Introduction
Urothelial carcinoma (UC) is a common tumor that is
identified most frequently in patients aged 50–80 years, and which
has a 2:1 male predominance (1). UC
can be located in the bladder, renal pelvis or ureter with a
relative frequency of 50:3:1 (2). The
natural history of upper tract urothelial carcinoma (UTUC) differs
from that of bladder cancer; 60% of UTUCs are invasive at diagnosis
compared with only 15–25% of bladder tumors (3,4). The
majority of UCs are detected in the early stage, such that the
patients often show long-term survival (5). For metastatic UC, systemic chemotherapy
is recommended (5).
Ureteral UC is a rare malignant tumor, which
accounts for ~6% of all tumors of the upper urinary tract (1). The diagnosis of ureteral UC is made via
radiography, endoscopy and pathology. Urinary obstruction is one of
the typical imaging features (2).
Although osteoblastic destruction is observed in the metastasis of
prostate cancer, UC can also be the reason for osteoblastic
metastasis. The treatment for metastatic ureteral UC is also
systemic chemotherapy, and the same regimens used for bladder UC
are recommended (5,6). The present study reports the case of a
66-year-old male presenting with osteoblastic metastases, in which
the primary tumor was finally diagnosed as a ureteral UC. However,
the lack of pathological evidence significantly delayed the
diagnosis of the primary tumor. Written informed consent was
obtained from the patient's family.
Case report
A 66-year-old man presented to the Outpatient
Department of the West China Hospital (Chengdu, Sichuan, China) on
December 25, 2012 due to a 1-year history of thoracodorsal pain and
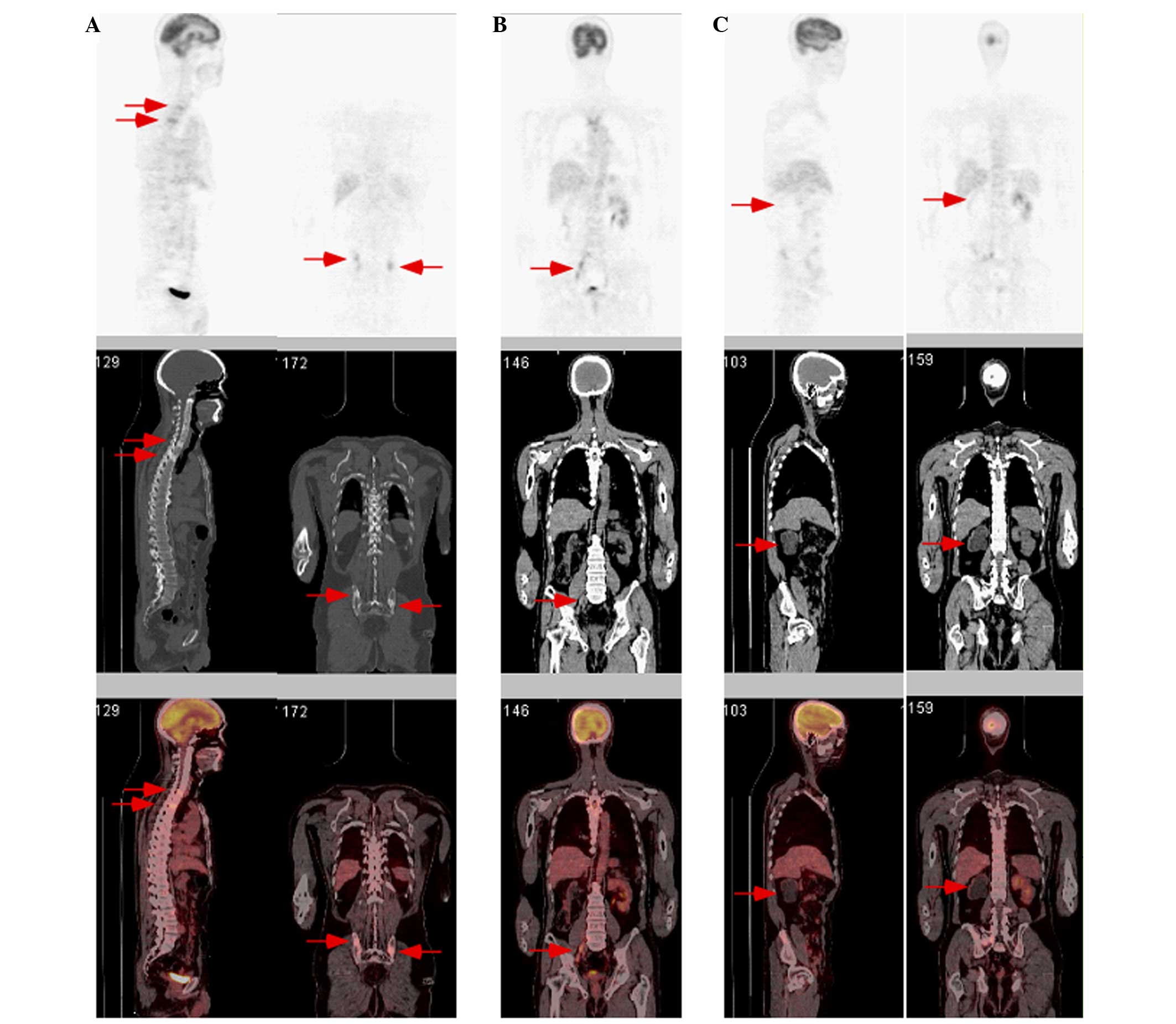
