Introduction
Osteosarcoma is the most frequent primary malignancy
of bone tissue, which mostly occurs in adolescents and children,
comprising almost 60% of all bone sarcomas (1,2).
Osteosarcoma exists as a local invasion of the bone and soft
tissue, and as distant metastases, which most often metastasize to
the lung (90%) and bone (8–10%), and are rarely observed in the
lymph nodes (3). Aggressive removal
of the primary tumor via limb sparing surgery or amputation is
typically required to ensure local control. Systemic chemotherapy
(prior to and following tumor removal) functions in the suppression
of metastasis and in the curative intent. The most common
chemotherapy regimens are comprised of drugs such as cisplatin
(CDDP), doxorubicin and high-dose methotrexate in combination
(4,5).
Despite neoadjuvant chemotherapy with a wide surgical resection of
the tumor, the survival rate for high-grade osteosarcomas remains
at only 50–80% (6) due to its early
metastasis and aggressive malignant potential. Nevertheless, a poor
response to chemotherapy is usually associated with a poor
prognosis (7), and increasing
evidence is showing that this therapeutic resistance is partially
due to the inherent resistance of a tumorigenic subpopulation of
cancer cells, referred to as cancer stem cells (CSCs).
CSCs were firstly isolated in myeloid leukemia
(8), and recent studies have
indicated that osteosarcoma is also driven by this small
subpopulation of cells (9), which
possess the potential for self-renewal and the ability to
differentiate and proliferate. Although the drug resistance
mechanisms of CSCs remain unclear, several studies have highlighted
that the adenosine triphosphate-binding cassette drug transporters
and chemotherapy-metabolizing enzyme expression may be involved, as
well as changes in cell cycle kinetics and anti-apoptotic protein
overexpression (10–12). Unlike in epithelial cancer, the
specific phenotypes of CSCs in osteosarcoma have not yet been
defined. As revealed by Adhikari et al (13), CD117 and Stro-1 are potential
candidates for osteosarcoma CSC markers that are associated with
metastasis and drug resistance, the most lethal characteristics of
the disease. To the best of our knowledge, the association between
tumor drug resistance in osteosarcoma cells and CSCs has rarely
been discussed; therefore, the present study used CDDP, which is
frequently applied for sarcoma treatment, to demonstrate the
resistance of osteosarcoma cells possessing CSC properties in a
mouse model.
In the present study, the effects of CDDP on CSCs
were assessed using the osteosarcoma 143B-TK cell line in in
vivo conditions. The results demonstrated that CDDP induced the
resistance of osteosarcoma cells possessing CSC properties in
vivo.
Materials and methods
Cell culture
The human osteosarcoma 143B-TK cell line was
obtained from the China Center for Type Culture Collection (Wuhan,
China) and cultured in Hyclone Dulbecco's minimum essential medium
(DMEM; GE Healthcare Life Sciences, Logan, UT, USA) with 10% fetal
bovine serum FBS and 1% penicillin/streptomycin (Thermo Fisher
Scientific Inc., Waltham, MA, USA). The cells were kept at 37°C in
a 5% CO2 humidified atmosphere, and harvested just as
they reached ~70% confluence.
In vivo animal model
Female, non-obese diabetic/severe combined
immunodeficiency (NOD/SCID) mice (3–5 weeks old) were obtained from
the Animal Biosafety Level-3 Laboratory of Wuhan University (Wuhan,
China) and housed in individually vented cages under specific
pathogen-free conditions, with a 12 h day/night cycle, and food and
water ad libitum. The osteosarcoma 143B-TK cell line was
cultured as aforementioned, and ~106 of the cells were
resuspended in 100 µl phosphate-buffered saline (PBS), which was
injected subcutaneously into the neck of each mouse. The animals
were evaluated for the incidence of tumor formation and checked
weekly for tumor progression; each measurement consisted of two
diameters, length (a) and width (b), and the size of tumors were
calculated by using the following formula: Volume = 0.2618 × a × b
× (a + b) (14). The mice that grew a
tumor mass were randomly divided into two groups when the tumors
reached to ~50-mm3. One group served as the
CDDP-resistant group and received an intraperitoneal injection of
CDDP (Thermo Fisher Scientific Inc.) at a dose of 5 mg/kg/week over
a period of 4 weeks. The other group served as the control group
and was administered 100 µl PBS only by intraperitoneal injection.
The mice were monitored for up to 90 days, after which they were
humanely euthanized with CO2. All experiments were
performed according to protocols approved by the Institutional
Animal Care and Use Committee and the Institutional Biosafety
Committee of the Animal Biosafety Level-3 Laboratory of Wuhan
University.
Hematoxylin and eosin (HE) staining of
the tumors
Tumors were obtained from the mice as
aforementioned, and then fixed with 4% paraformaldehyde overnight
and embedded in paraffin. Paraffin-embedded tissues sections (4-µm
thick) were deparaffinized and stained with hematoxylin
(Sigma-Aldrich, St. Louis, MO, USA) for 8 min. Subsequent to being
washed with PBS and Aqua Dest, the sections were incubated with
eosin (1:100 in Aqua Dest; Sigma, St. Louis, USA) for 3 min.
