Introduction
Lung cancer continues to be a major public health
concern in the USA and other Western countries (1). In 2015, the American Cancer Society
reported that 221,200 novel cases of lung cancer were diagnosed in
the USA, accounting for ~13% of all cancer diagnoses, and 27% of
all cancer-associated mortalities in the USA (2).
A strategy for the reduction of lung
cancer-associated mortality rates is to diagnose the disease while
it is at an early stage (3). A
solitary pulmonary nodule (SPN) is defined as a single lesion in
the lung completely surrounded by lung parenchyma with a diameter
<3 cm, which is observed on chest radiography and is often the
first identifiable manifestation of lung carcinoma (4,5). Previous
studies have demonstrated that 68–75% of SPNs are malignant
(6,7).
Timely and efficient assessment of these nodules is critical for
improved patient management (8).
In the USA, >150,000 novel SPNs are identified
each year using conventional chest radiography (9). Numerous pulmonary nodules are detected
annually using chest computed tomography (CT), since this technique
is becoming more widely used in USA patient populations (10). However, CT has limited diagnostic
accuracy, since the interpretation of the images relies principally
on the size of the lesion and other non-specific findings (11). Magnetic resonance imaging is not a
routine examination for the diagnosis of SPN, due to known
artefacts that result from tissue-air interfaces and relatively low
spatial resolution (12,13). Contrast-enhanced ultrasonography may
be used to diagnose pulmonary nodules, but it does not provide a
complete image of the lungs due to a variety of reasons, including
the lungs being full of gas and interference from the osseous
thorax (14). Thus, traditional
anatomic imaging does not characterize if the SPN is malignant or
benign, which leads to numerous unnecessary surgical lung biopsies
(15). Therefore, novel imaging
modalities are required to reduce the number of excisional lung
biopsies for the diagnosis of SPN.
Fluorine-18-fluorodeoxyglucose (18F-FDG)
positron emission tomography (PET) is considered to be the most
effective method in the differential diagnosis of indeterminate
SPN, but its use is limited due to the high cost of the equipment
and lack of availability, particularly in developing countries
(16). By contrast, single-photon
emission computed tomography (SPECT) is more widely used, since it
has a lower cost (17). Radioactive
tracers for SPECT, including technetium-99m
(99mTc)-octreotide acetate (18), 99mTc-depreotide,
99mTc-methoxyisobutylisonitrile (19) and 99mTc-tetrofosmin
(20), have been employed in the
diagnosis of SPN using diagnostic imaging procedures.
Previous studies have been performed using
99mTc-(polyethylene
glycol-4)3-(Arg-Gly-Asp)2 (99mTc-3P4-RGD2) as
a novel radioactive molecular probe (21–23). This
radiopharmaceutical demonstrated a high binding affinity to
integrin αvβ3 in vitro, and exhibited significantly
increased tumor uptake and improved in vivo kinetics in
animal models (24). In addition,
99mTc-3P4-RGD2 is easily prepared
using freeze-dried kits, has a high-labeling yield and
radiochemical purity, and does not exhibit adverse events in
vivo in nonhuman primates (24).
Previously, 99mTc-3P4-RGD2 has
been used for the noninvasive differentiation of breast neoplasms
(21), and the tracer has
demonstrated an impressive image quality with a high sensitivity
(83%) in detecting breast cancer.
The primary aim of the present study was to evaluate
the use of 99mTc-3P4-RGD2 SPECT in
patients with SPN. An additional aim was to compare the diagnostic
accuracy of visual and semiquantitative indices of SPECT and CT for
the noninvasive differentiation of benign versus malignant SPN
using receiver operating characteristic (ROC) analysis.
Materials and methods
Patients
The present prospective study consisted of a
consecutive series of 24 patients [14 men and 10 women; age range,
18–77 years; mean age ± standard deviation (SD), 48.79±15.53
years], who had a SPN diameter between 1 and 3 cm on a CT scan that
was performed between September 2012 and January 2014 at the
China-Japan Union Hospital, Jilin University (Changchun, China).
The diameter of all SPNs were calculated based on the maximum
lesion diameter observed on CT scanning performed prior to the
enrollment of the patients in the present study, which was assessed
by thoracic surgeons and radiologists (Department of Radiology,
China-Japan Union Hospital, Jilin University). A definitive
diagnosis was achieved using transthoracic fine-needle aspiration
biopsy or bronchoscopic biopsy. Patients with a malignant SPN
underwent surgical resection. Permission to use a novel
radiopharmaceutical was obtained from local independent ethics
committees and the Institutional Review Board of China-Japan Union
Hospital, Jilin University (Changchun, China). Written informed
consent to participate in the present scintimammography study was
obtained from all patients.
Radiopharmaceutical
The 99mTc-3P4-RGD2 conjugate
was generously provided by the Medical Isotopes Research Center of
Peking University (Beijing, China) as a freeze-dried kit.