a 2-week history of left-upper limb numbness. Positron emission
tomography/computed tomography (CT) performed at another hospital
on August 30, 2012 had revealed an increased bone density and
fluorodeoxyglucose (FDG) metabolism of the pelvis and several
vertebrae, increased FDG metabolism of enlarged lymph nodes inside
the abdominopelvic cavity and a non-functioning right kidney
(Fig. 1). Furthermore, enhanced
magnetic resonance imaging of the abdominopelvic cavity had
revealed several partly and unevenly enhanced nodules of the liver
(the largest was 1.4 cm in diameter), and an unevenly enhanced
stenotic right ureter with a thickened wall. However, the ureteral
endoscopy examination was negative, and pathological examinations
of the prostate, bone marrow and voided urine had not detected any
malignant cells.
In the Outpatient Operating Room of the West China
Hospital, the patient received another multipoint biopsy of the
prostate. However, a pathological examination of the prostatic
biopsy, performed by the Department of Pathology at our hospital,
found only low-grade prostatic intra-epithelial neoplasia and a few
focal atypical glands. The patient then received another bone
marrow biopsy (January 2,2013), and smears revealed a large number
of atypical cell clusters. A flow cytometric analysis of these
particular cells, performed on January 3, 2013, showed negative
results for cluster of differentiation (CD)45, CD2, CD5, CD7, CD16,
CD56, CD10, CD19, CD20, CD38, light-chain immunoglobulin, CD34,
human leukocyte antigen-antigen D related and CD117. However,
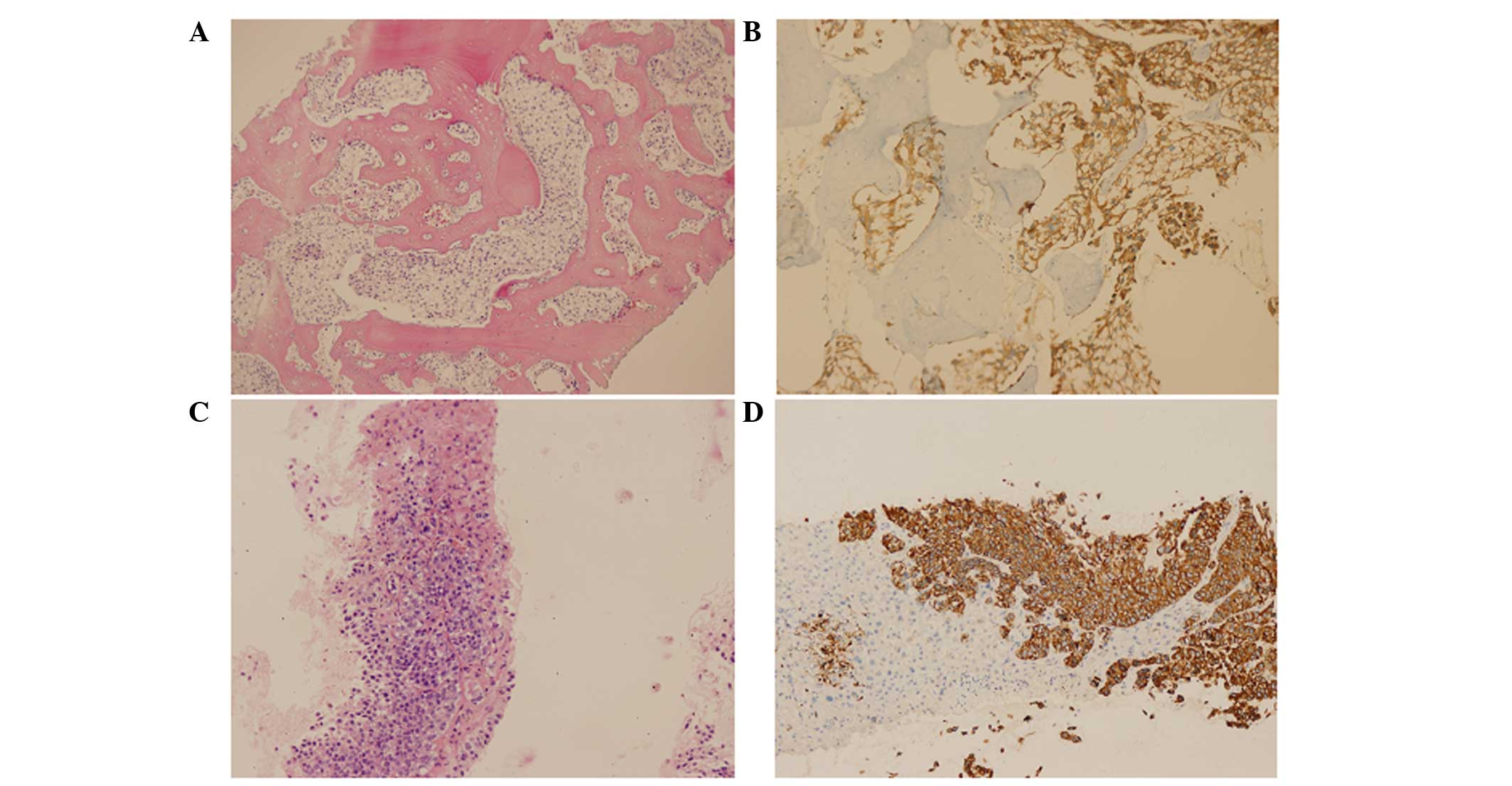
immunohistochemical staining performed by the Department of