Afterwards, the sections were washed with Aqua Dest, embedded in
the mounting medium, covered with a coverslip and analyzed by
transmission light microscopy (Olympus BX51; Olympus, Tokyo,
Japan).
Reverse transcription-polymerase chain
reaction (RT-qPCR)
Total RNA was isolated and dissolved in 20 µl RNA
enzyme-free water and then 2 µl of sample was reverse transcribed
per assay. qPCR was then performed using an ABI 7900 System
(Applied Biosystems; Thermo Fisher Scientific Inc.) in the presence
of SYBR Green. The following gene-specific primers were used:
Multi-drug resistance association protein-1 (MRP-1) forward,
5′-CTGGCTTGGTGTGAACTGAT-3′ and reverse, 5′-AGGCTCTGGCTTGGCTCTAT-3′;
multi-drug resistance gene-1 (MDR-1) forward,
5′-GGACAGAAACAGAGGATCGC-3′ and reverse, 5′-CCCGTCTTGATCATGTGGCC-3′;
octamer-binding transcription factor 4 (OCT4) forward,
5′-GAGTGAGAGGCAACCTGGAGAAT-3′ and reverse,
5′-ACCGAGGAGTACAGTGCAGTGAA-3′; SRY (sex determining region Y)-box 2
(SOX2) forward, 5′-TGGGTTCGGTGGTCAAGTCC-3′ and reverse,
5′-TGTGTGAGAGGGGCAGTGTG-3′; telomerase reverse transcriptase (TERT)
forward, 5′-AAGTTTGGAAGAACCCCACATT-3′ and reverse,
5′-AGGATGGTCTTGAAGTCTGAGG-3′; Nanog forward,
5′-GAGAAGAGTGTCGCAAAAAAGGA-3′ and reverse,
5′-TGAGGTTCAGGATGTTGGAGAGT-3′; and β-actin forward,
5′-CACCCAGCACAATGAAGATCAAGAT-3′ and reverse,
5′-CCAGTTTTTAAATCCTGAGTCAAGC-3′. The expression of the TERT, Nanog,
OCT4 and SOX2 marker genes is associated with CSCs, and β-actin was
used as endogenous normalization control. Target sequences were
amplified at 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 1 min. All assays were performed in triplicate.
The fold-change in mRNA expression was determined according to the
2ΔΔCq method (15).
Isolation of tumor cells
After the mice were sacrificed at the endpoint of
therapy, the tumors in each group were collected and immediately
immerged in DMEM supplemented with 10% FBS and 1%
penicillin/streptomycin. The tumors were transported in a container
filled with liquid nitrogen, and then the necrotic tissue of the
tumor was removed and the tumors were cut into small sections under
sterile conditions. Trypsin solution (0.25%) with
ethylenediaminetetraacetic acid (EDTA; 0.02%), at 10 times of the
volume of the tumor tissue, was added for digestion for 40 min at
37°C in a shaking incubator, and then the digestive fluid was
discarded and the cells were resuspended in 1 ml DMEM to obtain a
homogenized cell suspension. The suspension was diluted in DMEM
with 10% FBS and 1% penicillin/streptomycin and incubated under
standard conditions (37°C and 5% CO2) in a humidified
atmosphere. Just as the cells began to attach to the bottom of the
plate, the medium was replaced every second day.
Sphere formation assay
At ~70% confluence, the tumor cells in DMEM
supplemented with 10% FBS medium were dissociated with trypsin-EDTA
into single-cell suspensions. A total of 1×105
cells/well were seeded in ultra-low attachment 6-well plates
(Corning Inc., Corning, NY, USA) and inoculated into RPMI-1640
medium without serum, supplemented with 2% B27 solution, human
epidermal growth factor (EGF; 20 ng/ml) and human basic fibroblast
growth factor (b-FGF; 20 ng/ml). After 2 weeks, the resultant
spheroids that contained >20 cells were counted under an
inverted phase contrast microscope (Olympus IX51). Subsequently,
the spheres were dissociated and re-introduced into another 6-well
ultralow attachment plate to investigate their ability of
self-renewal through a secondary sphere formation assay.
Flow cytometry analysis
Isolated cells were cultured in DMEM and dissociated
with trypsin-EDTA into single-cell suspensions as aforementioned.
Subsequent to being washed with PBS twice, the cells were harvested
and incubated with cluster of differentiation (CD)117-phycoerthyrin
(dilution, 1:20; catalog no., 313203) and Stro-1 (dilution, 1:20;
catalog no., 340103) mouse monoclonal antibodies [in PBS (pH 7.2)
containing 0.09% sodium azide and 0.2% (w/v) bovine serum albumin
(Sigma-Aldrich); BioLegend, Inc., San Diego, CA, USA] on ice for 30
min in the dark. Afterwards, the cells were washed twice with PBS
and kept at 4°C in the dark prior to flow cytometry (BD
FACSCalibur; BD Biosciences Franklin Lakes, NJ, USA) and analyzed
using BD Cell Quest Pro software version 5.1 (BD Biosciences). The
expression of the cell markers was determined by comparison with an
isotype control.