99mTc-3P4-RGD2 was labeled with
Na99mTcO4 (China Institute of Atomic Energy, Beijing,
China) solution, followed by 20-min incubation at 100°C. Quality
control was conducted using radioactive thin-layer chromatography
(using an AR-2000 radio-TLC Imaging Scanner, Bioscan, Inc.,
Washington, DC, USA), which enabled to measure a high labeling
yield of ~95%. A molybdenum-technetium generator was provided by
Beijing Atom Hi-Tech Co., Ltd. (Beijing, China).
Acquisition of lung SPECT/CT images
with 99mTc-3P4-RGD2
99mTc-3P4-RGD2, with a mean radioactivity of 378±86
MBq, was administered via a single intravenous bolus injection in
the contralateral arm to the affected lung in each patient,
followed by a 10 ml saline flush (Sichuan Kelun Pharmaceutical Co.,
Ltd., Sichuan, China). Acquisition of the images was obtained at 60
min post-injection. During imaging, patients were in supine
position with raised arms. The SPECT/CT system (Precedence SPECT/CT
System; Philips Healthcare, Andover, MA, USA) consisted of a
dual-head variable-angle γ-camera equipped with low-energy
high-resolution collimators and a multi-slice spiral CT component
optimized for rapid rotation. Local X-ray scanning (Precedence
SPECT/CT System; Philips Healthcare) was performed to image the
region of SPN. The following CT scan was set at a matrix of 256×256
pixels, 130 kV, 17 mAs, B60s kernel and a 5-mm slice thickness.
Following the collection of CT images, the detecting bench was
positioned automatically for SPECT data collection. The matrix was
128×128 pixels and the photopeak was centered at 140 keV, with a
symmetrical 20% window. Imaging was performed using 6° angular
steps in a 20-sec time frame. The distance between the chest and
the detector was minimized as much as possible. Digital Imaging and
Communications in Medicine image files of each patient were saved
on optic disks (Philips Healthcare) and transferred to Extended
Brilliance Workspace 4.5 (Philips Healthcare) for centralized
reconstruction, reading and analysis. A fusion SPECT and CT image
was produced using Syntegra software (Philips Medical Systems B.V.,
Eindhoven, The Netherlands).
Evaluation criteria
Three experienced nuclear physicians (Department of
Nuclear Medicine, China-Japan Union Hospital, Jilin University) who
were blinded to the patients clinical information, physical
examinations and radiological findings interpreted the
99mTc-3P4-RGD2 scans individually. The
following qualitative interpretation grades were used: Grade 1, no
uptake; grade 2, uptake lower than mediastinum; grade 3, uptake
equivalent to mediastinum; grade 4, uptake between mediastinum and
liver; and grade 5, uptake higher than liver. For semiquantitative
analysis, a region of interest was drawn around the entire nodule
to the contralateral normal lung tissue. Tumor to non-tumor
localization ratios (T/NT) were determined. In the case of grade 1,
the T/NT was considered to be 1, due to the absence of uptake. The
results were reached by a consensus of the three nuclear
physicians.
The CT images were evaluated by two experienced
thoracic radiologists who were blinded to the clinical data of the
patients. In visual analysis, the image quality of the SPN,
including size, shape, conspicuity, margin and presence of
calcification, were evaluated. Five diagnostic groups were defined
to describe the possible malignancy of each nodule: 1, definitely
benign; 2, possibly benign; 3, indeterminate; 4, possibly
malignant; and 5, definitely malignant.
Statistical analysis
All numerical results were reported as the mean ±
SD. The statistical differences of the T/NT values between patients
with malignant and benign nodules were assessed using Student's t
test. T/NT values were also compared across the various
histological types of lung carcinoma. MedCalc software 12.7.7
(MedCalc Software bvba, Ostend, Belgium) was used to determine the
optimal visual interpretation grade of SPECT and CT and the cut-off
values of semiquantitative indices of SPECT for the detection of
SPN. Linear regression analysis was performed to determine the
powerful variables for the prediction of SPN. The incremental
diagnostic value of semiquantitative indice analysis was performed
using the area under the curve in ROC analysis. P<0.05 was
considered to indicate a statistically significant difference.
Results
Patients characteristics
Samples for histological examination were obtained
using transthoracic fine-needle aspiration biopsy in 18 patients
and bronchoscopy biopsy in 6 patients. In total, 17 out of 24 (71%)
SPNs were malignant and 7 (29%) were benign. Among the malignant
etiologies, 7 patients had squamous cell carcinoma, 6
adenocarcinoma and 4 small cell carcinoma. The benign aetiologies
consisted of 4 tuberculomas, 1 inflammatory pseudotumor, 1
hamartoma and 1 reactive hyperplasia of lymph node. The
characteristics of the 24 patients are detailed in Table I.
 | Table I.Characteristics of 24 patients with
malignant and benign solitary pulmonary nodules. |
Table I.
