysplastic or
myeloproliferative syndromes. A summary of patient characteristics
is present in Table I.
 | Table I.Clinical characteristics of patients
with acute myeloid leukaemia (n=95). |
Table I.
Clinical characteristics of patients
with acute myeloid leukaemia (n=95).
| Characteristic | Value |
|---|
| Gender, n |
|
| Male | 56 |
|
Female | 39 |
| Age (years) |
|
|
Range | 22–90 |
|
Median | 61 |
|
French-American-British subtype, n |
|
| M0 | 7 |
|
M1/M2 | 63 |
|
M4/M5 | 25 |
| White blood cells,
×109/l |
|
|
Range | 0.2–295 |
|
Median | 14 |
| Haemoglobin,
g/dl |
|
|
Range | 5.8–13.1 |
|
Median | 9.3 |
| Platelet count,
×109/l |
|
|
Range | 2–310 |
|
Median | 65 |
| Lactate
dehydrogenase, U/l |
|
|
Range | 108–4,565 |
|
Median | 340 |
| Blasts in bone
marrow, n |
|
|
<50% | 35 |
| ≥50% | 60 |
| Cytogenetics, n |
|
|
Favorable | 5 |
|
Intermediate | 39 |
|
Unfavorable | 51 |
| Chemotherapy, n |
|
|
Intensive | 56 |
|
Low-dose | 27 |
| Best
supportive care | 12 |
| Mutation status,
n | 60 |
| AML/ETO
(positive/negative) | 4/56 |
|
CBFb-MYH11
(positive/negative) | 2/58 |
| NPM1
(positive/negative) | 7/53 |
|
FLT3/ITD
(positive/negative) | 13/47 |
| Complete remission,
n |
|
| Yes
(total) | 51 |
| Yes
(after first-line therapy) | 36 |
| No | 44 |
| Duration of
remission, months |
|
|
Range | 2–54 |
|
Median | 20 |
| Time to relapse,
months |
|
|
Range | 3–23 |
|
Median | 12 |
| Survival,
months |
|
|
Range | 0–55 |
|
Median | 3 |
Induction chemotherapy
Following diagnosis, 56 patients were treated with
standard induction intensive chemotherapy (daunorubicin plus
cytarabine 3+7), 27 received low-dose chemotherapy (low-dose
cytarabine or azacitidine) and 12 received best supportive care
only. Following the completion of induction therapy, the response
to treatment was evaluated. Complete remission (CR) was defined by
the Cheson criteria (21). Bone
marrow samples were also reevaluated for miR-181 expression in 40
patients. Patients were followed up for a median of 21 months
(range, 1–40 months).
AML patients treated with
azacitidine
There were 17 newly diagnosed AML patients who were
not suitable for intensive chemotherapy and were treated with
azacitidine (4 women and 13 men; age range, 65–90 years; median
age, 75 years). Azacitidine was administered at a dose of 75
mg/m2 subcutaneously on days 1–7 of 28-day cycles.
Patients received 1–35 cycles of azacitidine (median, 7 cycles).
There were 6 patients who achieved CR, 6 who had partial response
or disease stabilisation and 5 who did not respond to therapy. At
the time of analysis, all patients, except one, had succumbed to
the disease.
Compliance with ethical standards
Research was conducted in compliance with the
Declaration of Helsinki. Approval for the study was obtained from
the Bioethics Committee of Wroclaw Medical University. Written
informed consent for the study was obtained from all
participants.