Statistical analysis
Statistical analyses were performed using the SPSS
13.0 statistical software package (SPSS Inc., Chicago, IL, USA).
Data are expressed as the mean ± standard deviation of three
independent experiments. The Student's t-test was used to
compare the means of the 2 groups. When >3 means were compared,
one-way analysis of variance, followed by multiple comparisons
among the means, was used. P<0.05 was considered to indicate a
statistically significant difference.
Results
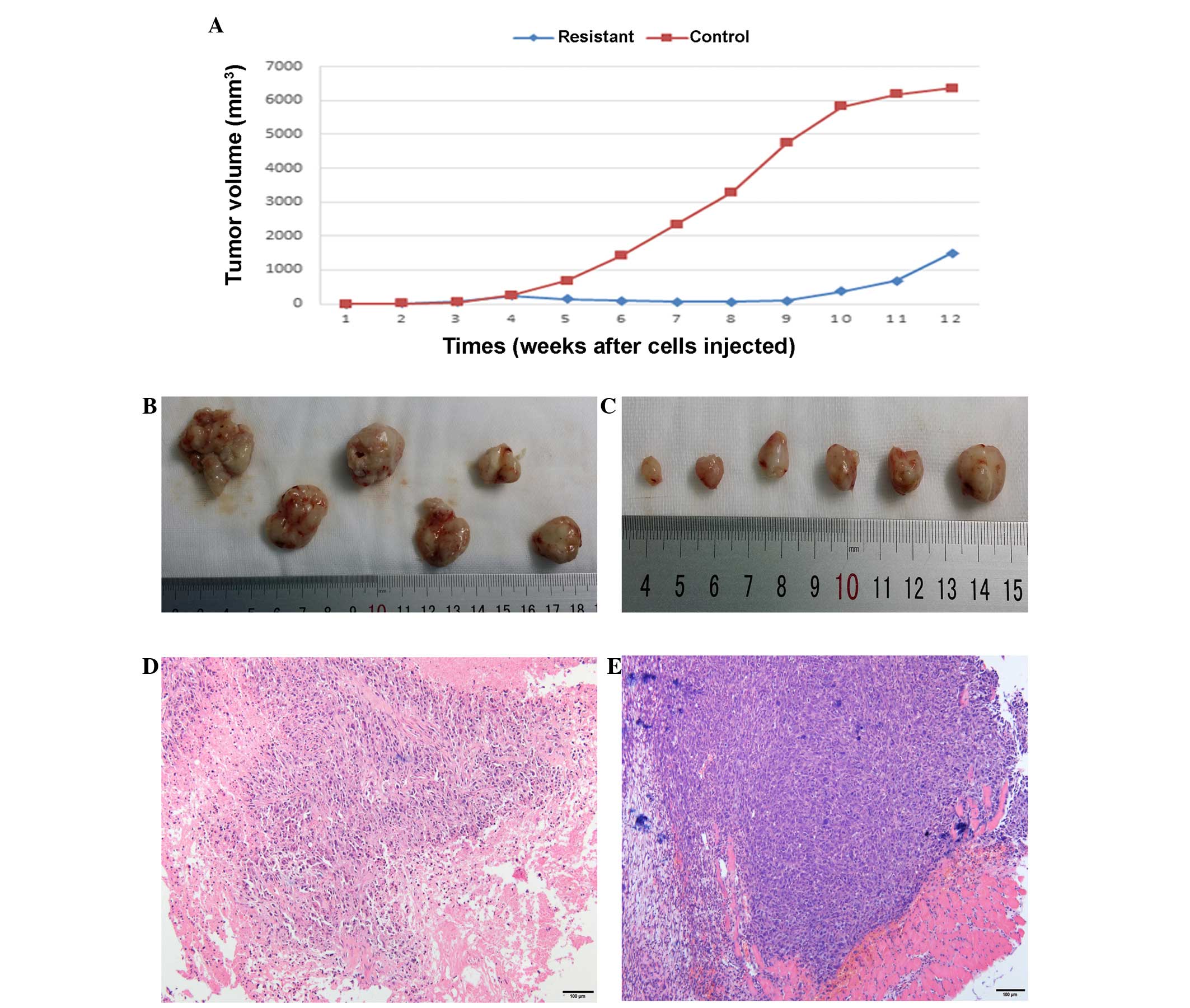
Metronomic CDDP therapy significantly
delays the growth of tumors in vivo
Female NOD/SCID mice were subcutaneously injected
with human osteosarcoma 143B-TK cells and only 12 mice (12/18)
finally formed a tumor xenograft. Half of these mice were treated
with metronomically scheduled CDDP (5 mg/kg every week applied
intraperitoneally), and drug treatment was started at the third
week after tumor cell implantation, just when tumors reached an
average volume of 50 mm3. Regularly CDDP treatment
resulted in a significant decrease in the size of the tumor and
delayed the tumor growth (Fig. 1).