Pathology on January 14, 2013 showed positive results for pan
cytokeratin (PCK), epithelial membrane antigen, CK7 and tumor
protein 63 (p63), and negative results for CK5/6, CK20, thyroid
transcription factor-1, prostate-specific antigen (PSA) and S-100
(Fig. 2A and B). Accordingly, the
patient was diagnosed with malignant bone metastases, and the
osteoblastic destruction was deemed not caused by prostate
cancer.
The patient was hospitalized and administered drugs
to control the pain (90 mg morphine hydrochloride sustained-release
tablets every 12 h) and nerve symptoms (thrice daily 0.3 g oral
gabapentin and once daily 10 mg intravenous dexamethasone for 4
days). The medical history revealed no family history of genetic
diseases, but the patient was a retired employee of an oil company,
therefore, contact was made with oil and various petroleum products
during this time. To search for the primary tumor, the patient
received thoracic and abdominal enhanced X-ray CT scans. The scans
revealed liver metastases (3.9×3.0 cm), and suggested a right
ureteral tumor. The patient subsequently underwent a percutaneous
liver biopsy. Immunohistochemical staining of the liver tissue,
performed on January 26, 2013, showed positive results for CK7 and
p63, a punctuate positive result for CD10, a weakly positive result
for homeobox protein CDX-2, a suspicious result for glypican-3, and
negative results for hepatocyte, Cam5.2, and Arginase (Fig. 2C and D). Meanwhile,
immunohistochemistry staining of the voided urine cytology (January
22, 2013) showed positive results for CK7 and CK20, and a negative
result for PSA. On January 26, 2013, the patient was diagnosed with
systemic multi-site metastases from ureteral UC.