Characteristics of 24 patients with
malignant and benign solitary pulmonary nodules.
| Patient no. | Pathology | Gender | Age, years | Nodule size,
cm | T/NT | SPECT grade | CT grade |
|---|
| 1 | Squamous cell
carcinoma | Female | 38 | 1.6 | 2.27 | 4 | 3 |
| 2 | Squamous cell
carcinoma | Female | 27 | 3.0 | 3.28 | 5 | 5 |
| 3 | Squamous cell
carcinoma | Male | 18 | 2.5 | 1.87 | 3 | 2 |
| 4 | Squamous cell
carcinoma | Female | 77 | 1.9 | 2.64 | 4 | 2 |
| 5 | Squamous cell
carcinoma | Female | 39 | 2.0 | 2.56 | 3 | 3 |
| 6 | Squamous cell
carcinoma | Female | 48 | 1.7 | 3.05 | 4 | 4 |
| 7 | Squamous cell
carcinoma | Male | 43 | 2.8 | 2.18 | 3 | 5 |
| 8 | Small cell
carcinoma | Male | 58 | 2.9 | 2.95 | 3 | 4 |
| 9 | Small cell
carcinoma | Male | 48 | 2.3 | 2.93 | 5 | 4 |
| 10 | Small cell
carcinoma | Male | 67 | 2.2 | 2.18 | 3 | 5 |
| 11 | Small cell
carcinoma | Male | 48 | 2.1 | 2.22 | 4 | 2 |
| 12 | Adenocarcinoma | Female | 57 | 2.9 | 2.07 | 3 | 4 |
| 13 | Adenocarcinoma | Female | 54 | 3.0 | 2.41 | 4 | 3 |
| 14 | Adenocarcinoma | Male | 32 | 1.1 | 3.27 | 5 | 4 |
| 15 | Adenocarcinoma | Male | 52 | 1.9 | 2.87 | 4 | 5 |
| 16 | Adenocarcinoma | Male | 68 | 2.7 | 2.38 | 4 | 3 |
| 17 | Adenocarcinoma | Male | 54 | 1.4 | 2.77 | 3 | 3 |
| 18 | Tuberculoma | Male | 37 | 1.4 | 1.04 | 1 | 2 |
| 19 | Tuberculoma | Male | 26 | 2.2 | 1.41 | 3 | 2 |
| 20 | Tuberculoma | Female | 51 | 2.1 | 2.18 | 1 | 3 |
| 21 | Tuberculoma | Female | 52 | 2.4 | 1.10 | 1 | 2 |
| 22 | Inflammatory
pseudotumor | Female | 67 | 1.1 | 3.24 | 5 | 4 |
| 23 | Reactive
hyperplasia of lymph node | Male | 35 | 2.3 | 1.19 | 1 | 2 |
| 24 | Hamartoma | Male | 75 | 1.2 | 1.06 | 1 | 1 |
T/NT
In all patients with SPN, the mean T/NT value ± SD
was 2.20±0.72 (range, 1.04–3.28). In patients with malignant SPNs,
the mean T/NT value ± SD was 2.58±0.43 (range, 1.87–3.28), and in
patients with benign SPNs it was 1.60±0.83 (range, 1.04–3.24)
(P=0.059). The semiquantitative analysis demonstrated a higher T/NT
ratio in malignant lesions compared with benign lesions, although
this was not statistically significant. Furthermore, the mean
nodule size for malignant and benign SPN was 2.24±0.59 cm, and
there was no significant correlation between the T/NT value and the
size of the nodule (P=0.92). The various histological types of
primary lung carcinoma did not affect the SPECT results [T/NT
ratios (Table I): Squamous cell
carcinoma, 2.55±0.49; adenocarcinoma, 2.63±0.43; and small cell
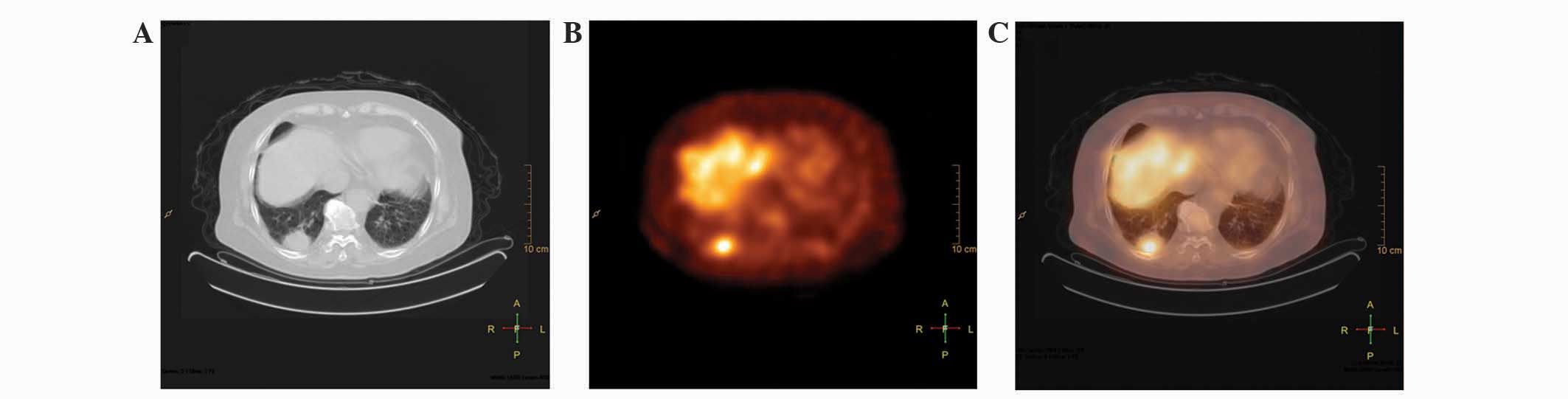
lung carcinoma, 2.57±0.43]. Representative examples of a high focal
uptake in carcinomas are presented in Fig. 1.