The tumor volume of the treated mice was slowly reduced to 60
mm3 at the eighth week after tumor cell implantation,
whereas the tumors in the control group exhibited rapid growth,
with at a growth rate of 40%. At around the ninth week after tumor
cell implantation, the tumor volume began to increase in the CDDP
treatment group despite ongoing treatment, with a tumor growth rate
of 85%, while tumors in the control group were constant in volume
at ~6,000 mm3, with a sharp slowing of tumor growth
(Fig. 1A). Treatment was stopped at
the seventh week and the two groups of mice were sacrificed in the
twelfth week; at this endpoint, tumors were collected and subjected
to histological and macroscopic analyses. Meanwhile, the tumor
cells were isolated from the tumor tissue for characterization and
cell experiments.
Effect of CDDP therapy on tumor
macroscopic appearance
Tumor tissues from the two groups were
macroscopically assessed at the therapy endpoint. The tumor tissue
in the CDDP treatment group appeared pale and dingy, was harder and
had less blood supply on the surface of the tissues compared with
the untreated control tumors (Fig. 1B and
C). For further evaluation of the changes induced by in
vivo passaging and CDDP treatment, HE staining analyses were
performed in the treatment and control groups; sections were
stained with HE and analyzed by transmitted light microscopy. The
tissue structure in the original 143B-TK xenografts (established
from the control group) was compact and homogeneous, with a large
number of tumor cells that were arranged irregularly and no hybrid
connective tissue between the tumor cells (Fig. 1E). The resistant tumors (treatment
group) exhibited an inhomogeneous structure and the number of
osteosarcoma cells was significantly reduced, with hyperplasia of
the connective tissue (Fig. 1D). This
indicated that CDDP exhibited a strong toxic effect on the tumor
cells; resistance tissues underwent a series of corresponding
changes in structure compared with the control tissues in the mouse
model.
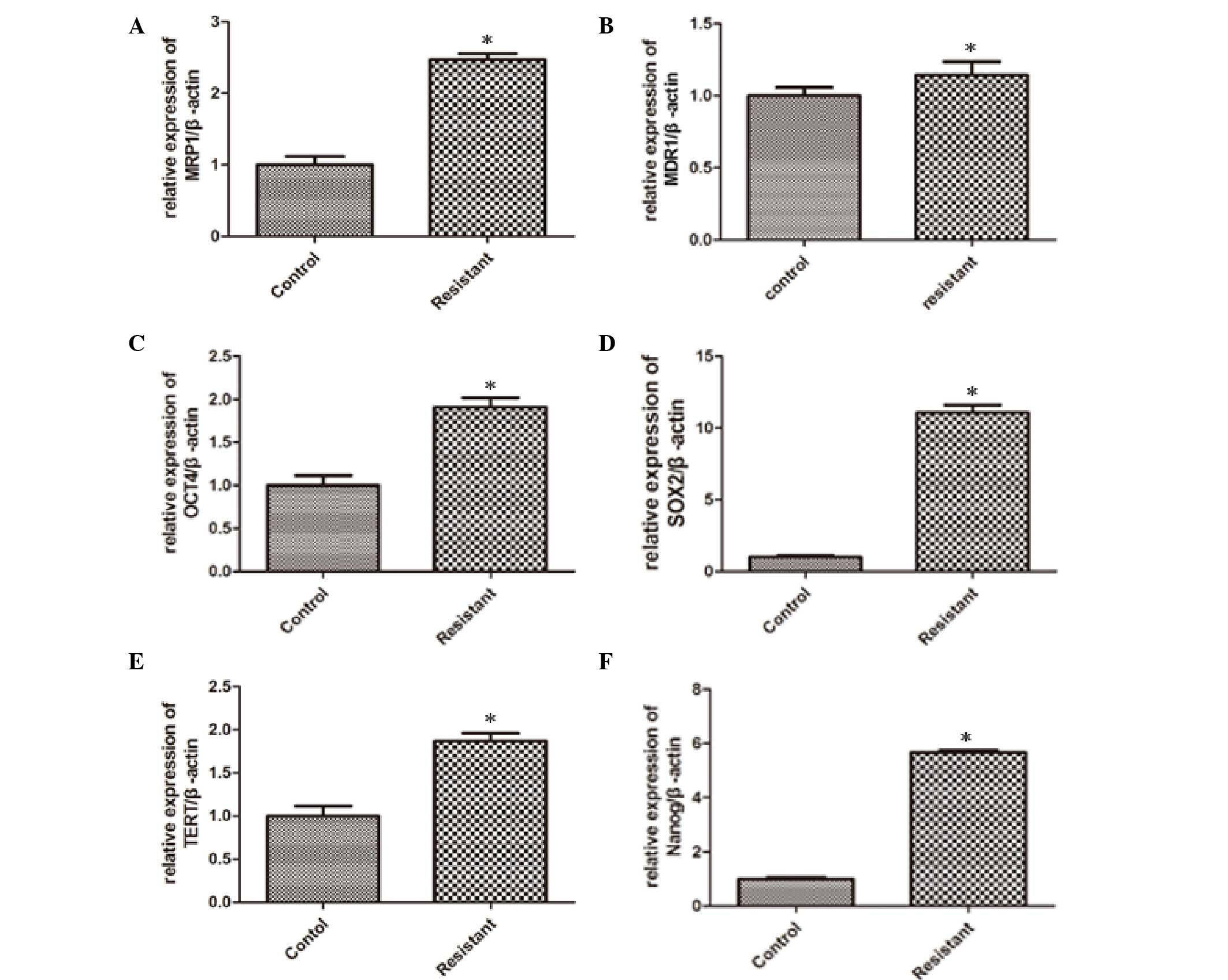
Expression profiles of drug resistance
and stemness genes in response to CDDP therapy in vivo
For characterization of stemness as a possible cause
of tumor cell drug resistance and recurrence, the drug resistance
genes, MDR1 and MRP1, and the well-established stemness genes,
OCT4, SOX2, TERT and Nanog, were detected by qPCR after samples
were extracted from the tumor tissue. The analysis results revealed
that the expression levels of MRP1 (Fig.