Due to the patient's poor performance status, only
two cycles of intravenous gemcitabine (1,800 mg, days 1 and 8,
every 21 days plus cisplatin (500 mg, day 1 every 21 days) were
administered. The patient succumbed to disease progression 4 months
later (June 2013). From diagnosis to mortality, the patient
survived for ~6 months.
Discussion
The present study describes a delayed diagnosis of
ureteral UC due to the lack of pathological evidence. As the
patient refused to undergo endoscopic examinations again, it is
unknown whether there were synchronous or metachronous ureteral
tumors in other locations of the urinary system.
Ureteral UC is a rare malignant tumor. The most
common clinical manifestations of ureteral UC are hematuria,
increased urinary frequency, dysuria and pain. Pyuria and a
palpable mass are much less frequently observed (1). Bone is one of the common metastatic
sites of ureteral UC (7,8). Due to the drainage through the pelvic
veins into the lumbar plexus, the bone metastases of ureteral UC
most commonly affect the spine (8).
Although information on the type of bone destruction of ureteral UC
is limited, an osteoblastic or a mixed osteolystic-osteoblastic
pattern of bone destruction of transitional cell carcinoma from the
bladder has been reported (9). UC
should be therefore be preferentially considered as a primary tumor
for osteoblastic metastasis of the spine besides prostate
cancer.
However, all the typical clinical manifestations of
ureteral UC were not observed in the present patient. The patient
was not aware of the condition until thoracodorsal pain occurred
when the disease progressed to bone metastases. The lack of
pathological evidence significantly delayed the diagnosis of the
primary tumor (the negative result of the first bone marrow biopsy
may have been caused by an unsuitable puncture site), even though
radiographic examination significantly suggested ureteral UC, and
the bone metastases of the patient were osteoblastic and mainly
involved the spine.
Currently, imaging and endoscopy, combined with
pathological examination, are the main diagnostic approaches for UC
(10–12). According to the present study, a more
efficient diagnostic method is required. However, pathological
evidence remains the current golden criterion for a ureteral UC
diagnosis. Hence, the difficulty in achieving pathological evidence
(as reported in the present study) will delay the diagnosis, no
matter which diagnostic method the patient received or how
efficient this was. Notably, in other tumors with the same
situation (e.g., leptomeningeal metastasis), the National
Comprehensive Cancer Network guidelines allow clinicians to make
the diagnosis without pathological evidence (13). The guidelines offer multiple
diagnostic criteria for such a unique situation. Accordingly, the
possibility of new diagnostic criterion that do not rely on the
pathology of primary foci should be considered in ureteral UC.
According to the medical history of the patient, the
risk of UC was high in the present study. Although there is
currently insufficient evidence to recommend a screening test for
the whole UC population (14,15), a screening test should be recommended
for the sub-population who are at high risk. Recently, UC of the
bladder and the upper tract have been noted to represent two
distinct diseases with practical, anatomical, biological and
molecular differences (16). Hence, a
screening test of UC should consider the differences between the
two distinct diseases. From cytology to biomarkers, a number of
novel approaches to screen UC in high-risk patients have been
investigated (17,18). Compared with cytology, using
cost-efficient high-performing urinary biomarkers may be more
beneficial in these particular patients (14). Moreover, cytology may not be
appropriate to be used as a single screening test on the basis of
the current study results. Hence, more attention should be aimed at
the investigation of using combination strategies for the screening
test in the sub-population at high risk.
In conclusion, the present study reports the case of
a patient with ureteral UC presenting with osteoblastic metastases,
in which the diagnosis was delayed due to the lack of symptoms and
pathological evidence. Considering the possibility of asymptomatic
ureteral UC and negative pathology, a suitable screening test
should be recommended for high-risk patients. Additionally, a more
efficient diagnostic method is required. Moreover, the possibility
of new diagnostic criteria that do not rely on the pathology of
primary foci in ureteral UC should be considered due to the
difficulty in achieving pathological evidence in certain
patients.
References
|
1
|
Bennington J and Beckwith JB: Tumors of
the kidney, renal pelvis and ureterAtlas of Tumor Pathology. 2nd
series, fasc 12. Armed Forces Institute of Pathology; pp. 320–322.
1975
|
|
2
|
Winalski CS, Lipman JC and Tumeh SS:
Ureteral Neoplasms. Radiographics. 10:271–283. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Babjuk M, Oosterlinck W, Sylvester R,
Kaasinen E, Böhle A, Palou-Redorta J and Rouprêt M: European
Association of Urology (EAU): EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder, the 2011 update. Eur Urol.