Isolation and expression analysis of
miRs
Bone marrow mononuclear cells were isolated by
Ficoll-Hypaque density gradient centrifugation. Total RNA and miRs
were extracted from collected AML mononuclear cells using the
mirVana™ miRNA Isolation Kit (Ambion; Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the protocol of the
manufacturer. Subsequently, 5 µl of total miRNA was used as a
template for the synthesis of cDNA using TaqMan MicroRNA
Transcription Reaction Kit (Applied Biosystems; Thermo Fisher
Scientific, Inc.) and 3 µl of specific miRNA primers from the
TaqMan MicroRNA Assays (Applied Biosystems; Thermo Fisher
Scientific, Inc.). Individual reactions were conducted in a 15 µl
total volume with the following thermal condition: 16°C for 30 min,
42°C for 30 min and 85°C for 5 min. TaqMan MicroRNA Assays for
miR-181 (hsa-miR-181) and RNU48 were used. The expression levels of
each miR were measured by the relative quantitative polymerase
chain reaction (qPCR) method using TaqMan Gene Expression Assays
and TaqMan Fast Universal PCR Master Mix (Applied Biosystems;
Thermo Fisher Scientific, Inc.). All reactions were performed in
triplicate in a total volume of 20 µl on 96-well plates. qPCR was
performed on 7900HT Fast Real-Time PCR System (Applied Biosystems;
Thermo Fisher Scientific, Inc.) under the following thermal cycling
conditions: 20 sec at 95°C; and 40 cycles of 1 sec at 95°C and 20
sec at 60°C. For quantification, the samples were normalised
against the expression of RNU48 miR. Relative quantification
factors for the examined miRs were calculated using the ΔΔCq method
(22).
Statistical analysis
The differences in the mean levels of gene
expression between the study and control patients were assessed
using the Student's t-test (for independent samples). To
examine the time to mortality or remission, a Cox regression
analysis was applied (23). The
differences between the gene expression levels before and after
treatment were analysed using robust regression and multivariate
approach (24). The computation was
performed in R software (25) and
based on the simulation technique known as Gibbs sampling in
WinBUGS platform (26). Kaplan-Meier
survival curves were used to determine any significant relationship
between miR-181 expression and clinical outcome. P<0.05
indicated a statistically significant difference.
Results
miR-181 expression and clinical
characteristics
miR-181 expression was found to be significantly
higher in AML patients compared to the control group (P=0.019), and
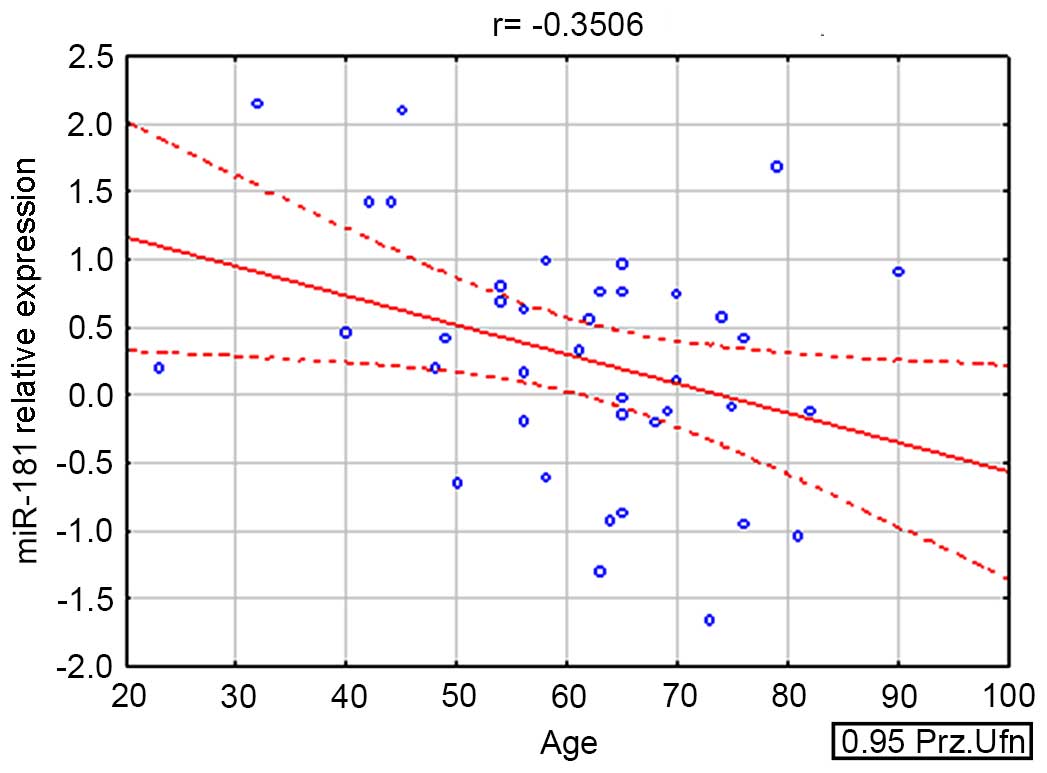
was also indirectly correlated with the age of the patient
(r=−0.350563; P=0.032) (Fig. 1).