2A), MDR1 (Fig. 2B), OCT4
(Fig. 2C), SOX2 (Fig. 2D), TERT (Fig. 2E) and Nanog (Fig. 2F) were significantly elevated from
0.15- to 10-fold in resistant tumors tissues in comparison to
tumors established from untreated mice, which indicated that the
proportion of stem cells was increased in osteosarcoma tissues from
CDDP-treated mice. In the CDDP group, MRP-1 expression showed a
significant increase (P=0.026), MDR-1 expression increased slightly
(P=0.041) and the expressions of the stem cell-related genes OCT-4,
SOX-2, TERT and Nanog were elevated significantly (P=0.021, 0.029,
0.043 and 0.031, respectively) compared with the control group. The
particular mechanism of this phenomenon remains unclear, and we
speculated that there were a number of CSCs in the osteosarcoma
tissues that had a stronger tolerance to CDDP. In other words, CDDP
could select and enrich the CSCs in osteosarcoma tissues in
vivo.
Cells resistant to CDDP show higher
expression of CSC markers CD117 and Stro-1
To confirm the percentage of putative osteosarcoma
stem cells, cells cultured from the tumor tissues of the two groups
were tested by flow cytometry for the expression of surface markers
CD117 and Stro-1, known markers of CSCs. The percentage of
CD117+Stro-1+ cells in the resistant group
was 0.5% (Fig. 3A), where <0.1% of
CD117+Stro-1+ cells were detected in the
control group (Fig. 3B). Despite the
percentage of double-positive markers being low in the resistant
group, a distinct difference was found in the CDDP untreated cells,
which provided direct evidence for CDDP-resistant osteosarcoma
cells that possess CSC properties.
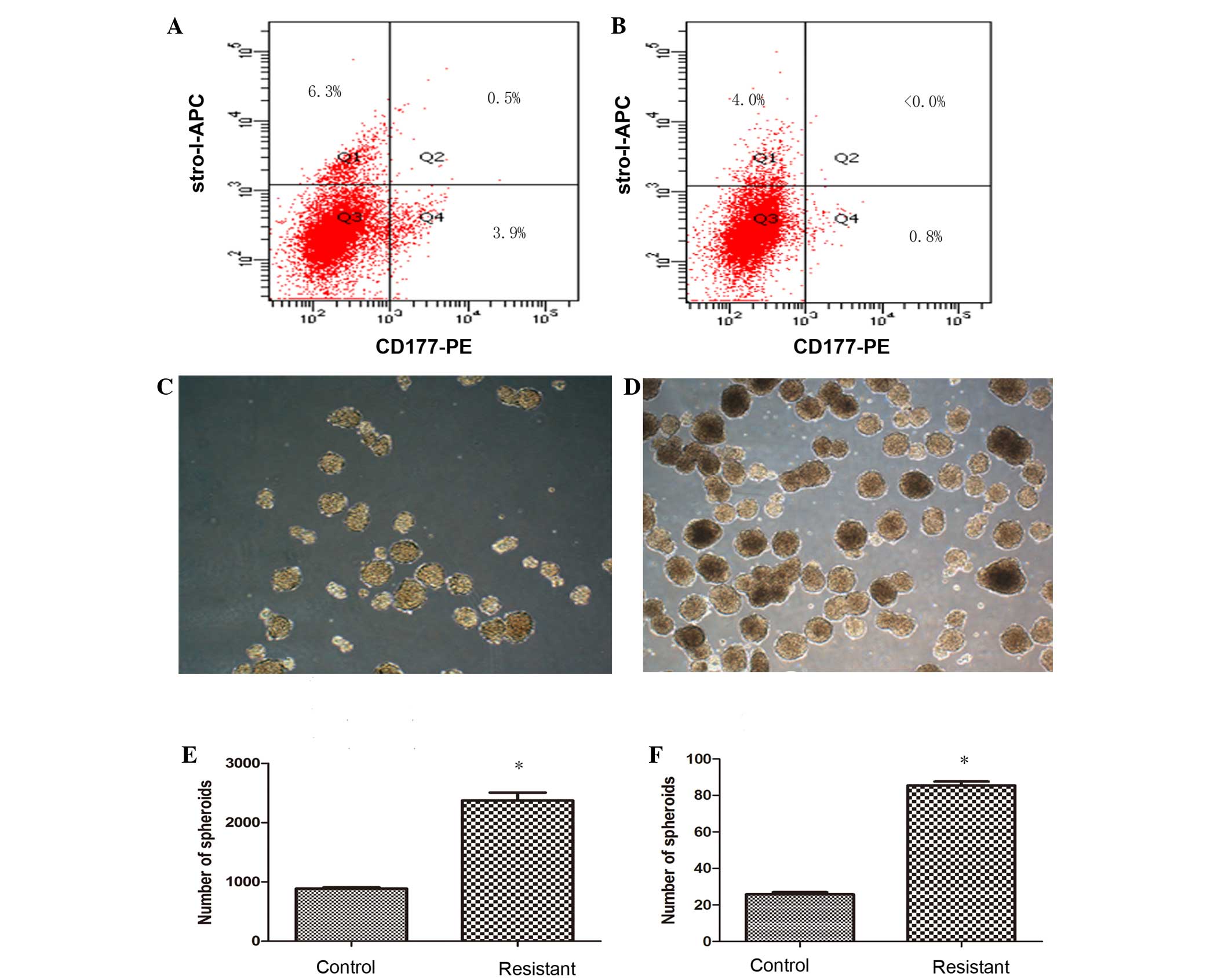 | Figure 3.Cisplatin (CDDP)-resistant cells
exhibit increased cancer stem cell (CSC) properties. The surface
markers of CSCs were tested by flow cytometry in cells cultured
from the of tumor tissue, and the percentage of
CD117+Stro-1+ cells in the (A) resistant
group (0.5%) showed higher expression of CD117 and Stro-1 compare
with the cells of the (B) control group (<0.0%), while single
positive CD117 or Stro-1, and double-negative
CD117−Stro1− cells in the resistant group
were exhibited at 3.9, 6.3 and 89.3%, respectively, compared with
0.8, 4.0 and 95.2% in the control group Differences in size and
number appeared in the forming spherical colonies in the (C)
control and (D) resistant groups. The ability to generate spheroids
in a serum-starved sphere formation assay was increased in the (E)
CDDP-resistant cells, and this result was also similar in (F) the
formation of secondary spheres. Results represent the mean ±
standard deviation of three repeated experiments. *P<0.05 vs.
control. APC, allophycocyanin; PE, phycoerythrin. |
CDDP-resistant cells exhibit an
increased ability to form sarcospheres
For characterization of cell dependency on essential