Optimal cut-off values
The optimal cut-off values for CT interpretation,
SPECT visual analysis and semiquantitative analysis were visual
score = 3, visual score = 2 and T/NT = 1.64, respectively. The
sensitivity, specificity, accuracy, positive predictive value and
negative predictive value of the three diagnostic analyses are
indicated in Table II.
 | Table II.Diagnostic test parameters of three
diagnostic methods for the characterization of solitary pulmonary
nodules. |
Table II.
Diagnostic test parameters of three
diagnostic methods for the characterization of solitary pulmonary
nodules.
| Parameters | CT
interpretation | Visual analysis of
SPECT | Semiquantitative
analysis of SPECT |
|---|
| Accuracy, % (95%
CI) | 79.2
(59.5–90.8) | 91.7
(74.2–97.7) | 91.7
(74.2–97.7) |
| Sensitivity, % (95%
CI) | 82.4
(59.0–93.8) | 100.0
(81.6–100.0) | 100.0
(81.6–100.0) |
| Specificity, % (95%
CI) | 71.4
(35.9–91.8) | 71.4
(35.9–91.8) | 71.4
(35.9–91.8) |
| PPV, % (95%
CI) | 87.5
(64.0–96.5) | 89.5
(68.6–97.1) | 89.5
(68.6–97.1) |
| NPV, % (95%
CI) | 62.5
(30.6–86.3) | 100.0
(56.6–100.0) | 100.0
(56.6–100.0) |
Visual analysis
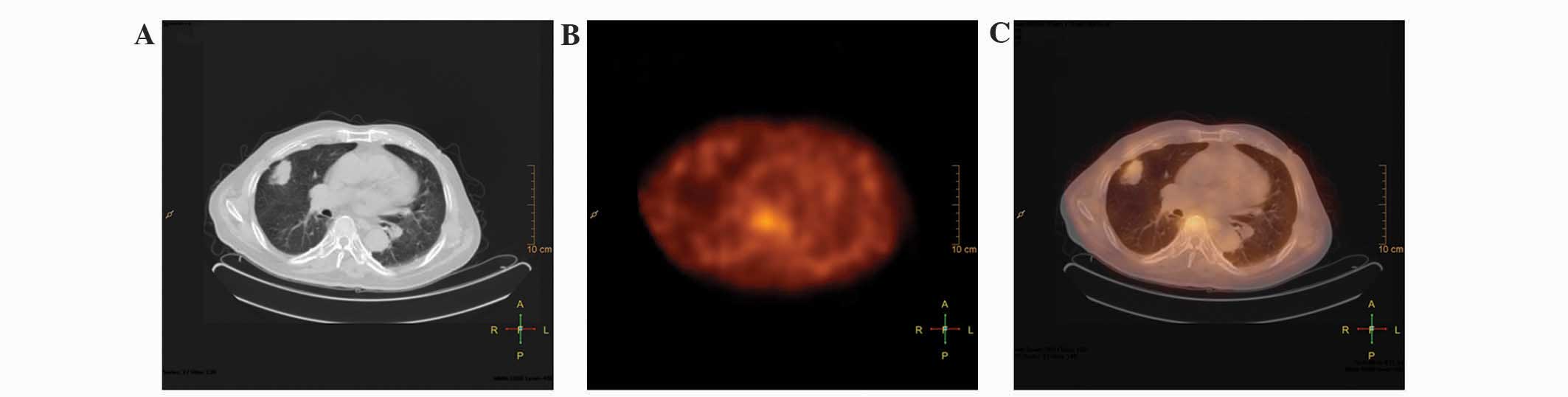
The CT scan resulted in 2 false-positive findings (1
tuberculoma and 1 inflammatory pseudotumor) and 3 false-negative
findings (2 squamous cell carcinoma and 1 small cell carcinoma)
(Fig. 1). Although SPECT visual and
semiquantitative analyses also yielded 2 false-positive findings (1
tuberculoma in a different patient and 1 inflammatory pseudotumor
in patient 22), it resulted in no false-negative findings (Fig. 2).