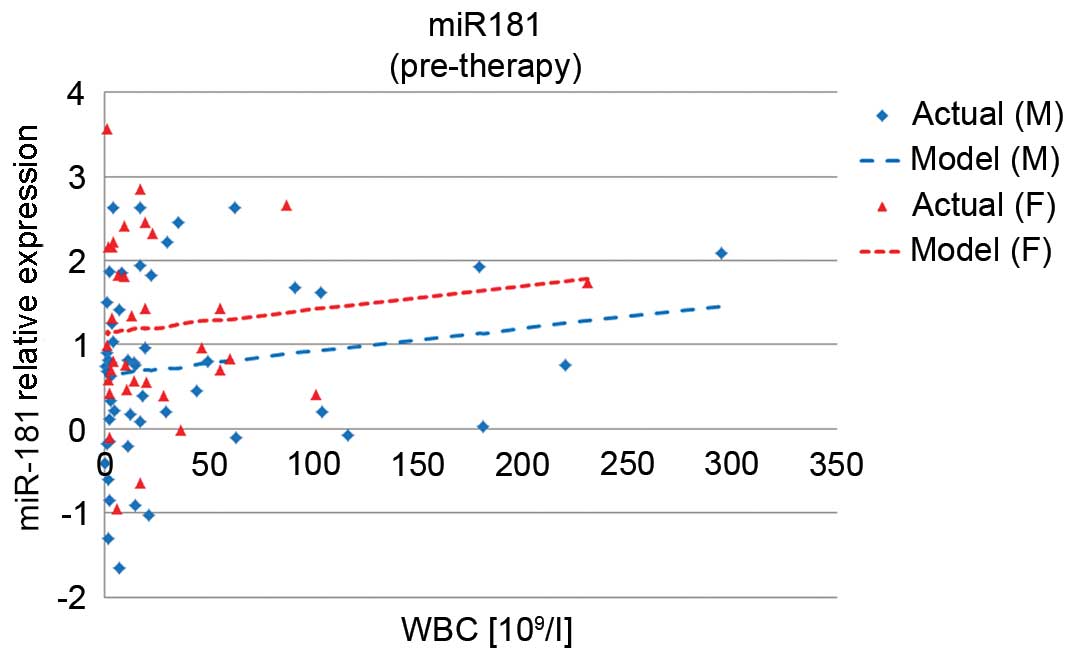
miR-181 expression was correlated with higher white blood cell
count (P=0.0069). It was also associated with gender and was
significantly higher in women than in men (P=0.001) (Fig. 2).
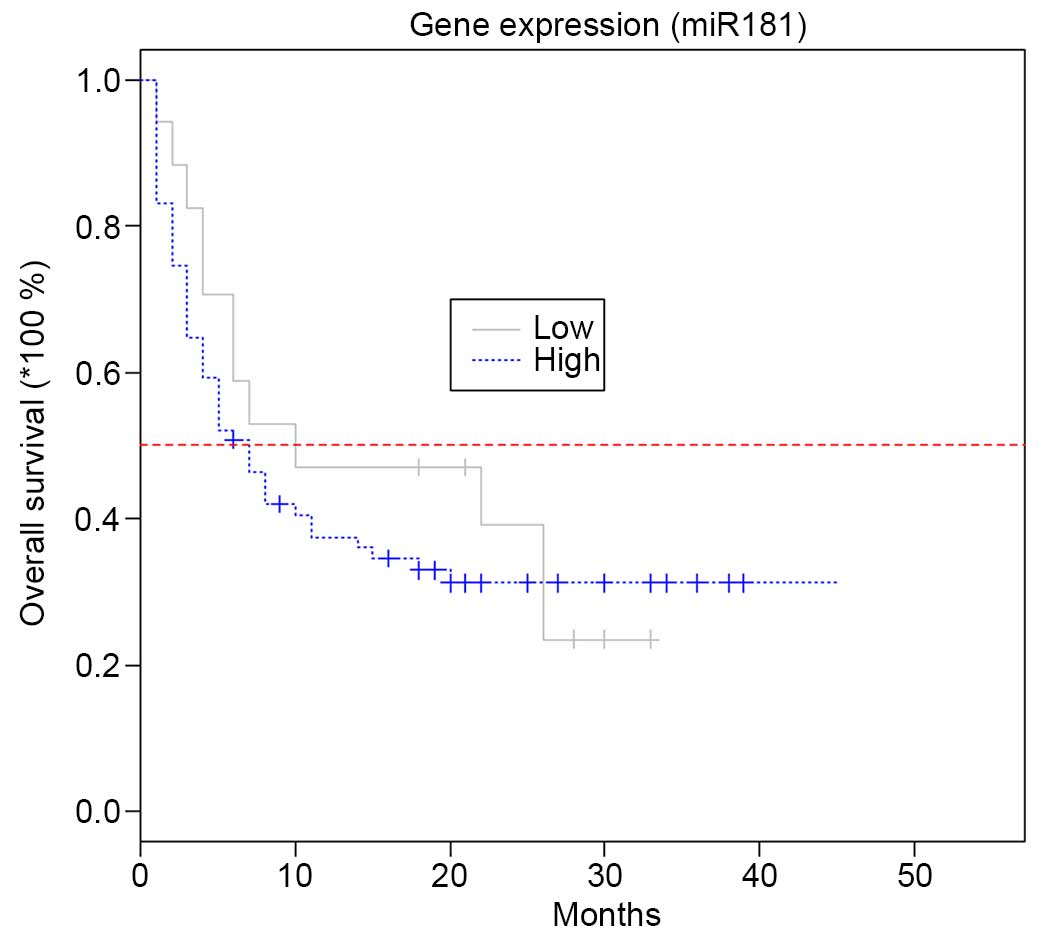
Patients with higher miR-181 expression at diagnosis
had better prognosis than those with lower miR-181 expression