matrix signaling, the ability to generate spherical clones and the
ability for self-renewal were evaluated in a serum-starved sphere
formation assay. Within several days after being cultured in
RPMI-1640 medium without serum, the cells started to form spherical
colonies, and significant differences between control and resistant
group cells could be observed after 2 weeks (Fig. 3C and D), as the spheroids in the
control group appeared smaller in size and fewer in number, and the
spheroids in resistant group were more compact and homogeneous. The
spheroids eventually formed at a frequency of ~1/42 (2,373.33±133.2
colonies/1×105 cells) for the resistant group, and 1/113
(887±14.45 colonies/1×105 cells) for the control group
(Fig. 3E). To investigate cell
self-renewal potential in vitro, cultured spheres were
dissociated into single cells and allowed to grow in serum-starved
medium supplemented with B27, human EGF and human b-FGF repeatedly.
Sarcospheres from each group showed expansion in the suspension
culture, which led to cell differentiation and self-renewal through
the formation of secondary spheres. The frequency of sphere
formation was similar to that of the primary sphere results, with
~1/117 (85.5±2.15 colonies/1×104 cells) for the
resistant group compared with 1/388 (25.75±1.35
colonies/1×104 cells) for the control group (Fig. 3F). The number of generate spheroids
and secondary spheres in CDDP-resistant cells was increased
significantly (P=0.039 and 0.045, respectively) compared with the
control group.
Discussion
Osteosarcoma is a highly malignant form of bone
cancer with the characteristic of osteoid produced in tumor tissues
(1,2,16). Despite
numerous improvements in the therapeutic strategies for
osteosarcoma, problems remain during the course of treatment, such
as therapeutic resistance, cancer recurrence and metastatic
disease, and usually tumor drug resistance is closely associated
with recurrence. Currently, there are a large number of studies
regarding the development and use of xenografts and allograft
models of human and osteosarcoma cells injected into
immunocompromised mice. These models have yielded tumors
histologically resembling human cancer and have produced cell lines
to complement human osteosarcoma studies (17). The advantages of a model using the
injection of a subcutaneous cell suspension are its high incidence
rate and good reproducibility (18,19). A
multitude of studies of chemotherapy resistance are based on cells,
and osteosarcoma models are rarely used for evaluating the
histological and molecular characteristics of drug resistance. In
the present study, CDDP was used to create an animal model of drug
resistance, and it was found that CDDP can significantly reduce the
tumor volume and result in a significant delay in tumor growth.