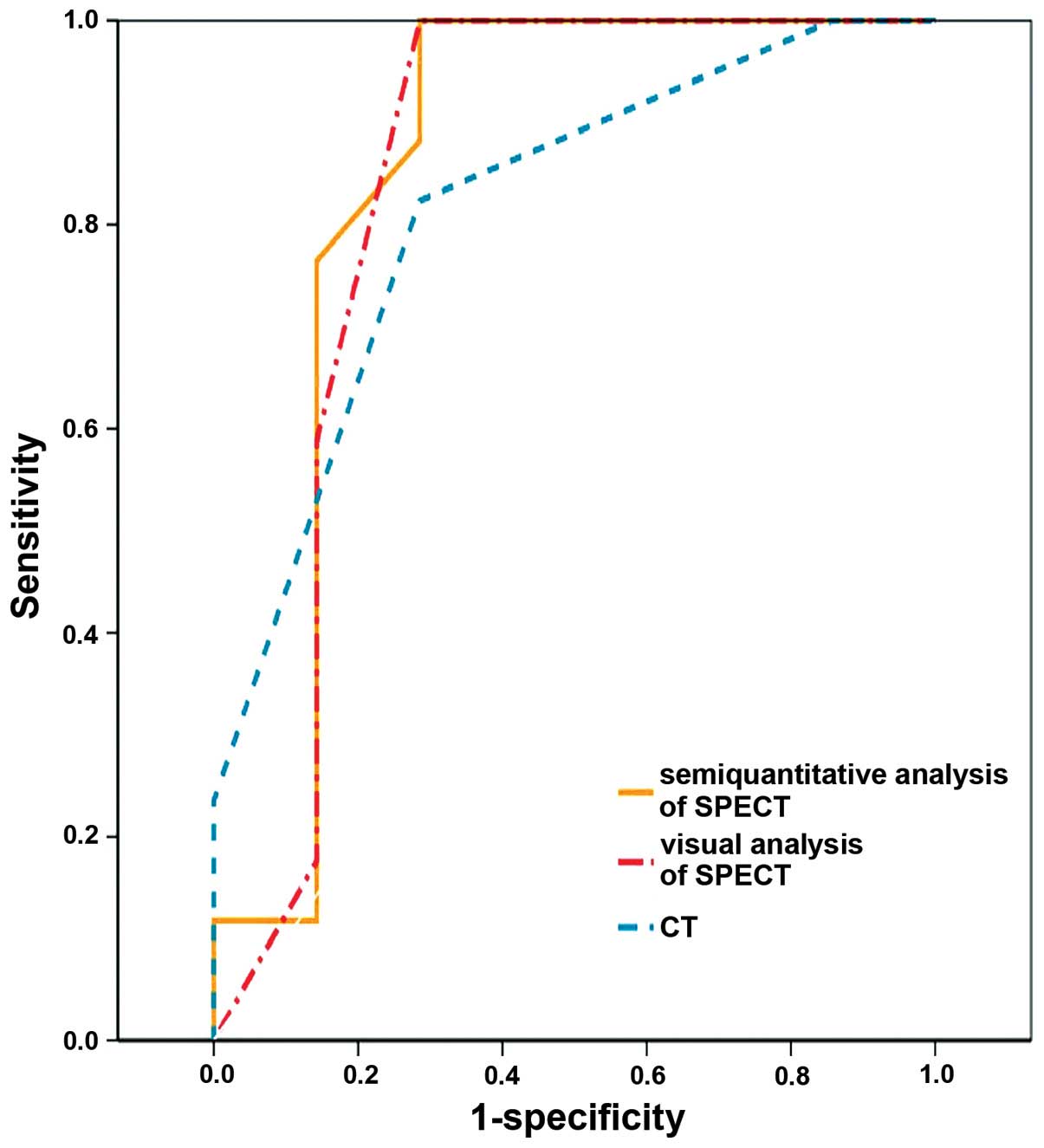
ROC analysis
The empirical ROC areas, which estimated overall
diagnostic performance, did not differ significantly among the
three diagnostic methodologies (Fig.
3): Semiquantitative analysis of SPECT, 0.849 [95% confidence
interval (CI), 0.618–1.000]]; visual analysis of SPECT, 0.840 (95%
CI, 0.600–1.000]; CT interpretation, 0.815 (95% CI, 0.626–1.000);
semiquantitative analysis of SPECT vs. visual analysis of SPECT
(P=0.521); semiquantitative analysis of SPECT vs. CT interpretation
(P=0.588); and visual analysis of SPECT vs. CT interpretation
(P=0.564).