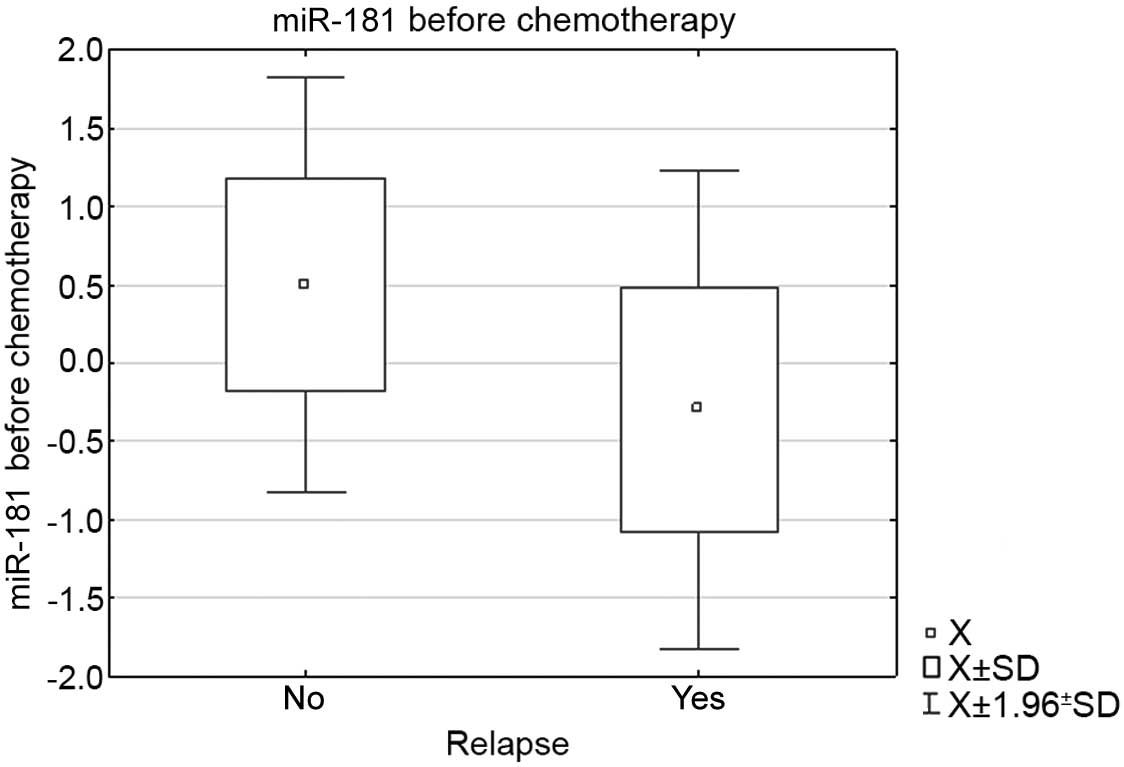
(P=0.048). In the group of AML patients who relapsed following
remission, the mean miR-181 expression level at diagnosis was lower
than in the group that maintained CR (−0.299657 vs. 0.500855;
P=0.012) (Fig. 3). The change in
miR-181 expression following chemotherapy did not influence
patients' clinical outcomes (P=0.065).