Moreover, the products of the multi-drug resistance genes MDR1 and
MRP1, which act as an energy-dependent elimination pump that can
convey cytotoxic drugs out of tumor cells (20), have previously been perceived as
prognostic factors in osteosarcoma (21). In the present study, RT-qPCR revealed
that the MDR1 and MRP1 genes were overexpressed in the CDDP
treatment group compared with in the untreated tissues, indicating
that MDR1 and MRP1 expression levels increase under
chemotherapeutic treatment, which is similar to the results
reported by other studies regarding the treatment of osteosarcoma
(22–24). This result also certified that the
establishment of an animal resistance model induced by CDDP via the
aforementioned method is feasible and effective.
Recent studies have suggested that CSCs are involved
in the mechanisms of drug resistance (25–27),
providing a novel theoretical basis to solve the problem of
chemotherapy resistance during the eradication of osteosarcoma. At
present, identifying and isolating CSCs mainly relies on surface
markers or side population sorting. However, isolating and
purifying CSCs is occasionally difficult due to their continuous
differentiation and scarcity. Previous studies have shown that OCT4
expression is vital in the maintenance of stem cell pluripotency
(28), and that SOX2 and Nanog
expression contributes to plasticity, self-renewal and stemness
(29). The present results revealed
that OCT4, SOX2 and Nanog expression was increased in the CDDP
treatment group compared with the untreated control group. This
indicated that the CDDP-resistant cells in tumor tissues possessed
CSC characteristics that increased the expression of the stem
cell-related genes and the expression of the surface markers, CD117
and Stro-1, as determined by an increased percentage of
CD117+Stro-1+ tumor cells isolated from the
drug treatment tissues. Although the level of
CD117+Stro-1+ cells in the tumor tissues, as
detected by immunohistochemistry analysis, was unsatisfactory
[albeit ascended from <0.1% (Fig.
3B) to 5% (Fig. 3A)], we
speculate on the possibility that CD117 and Stro-1 are expressed at
low levels in osteosarcoma and that CSCs are rare in the tumor.
Similar results were also found in the sphere formation assay,
which showed that CDDP-resistant cells exhibited an increased
ability to form spheres and secondary spheres compared with the
untreated cells, meaning that the cells possessed the potential for
self-renewal and that the ability for tumorigenicity were
strengthened by CDDP though this method. It is known that CD117
(c-kit) is the receptor for stem cell factor and a
proto-oncoprotein, and that Stro-1 is a cell surface marker for
mesenchymal stem cells (30),
nonetheless, it remains unknown whether CD117 or Stro-1 plays an
active role in the properties of osteosarcoma-initiating cells.
Adhikara et al (13)
documented that CD117 and Stro-1 were preferentially expressed in
drug-resistant cells and spheres. High
CD117+Stro-1+ expression and the results of a
sphere formation assay in CDDP-treated cells in the present study
confirmed that the resistance tumor possessed a strong ability for
oncogenicity and self-renewal; in other words, CDDP induced and
enriched the CSCs in the xenograft tissues.
In conclusion, in the present study, acquired in
vivo chemoresistance against CDDP treatment was studied in a
human osteosarcoma 143B-TK xenograft mouse model. The results
showed that CDDP could induce the stem cell-related genes and the
expression of the surface markers, enhance the characterization of
stem cells and enrich the CSCs in the xenograft tissues. This
suggests an association between chemoresistance and the properties
of CSCs, indicating that osteosarcoma cells probably acquired the
ability to evade the chemotherapy drug, CDDP, through upregulating
the CSC-related gene. In addition, this result also indicates that
osteosarcoma recurrence is probably associated with CSCs. Although
there was a lack of data on the differentiation and metastasis of
the tumor, which requires investigation in further research,
CDDP-resistant osteosarcoma cells showed CSC properties in the
mouse model. Further study on how the specific mechanisms of
resistance may impact cell plasticity and differentiation remain to
be investigated in future.
Acknowledgements
The study was supported by a grant from the Wuhan
science and Technology Council of Hubei Province (no.
2014062801011264).