Discussion
99mTc-3P4-RGD2 is a
well-designed, dimeric RGD peptide that exhibits increased uptake
in mouse cancer xenografts using scintigraphy (25). The uptake of the RGD-containing
peptide, quantified by in vitro and in vivo studies,
has been observed to be proportional to integrin density and tumor
size (26–29). In previous studies, the present
authors group applied this novel tracer for the noninvasive
differentiation of palpable and nonpalpable breast lesions
(21), and the tracer demonstrated an
impressive image quality with a high sensitivity in detecting
breast cancer.
Chest CT, as a conventional imaging method,
continues to be important in the evaluation of patients with SPN
(30). Radiological findings that
suggest SPN malignancy are thickness of the cavity wall and the
presence of a speculated or nodular edge, whereas central,
laminated or diffuse calcifications are more likely to be
associated with a benign etiology (31). However, despite the advances in
anatomic and morphological imaging, numerous nodules remain
indeterminate, due to the various criteria employed for their
characterization as benign or malignant nodules (7). Invasive and expensive diagnostic
procedures, including bronchoscopy and surgical exploration, are
often undertaken to obtain a specific SPN diagnosis (32). The observation that 60% of removed
nodules are benign lesions indicates the requirement for a simple,
efficient and noninvasive approach for the differentiation of
benign versus malignant nodules (33).
The present study performed a comparison of
99mTc-3P4-RGD2 SPECT and CT for
evaluating SPN. The sensitivity of
99mTc-3P4-RGD2 SPECT was superior
to that of CT in the present study; however, the difference in
performance of the diagnostic methods was not statistically
significant. In total, 17.6% of the nodules were characterized as
malignant nodules using
99mTc-3P4-RGD2 SPECT. In addition,
5 SPNs were classified as indeterminate using CT, but were
correctly diagnosed using
99mTc-3P4-RGD2 SPECT. Visual
analysis yielded the same result as semiquantitative analysis for
99mTc-3P4-RGD2 SPECT, indicating
that visual analysis may be sufficient for the characterization of
SPN.
When grade 2 was used as a cut-off point for visual
analysis, SPECT revealed that all malignant SPNs exhibited focal
99mTc-3P4-RGD2 accumulation with
varying intensities. However, the negative predictive value of 100%
was possibly due to the low number of SPNs characterized (n=7).
Thus, an additional study with a larger patient population is
required to determine the correct negative predictive value. The
sensitivity reported in the present study for
99mTc-3P4-RGD2 SPECT is comparable
to that of 18F-FDG PET/CT (83–100%) (34,35). This
high sensitivity may be explained by a high prevalence of malignant
tumors in the present cohort. Furthermore, the low
99mTc-3P4-RGD2 uptake in the
thoracic region also exhibited a high sensitivity for the detection
of malignant nodules. Previous studies have recommended targeting
99mTc-3P4-RGD2 to regions of
angiogenesis during tumor development, since a tumor size of
0.2–0.3 cm may exhibit angiogenesis (36). Hypothetically, a pharmaceutical with
enough binding affinity such as
99mTc-3P4-RGD2 may detect the
majority of tumors with a diameter >1 cm, and may also be the
cause of high sensitivity.
In the present study, 1 case of tuberculoma and 1
case of inflammatory pseudotumor exhibited focal
99mTc-3P4-RGD2 uptake. Previous
studies have demonstrated that integrin αvβ3 is only observed on
the luminal surface of endothelial cells during angiogenesis
(37); however, angiogenesis is
nonspecific in various pathological events (38). By contrast to other benign lesions,
inflammation always exhibits a high cell density and vascularity,
which are most likely to be responsible for the increased uptake of
the tracer (39). Furthermore,
integrin αvβ3 exists on neutrophils, monocytes and vascular smooth
muscle cells (40,41); therefore, also contributing to the
false-positive results using
99mTc-3P4-RGD2 SPECT.
In conclusion, the present results suggest that a
99mTc-3P4-RGD2 SPECT scan is the
most beneficial method for the detection of malignant SPN, and
visual analysis appears to be sufficient for the characterization
of SPN.
Acknowledgements
The authors would like to thank the National Natural
Science Foundation of China (Beijing, China; grant no. 81271606)
and the Research Fund of Science and Technology Department of Jilin
Province (Changchun, China; grant no. 20150520154JH) for
financially supporting the present study.
References
|
1
|
Kristiansen C, Schytte T, Hansen KH,
Holtved E and Hansen O: Academy of Geriatric Cancer Research
(AgeCare): Trends in lung cancer in elderly in Denmark, 1980–2012.