miR-181 expression and azacitidine
therapy
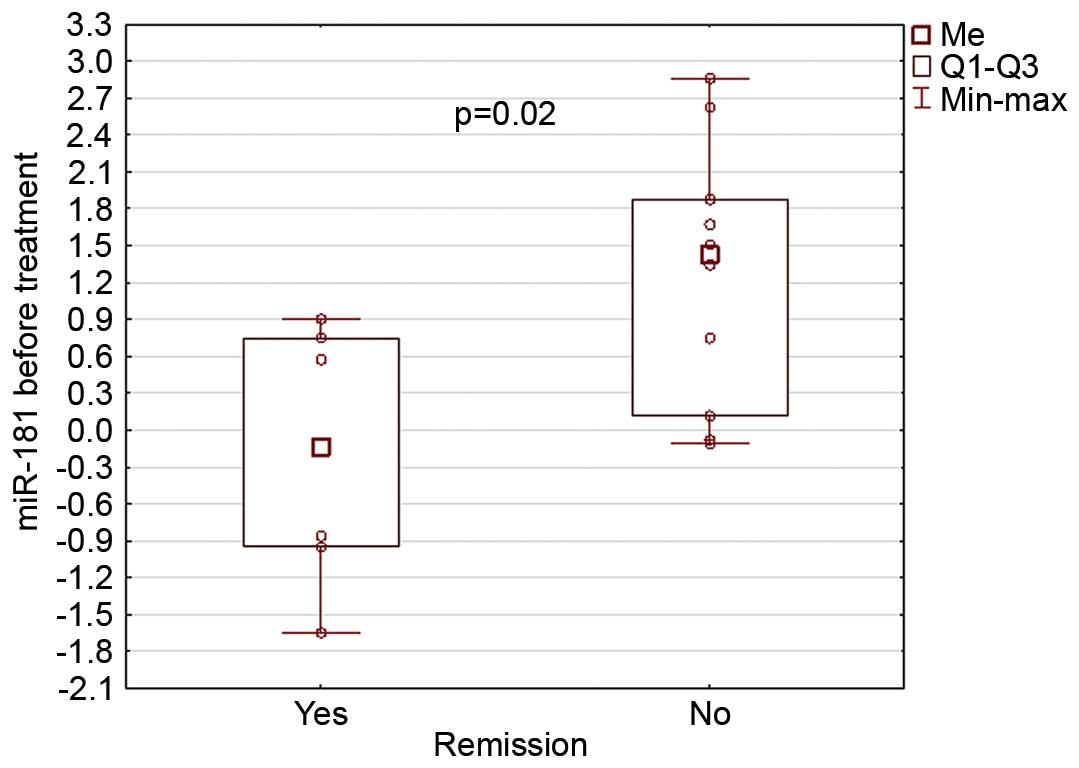
At the time of AML diagnosis, miR-181 expression was
correlated with the response to azacitidine treatment: Patients who
responded to azacitidine therapy had lower miR-181 expression at
diagnosis than those without remission (−0.21 vs. 1.26; P=0.02)
(Fig. 4).