59:997–1008. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Margulis V, Shariat SF, Matin SF, Kamat
AM, Zigeuner R, Kikuchi E, Lotan Y, Weizer A, Raman JD and Wood CG:
Upper Tract Urothelial Carcinoma Collaboration: Outcomes of radical
nephroureterectomy: A series from the Upper Tract Urothelial
Carcinoma Collaboration. Cancer. 115:1224–1233. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Clark PE, Spiess PE, Agarwal N, et al:
Bladder Cancer: National Comprehensive Cancer Network guidelines.
https://www.nccn.org/professionals/physician_gls/f_guidelines.asp#bladderAccessed.
May 21–2015
|
|
6
|
Rouprêt MI, Babjuk M, Compérat E, Zigeuner
R, Sylvester RJ, Burger M, Cowan NC, Böhle A, Van Rhijn BW,
Kaasinen E, et al: European Association of Urology guidelines on
upper urinary tract urothelial cell carcinoma: 2015 update. Eur
Urol. 68:868–879. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Batata MA, Whitmore WF, Hilaris BS, Tokita
N and Grabstald H: Primary carcinoma of the ureter: A prognostic
study. Cancer. 35:1626–1632. 1975. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Saitoh H, Hida M, Nakamura K and Satoh T:
Distant metastasis of urothelial tumors of the renal pelvis and
ureter. Tokai J Exp Clin Med. 7:355–364. 1982.PubMed/NCBI
|
|
9
|
Goldman SM, Fajardo AA, Naraval RC and
Madewell JE: Metastatic transitional cell carcinoma from the
bladder: Radiographic manifestions. AJR Am J Roentgenol.
132:419–425. 1979. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Krabbe LM, Bagrodia A, Westerman ME and
Margulis V: Diagnosis and management of upper tract urothelial
carcinoma. Minerva Urol Nefrol. 66:37–48. 2014.PubMed/NCBI
|
|
11
|
Yakoubi R, Colin P, Seisen T, Léon P,
Nison L, Bozzini G, Shariat SF and Rouprêt M: Radical
nephroureterectomy versus endoscopic procedures for the treatment
of localised upper tract urothelial carcinoma: A meta-analysis and
a systematic review of current evidence from comparative studies.
Eur J Surg Oncol. 40:1629–1634. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Raman SP and Fishman EK: Bladder
malignancies on CT: The underrated role of CT in diagnosis. AJR Am
J Roentgenol. 203:347–354. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Brem SS, Bierman PJ, Brem H, Butowski N,
Chamberlain MC, Chiocca EA, DeAngelis LM, Fenstermaker RA, Friedman
A, Gilbert MR, et al: Central nervous system cancers. J Natl Compr
Canc Netw. 9:352–400. 2011.PubMed/NCBI
|
|
14
|
Larre S, Catto JW, Cookson MS, Messing EM,
Shariat SF, Soloway MS, Svatek RS, Lotan Y, Zlotta AR and Grossman
HB: Screening for bladder cancer: Rationale, limitations, whom to
target, and perspectives. Eur Urol. 63:1049–1058. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Xylinas E, Kluth LA, Rieken M, Karakiewicz
PI, Lotan Y and Shariat SF: Urine markers for detection and
surveillance of bladder cancer. Urol Oncol. 32:222–229. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Green DA, Rink M, Xylinas E, Matin SF,
Stenzl A, Roupret M, Karakiewicz PI, Scherr DS and Shariat SF:
Urothelial carcinoma of the bladder and the upper tract: Disparate
twins. J Urol. 189:1214–1221. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Zincke H, Aguilo JJ, Farrow GM, Utz DC and
Khan AU: Significance of urinary cytology in the early detection of
transitional cell cancer of the upper urinary tract. J Urol.
116:781–783. 1976.PubMed/NCBI
|
|
18
|
Ho KJ and Kuo SH: Urinary
beta-glucuronidase activity as an initial screening test for
urinary tract malignancy in high risk patients. Comparison with
conventional urine cytologic evaluation. Cancer. 76:473–478. 1995.
View Article : Google Scholar : PubMed/NCBI
|