Acta Oncol. 55(Suppl 1): 46–51. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Novaes FT, Cataneo DC, Ruiz RL Junior,
Defaveri J, Michelin OC and Cataneo AJ: Lung cancer: Histology,
staging, treatment and survival. J Bras Pneumol. 34:595–600. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tan BB, Flaherty KR, Kazerooni EA and
Iannettoni MD: American College of Chest Physicians: The solitary
pulmonary nodule. Chest. 123(Suppl 1): 89S–96S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Winer-Muram HT: The solitary pulmonary
nodule. Radiology. 239:34–49. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gould MK, Sanders GD, Barnett PG, Rydzak
CE, Maclean CC, McClellan MB and Owens DK: Cost-effectiveness of
alternative management strategies for patients with solitary
pulmonary nodules. Ann Intern Med. 138:724–735. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Schrevens L, Lorent N, Dooms C and
Vansteenkiste J: The role of PET scan in diagnosis, staging, and
management of non-small cell lung cancer. Oncologist. 9:633–643.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Spiro SG, Gould MK and Colice GL: American
College of Chest Physicians: Initial evaluation of the patient with
lung cancer: Symptoms, signs, laboratory tests, and paraneoplastic
syndromes: ACCP evidenced-based clinical practice guidelines (2nd
edition). Chest. 132(Suppl 3): 149S–160S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Ost D, Fein AM and Feinsilver SH: Clinical
practice. The solitary pulmonary nodule. N Engl J Med.
348:2535–2542. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Fujimoto K: Usefulness of
contrast-enhanced magnetic resonance imaging for evaluating
solitary pulmonary nodules. Cancer Imaging. 8:36–44. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Webb WR, Gatsonis C, Zerhouni EA, Heelan
RT, Glazer GM, Francis IR and McNeil BJ: CT and MR imaging in
staging non-small cell bronchogenic carcinoma: Report of the
Radiologic Diagnostic Oncology Group. Radiology. 178:705–713. 1991.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Bergin CJ, Glover GH and Pauly JM: Lung
parenchyma: Magnetic susceptibility in MR imaging. Radiology.
180:845–848. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Suga K, Ogasawara N, Okada M, Hara A and
Matsunaga N: Potential of noncontrast electrocardiogram-gated
half-Fourier fast-spin-echo magnetic resonance imaging to monitor
dynamically altered perfusion in regional lung. Invest Radiol.
37:615–625. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Sartori S, Postorivo S, Vece FD, Ermili F,
Tassinari D and Tombesi P: Contrast-enhanced ultrasonography in
peripheral lung consolidations: What's its actual role? World J
Radiol. 5:372–380. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
van de Luijtgaarden ACI, de Rooy JW, de
Geus-Oei LF, van der Graaf WT and Oyen WJ: Promises and challenges
of positron emission tomography for assessment of sarcoma in daily
clinical practice. Cancer Imaging. 8:S61–S68. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Sim YT, Goh YG, Dempsey MF, Han S and Poon
FW: PET-CT evaluation of solitary pulmonary nodules: correlation
with maximum standardized uptake value and pathology. Lung.
191:625–632. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
MacManus MP, Hicks RJ, Ball DL, Ciavarella
F, Binns D, Hogg A, Kalff V, Ware R, Wirth A, Salminen E and
McKenzie A: Imaging with F-18 FDG PET is superior to Tl-201 SPECT
in the staging of non-small cell lung cancer for radical radiation
therapy. Australas Radiol. 45:483–490. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wang L, Yin X, Wang F, Gu J, Lu L, Wu Q,
Shen B and Li XF: The usefulness of combined diagnostic CT and
(99m)Tc-octreotide somatostatin receptor SPECT/CT imaging on
pulmonary nodule characterization in patients. Cancer Biother
Radiopharm. 28:731–736. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Schuurmans MM, Ellmann A, Bouma H, Diacon
AH, Dyckmans K and Bolliger CT: Solitary pulmonary nodule
evaluation with 99mTc-methoxy isobutyl isonitrile in a
tuberculosis-endemic area. Eur Respir J. 30:1090–1095. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Spanu A, Schillaci O, Pirina P, Arru A and
Madeddu G, Chessa F, Marongiu P, Solinas ME and Madeddu G:
99mTc-tetrofosmin SPECT in solitary pulmonary nodule evaluation.
Oncol Rep. 16:763–769. 2006.PubMed/NCBI
|
|
21
|
Liu L, Song Y, Gao S, Ji T, Zhang H, Ji B,
Chen B, Jia B, Wang F, Xu Z and Ma Q: (99)mTc-3PRGD2
scintimammography in palpable and nonpalpable breast lesions. Mol
Imaging. 13:1–7. 2014.PubMed/NCBI
|
|
22
|
Jin X, Meng Y, Zhu Z, Jing H and Li F:
Elevated 99mTc 3PRGD2 activity in benign metastasizing leiomyoma.