Furthermore, miR-181 expression level prior to
therapy determined patients' clinical outcomes. Its expression was
indirectly correlated with survival after azacitidine therapy
(r=−0.503172; P=0.048); lower expression determined longer survival
(median survival 22 vs. 5 months). On multivariate analysis, low
miR-181 expression at diagnosis was an independent predictive
factor for response to azacitidine and prolonged survival (P=0.012;
Fig. 5).
Discussion
Currently, an increasing number of studies on miRs
in solid tumours and haematological malignancies are being
conducted, with which our knowledge regarding the role of miRs in
pathogenesis and the clinical course of the disease is still
increasing. Dysregulation of the expression of various miRs is
associated with the development and progression of AML (20,27–30).
The important role of miR-181 in AML has been
already described in previous studies. Isken et al (31) and Debernardi et al (20) reported elevated expression of miR-181
in M1 and M2 FAB AML subtypes, while in M4 and M5 patients the
miR-181, expression was lower. In the present study, higher levels
of miR-181 expression were identified in the AML group compared to
the control group; however, no association between expression and
FAB AML subtype was observed. In addition, the current results
suggest an indirect correlation of miR-181 expression with the age
of AML patients. Furthermore, higher miR-181 expression was
observed in women and associated with white blood cell count.
The role of miR-181 as a potential prognostic marker
has been demonstrated in particular groups of AML subsets. A study
by Li et al (16) revealed
downregulation of miR-181 expression in cytogenetically abnormal
AML cases, while its increased expression was associated with
better prognosis. The results of the present study are in line with
previously published studies, such as the study by Schwind et
al (32) who confirmed a higher
CR rate, and longer overall survival and disease-free survival
times in patients with higher miR-181 expression levels. In the
present study, lower miR-181 expression at diagnosis was associated
with a higher risk of disease relapse.
Although high expression of miR-181 was observed in
the total AML patient population and was correlated with better
survival, low expression of miR-181 was observed in an older
population treated with the hypomethylating drug azacitidine and
was associated with prolonged survival. The novelty of our results
relates to the relationship between miR-181 expression and response
to azacitidine therapy in the subset of elderly patients with AML.
In the aforementioned patient population, characterised by poor
overall survival, no standard chemotherapy options are available.
Azacitidine is a cytidine analogue which, in low doses, causes DNA
demethylation by inactivation of the DNA methyltransferase-1 enzyme
(33). Recently, it has been shown
that azacitidine is an effective and safe induction therapy for
elderly AML patients who are not suitable to undergo intensive
treatment (34). However, there is
still a group of patients who do not benefit from hypomethylating
therapy.
To the best of our knowledge, the present study is
the first to show a correlation between miR-181 expression and
azacitidine efficacy in AML patients; lower expression was an
independent predictor for good response to treatment and prolonged
survival. With regard to the feasibility of miR-181 expression
measurement, it is an easy tool in the selection of particular
patient groups potentially benefiting from this kind of
treatment.
In summary, the current study confirmed the presence
of upregulated miR-181 expression in AML patients. Furthermore, for
the first time, the ability of miR-181 expression to determine
response to azacitidine in elderly patients has been shown. In
addition, miR-181 may be used as a stratification tool to qualify
patients for this kind of treatment, and to select potential
responders to azacitidine therapy. Nevertheless, these results must
be validated in a larger population of AML patients.
Acknowledgements
This study was supported by the Wroclaw Medical
University Young Scientist Grant (no. Pbmn140).
References
|
1
|
Shivarov V and Bullinger L: Expression
profiling of leukemia patients: Key lessons and future directions.
Exp Hematol. 42:651–660. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ross K, Gillespie-Twardy AL, Agha M,
Raptis A, Hou JZ, Farah R, Redner RL, Im A, Duggal S, Ding F, et
al: Intensive chemotherapy in patients aged 70 years or older newly
diagnosed with acute myeloid leukemia. Oncol Res. 22:85–92. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wang ES: Treating acute myeloid leukemia
in older adults. Hematology Am Soc Hematol Educ Program.
2014:14–20. 2014.PubMed/NCBI
|
|
4
|
Burnett AK, Russell N, Hills RK,
Panoskaltsis N, Khwaja A, Hemmaway C, Cahalin P, Clark RE and
Milligan D: A randomised comparison of the novel nucleoside
analogue sapacitabine with low-dose cytarabine in older patients
with acute myeloid leukaemia. Leukemia. 29:1312–1319. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mayer J, Arthur C, Delaunay J, Mazur G,
Thomas XG, Wierzbowska A, Ravandi F, Berrak E, Jones M, Li Y and
Kantarjian HM: Multivariate and subgroup analyses of a randomized,
multinational, phase 3 trial of decitabine vs treatment choice of
supportive care or cytarabine in older patients with newly
diagnosed acute myeloid leukemia and poor- or intermediate-risk
cytogenetics. BMC Cancer. 14:692014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kantarjian HM, Thomas XG, Dmoszynska A,
Wierzbowska A, Mazur G, Mayer J, Gau JP, Chou WC, Buckstein R,
Cermak J, et al: Multicenter, randomized, open-label, phase III
trial of decitabine versus patient choice, with physician advice,
of either supportive care or low-dose cytarabine for the treatment
of older patients with newly diagnosed acute myeloid leukemia. J
Clin Oncol. 30:2670–2677. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Ramos F, Thépot S, Pleyer L, Maurillo L,
Itzykson R, Bargay J, Stauder R, Venditti A, Seegers V,
Martínez-Robles V, et al: Azacitidine frontline therapy for unfit
acute myeloid leukemia patients: Clinical use and outcome
prediction. Leuk Res. 39:296–306. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Lao Z, Yiu R, Wong GC and Ho A: Treatment
of elderly patients with acute myeloid leukemia with azacitidine
results in fewer hospitalization days and infective complications
but similar survival compared with intensive chemotherapy. Asia Pac
J Clin Oncol. 11:54–61. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Huang J, Lyu H, Wang J and Liu B: MicroRNA
regulation and therapeutic targeting of survivin in cancer. Am J
Cancer Res. 5:20–31. 2014.PubMed/NCBI
|
|
10
|
Larson RA: Micro-RNAs and copy number
changes: New levels of gene regulation in acute myeloid leukemia.
Chem Biol Interact. 184:21–25. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Yahya SM and Elsayed GH: A summary for
molecular regulations of miRNAs in breastcancer. Clin Biochem.
48:388–396. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li YQ, Ren XY, He QM, Xu YF, Tang XR, Sun
Y, Zeng MS, Kang TB, Liu N and Ma J: MiR-34c suppresses tumor
growth and metastasis in nasopharyngeal carcinoma by targeting MET.
Cell Death Dis. 6:e16182015. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Drebber U, Lay M, Wedemeyer I, Vallböhmer
D, Bollschweiler E, Brabender J, Mönig SP, Hölscher AH, Dienes HP
and Odenthal M: Altered levels of the onco-microRNA 21 and the
tumor-supressor microRNAs 143 and 145 in advanced rectal cancer
indicate successful neoadjuvant chemoradiotherapy. Int J Oncol.
39:409–415. 2011.PubMed/NCBI
|
|
14
|
Weng H, Lal K, Yang FF and Chen J: The
pathological role and prognostic impact of miR-181 in acute myeloid
leukemia. Cancer Genet. 208:225–229. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Jinlong S, Lin F, Yonghui L, Li Y, Li Y
and Weidong W: Identification of let-7a-2-3p or/and miR-188-5p as
prognostic biomarkers in cytogenetically normal acute myeloid
leukemia. PLoS One. 10:e01180992015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Li Z, Huang H, Li Y, Jiang X, Chen P,
Arnovitz S, Radmacher MD, Maharry K, Elkahloun A, Yang X, et al:
Up-regulation of a HOXA-PBX3 homeobox-gene signature following
down-regulation of miR-181 is associated with adverse prognosis in
patients with cytogenetically abnormal AML. Blood. 119:2314–2324.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chu B, Zhong L, Dou S, Wang J, Li J, Wang
M, Shi Q, Mei Y and Wu M: miRNA-181 regulates embryo implantation
in mice through targeting leukemia inhibitory factor. J Mol Cell
Biol. 7:12–22. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Huang P, Ye B, Yang Y, Shi J and Zhao H:
MicroRNA-181 functions as a tumor suppressor in non-small cell lung
cancer (NSCLC) by targeting Bcl-2. Tumour Biol. 36:3381–3387. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Su R, Lin HS, Zhang XH, Yin XL, Ning HM,
Liu B, Zhai PF, Gong JN, Shen C, Song L, et al: MiR-181 family:
Regulators of myeloid differentiation and acute myeloid leukemia as
well as potential therapeutic targets. Oncogene. 34:3226–3239.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Debernardi S, Skoulakis S, Molloy G,
Chaplin T, Dixon-McIver A and Young BD: MicroRNA miR-181a
correlates with morphological sub-class of acute myeloid leukaemia
and the expression of its target genes in global genome-wide
analysis. Leukemia. 21:912–916. 2007.PubMed/NCBI
|
|
21
|
Cheson BD, Bennett JM, Kopecky KJ, Büchner
T, Willman CL, Estey EH, Schiffer CA, Doehner H, Tallman MS, Lister
TA, et al: Revised recommendations of the International Working
Group for Diagnosis, standardization of response criteria,
treatment outcomes and reporting standards for therapeutic trials
in acute myeloid leukemia. J Clin Oncol. 21:4642–4649. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Cox DR: Regression models and life-tables.