Clin Nucl Med. 38:117–119. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Huang C, Zheng Q and Miao W: Study of
novel molecular probe 99mTc-3PRGD2 in the diagnosis of rheumatoid
arthritis. Nucl Med Commun. 36:1208–1214. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jia B, Liu Z, Zhu Z, Shi J, Jin X, Zhao H,
Li F, Liu S and Wang F: Blood clearance kinetics, biodistribution,
and radiation dosimetry of a kit-formulated integrin αvβ3-selective
radiotracer 99mTc-3PRGD 2 in non-human primates. Mol Imaging Biol.
13:730–736. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhou Y, Shao G and Liu S: Monitoring
breast tumor lung metastasis by U-SPECT-II/CT with an integrin
αvβ3-targeted radiotracer 99mTc-3P-RGD2. Theranostics. 2:577–588.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wang L, Shi J, Kim YS, Zhai S, Jia B, Zhao
H, Liu Z, Wang F, Chen X and Liu S: Improving tumor-targeting
capability and pharmacokinetics of (99m)Tc-labeled cyclic RGD
dimers with PEG(4) linkers. Mol Pharm. 6:231–245. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Zhang X, Xiong Z, Wu Y, Cai W, Tseng JR,
Gambhir SS and Chen X: Quantitative PET imaging of tumor integrin
alphavbeta3 expression with 18F-FRGD2. J Nucl Med. 47:113–121.
2006.PubMed/NCBI
|
|
28
|
Liu Z, Jia B, Shi J, Jin X, Zhao H, Li F,
Liu S and Wang F: Tumor uptake of the RGD dimeric probe
(99m)Tc-G3-2P4-RGD2 is correlated with integrin αvβ3 expressed on
both tumor cells and neovasculature. Bioconjug Chem. 21:548–555.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Beer AJ, Haubner R, Sarbia M, Goebel M,
Luderschmidt S, Grosu AL, Schnell O, Niemeyer M, Kessler H, Wester
HJ, et al: Positron emission tomography using
[18F]Galacto-RGD identifies the level of integrin α(v)β3
expression in man. Clin Cancer Res. 12:3942–3949. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wang X, Cao A, Peng M, Hu C, Liu D, Gu T
and Liu H: The value of chest CT scan and tumor markers detection
in sputum for early diagnosis of peripheral lung cancer. Zhongguo
Fei Ai Za Zhi. 7:58–63. 2004.PubMed/NCBI
|
|
31
|
Maehara Y, Matsumoto M, Matsuura M,
Kawasima M, Tateno K and Sakaino K: Pleural thickening associated
with solitary pulmonary nodule: Evaluation with thin-slice CT.
Nihon Igaku Hoshasen Gakkai Zasshi. 49:253–258. 1989.(In Japanese).
PubMed/NCBI
|
|
32
|
Schwarz C, Schönfeld N, Bittner RC,
Mairinger T, Rüssmann H, Bauer TT, Kaiser D and Loddenkemper R:
Value of flexible bronchoscopy in the pre-operative work-up of
solitary pulmonary nodules. Eur Respir J. 41:177–182. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Midthun DE: Solitary pulmonary nodule:
Time to think small. Curr Opin Pulm Med. 6:364–370. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Kim SK, Allen-Auerbach M, Goldin J, Fueger
BJ, Dahlbom M, Brown M, Czernin J and Schiepers C: Accuracy of
PET/CT in characterization of solitary pulmonary lesions. J Nucl
Med. 48:214–220. 2007.PubMed/NCBI
|
|
35
|
Jeong SY, Lee KS, Shin KM, Bae YA, Kim BT,
Choe BK, Kim TS and Chung MJ: Efficacy of PET/CT in the
characterization of solid or partly solid solitary pulmonary
nodules. Lung Cancer. 61:186–194. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Folkman J: Angiogenesis. Annu Rev Med.
57:1–18. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Brooks PC, Clark RA and Cheresh DA:
Requirement of vascular integrin alpha v beta 3 for angiogenesis.
Science. 264:569–571. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Mousa SA: Alphav Vitronectin receptors in
vascular-mediated disorders. Med Res Rev. 23:190–199. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Thurston G, Murphy TJ, Baluk P, Lindsey JR
and McDonald DM: Angiogenesis in mice with chronic airway
inflammation: Strain-dependent differences. Am J Pathol.
153:1099–1112. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Antonov AS, Antonova GN, Munn DH, Mivechi
N, Lucas R, Catravas JD and Verin AD: αVβ3 integrin regulates
macrophage inflammatory responses via PI3 kinase/Akt-dependent
NF-κB activation. J Cell Physiol. 226:469–476. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Horton MA: The alpha v beta 3 integrin
‘vitronectin receptor’. Int J Biochem Cell Biol. 29:721–725. 1997.
View Article : Google Scholar : PubMed/NCBI
|