J R Stat Soc Series B Stat Methodol. 34:187–220. 1972.
|
|
24
|
Congdon P: Robust Regression
MethodsApplied Bayesian Modelling. Wiley; Chichester: pp. 118–126.
2003
|
|
25
|
R Core Team, . A language and environment
for statistical computing. Version 3.0.3. 2014. Vienna: R
Foundation for Statistical Computing. http://www.r-project.org/
|
|
26
|
Spiegelhalter D, Thomas A, Best N and Lunn
D: WinBUGS. Version 1.4.3. 2003. Cambridge: Imperial College School
of Medicine & Medical Research Council - Biostatistics Unit.
https://www.mrc-bsu.cam.ac.uk/bugs/winbugs/2003
|
|
27
|
Butrym A, Rybka J, Baczyńska D, Tukiendorf
A, Kuliczkowski K and Mazur G: Expression of microRNA-331 can be
used as a predictor for response to therapy and survival in acute
myeloid leukemia patients. Biomark Med. 9:453–460. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Cammarata G, Augugliaro L, Salemi D,
Agueli C, La Rosa M, Dagnino L, Civiletto G, Messana F, Marfia A,
Bica MG, et al: Differential expression of specific microRNA and
their targets in acute myeloid leukemia. Am J Hematol. 85:331–339.
2010.PubMed/NCBI
|
|
29
|
Grenda A, Budzyński M and Filip AA:
Biogenesis of microRNAs and their role in the development and
course of selected hematologic disorders. Postepy Hig Med Dosw
(Online). 67:174–185. 2013.(In Polish). View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Butrym A, Rybka J, Baczyńska D, Tukiendorf
A, Kuliczkowski K and Mazur G: Low expression of microRNA-204
(miR-204) is associated with poor clinical outcome of acute myeloid
leukemia (AML) patients. J Exp Clin Cancer Res. 34:682015.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Isken F, Steffen B, Merk S, Dugas M,
Markus B, Tidow N, Zühlsdorf M, Illmer T, Thiede C, Berdel WE, et
al: Identification of acute myeloid leukaemia associated microRNA
expression patterns. Br J Haematol. 140:153–161. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Schwind S, Maharry K, Radmacher MD, Mrózek
K, Holland KB, Margeson D, Whitman SP, Hickey C, Becker H, Metzeler
KH, et al: Prognostic significance of expression of a single
microRNA, miR-181a, in cytogenetically normal acute myeloid
leukemia: A cancer and leukemia group B study. J Clin Oncol.
28:5257–5264. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Derissen EJ, Beijnen JH and Schellens JH:
Concise drug review: Azacitidine and decitabine. Oncologist.
18:619–624. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Dombret H, Seymour JF, Butrym A,
Wierzbowska A, Selleslag D, Jang JH, Kumar R, Cavenagh J, Schuh AC,
Candoni A, et al: International phase 3 study of azacitidine vs
conventional care regimens in older patients with newly diagnosed
AML with >30% blasts. Blood. 126:291–299. 2015. View Article : Google Scholar : PubMed/NCBI
|