References
|
1
|
Bielack SS, Kempf-Bielack B, Delling G,
Exner GU, Flege S, Helmke K, Kotz R, Salzer-Kuntschik M, Werner M,
Winkelmann W, et al: Prognostic factors in high-grade osteosarcoma
of the extremities or trunk: An analysis of 1,702 patients treated
on neoadjuvant cooperative osteosarcoma study group protocols. J
Clin Oncol. 20:776–790. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cormier JN and Pollock RE: Soft tissue
sarcomas. CA Cancer J Clin. 54:94–109. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Luetke A, Meyers PA, Lewis I and Juergens
H: Osteosarcoma treatment-where do we stand? A state of the art
review. Cancer Treat Rev. 40:523–532. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Janeway KA and Grier HE: Sequelae of
osteosarcoma medical therapy: A review of rare acute toxicities and
late effects. Lancet Oncol. 11:670–678. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Picci P, Ferrari S, Bacci G and
Gherlinzoni F: Treatment recommendations for osteosarcoma and adult
soft tissue sarcomas. Drugs. 47:82–92. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Meyers PA: Muramyl tripeptide
(mifamurtide) for the treatment of osteosarcoma. Expert Rev
Anticancer Ther. 9:1035–1049. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Gorlick R, Anderson P, Andrulis I, Arndt
C, Beardsley GP, Bernstein M, Bridge J, Cheung NK, Dome JS, Ebb D,
et al: Biology of childhood osteogenic sarcoma and potential
targets for therapeutic development: Meeting summary. Clin Cancer
Res. 9:5442–5453. 2003.PubMed/NCBI
|
|
8
|
Bonnet D and Dick JE: Human acute myeloid
leukemia is organized as a hierarchy that originates from a
primitive hematopoietic cell. Nat Med. 3:730–737. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park CY, Tseng D and Weissman IL: Cancer
stem cell-directed therapies: Recent data from the laboratory and
clinic. Mol Ther. 17:219–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Dean M, Fojo T and Bates S: Tumour stem
cells and drug resistance. Nat Rev Cancer. 5:275–284. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Korkaya H and Wicha MS: Selective
targeting of cancer stem cells: A new concept in cancer
therapeutics. BioDrugs. 21:299–310. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ma S, Lee TK, Zheng BJ, Chan KW and Guan
XY: CD133+ HCC cancer stem cells confer chemoresistance
by preferential expression of the Akt/PKB survival pathway.
Oncogene. 27:1749–1758. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Adhikari AS, Agarwal N, Wood BM, Porretta
C, Ruiz B, Pochampally RR and Iwakuma T: CD117 and Stro-1 identify
osteosarcoma tumor-initiating cells associated with metastasis and
drug resistance. Cancer Res. 70:4602–4612. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Luu HH, Kang Q, Park JK, Si W, Luo Q,
Jiang W, Yin H, Montag AG, Simon MA, Peabody TD, et al: An
orthotopic model of human osteosarcoma growth and spontaneous
pulmonary metastasis. Clin Exp Metastasis. 22:319–329. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Savage S.A..Mirabello L: Using
epidemiology and genomics to understand osteosarcoma etiology.
Sarcoma, 2011. 2011.548151
|
|
17
|
Ek ET, Dass CR and Choong PF: Commonly
used mouse models of osteosarcoma. Crit Rev Oncol Hematol. 60:1–8.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chen X, Li Y, Xiong K, Aizicovici S, Xie
Y, Zhu Q, et al: Cancer gene therapy by direct tumor injections of
a nonviral T7 vector encoding a thymidine kinase gene. Human Gene
Ther. 1998.9(5): 729–36. View Article : Google Scholar
|
|
19
|
Crnalic S, Hakansson I, Boquist L,
Lofvenberg R and Brostrom LA: A novel spontaneous metastasis model
of human osteosarcoma developed using orthotopic transplantation of
intact tumor tissue into tibia of nude mice. Clin Exp Metastasis.
1997.15(2): 164–72. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vergote J, Moretti JL, de Vries EG and
Garnier-Suillerot A: Comparison of the kinetics of active efflux of
99mTc-MIBI in cells with P-glycoprotein-mediated and
multidrug-resistance protein-associated multidrug-resistance
phenotypes. Eur J Biochem. 252:140–146. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Suto R, Abe Y, Nakamura M, Ohnishi Y,
Yoshimura M, Lee YH, Imanishi T, Yamazaki H, Kijima H, Tokunaga T,
et al: Multidrug resistance mediated by overexpression of
P-glycoprotein in human osteosarcoma in vivo. Int J Oncol.
12:287–291. 1998.PubMed/NCBI
|
|
22
|
Chan HS, Grogan TM, Haddad G, DeBoer G and
Ling V: P-glycoprotein expression: Critical determinant in the
response to osteosarcoma chemotherapy. J Natl Cancer Inst.
89:1706–1715. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Burak Z, Moretti JL, Ersoy O, Sanli U,
Kantar M, Tamgac F and Basdemir G: 99mTc-MIBI imaging as a
predictor of therapy response in osteosarcoma compared with
multidrug resistance-associated protein and P-glycoprotein
expression. J Nucl Med. 44:1394–1401. 2003.PubMed/NCBI
|
|
24
|
Dutour A, Leclers D, Monteil J, Paraf F,
Charissoux JL, Rousseau R and Rigaud M: Non-invasive imaging
correlates with histological and molecular characteristics of an
osteosarcoma model: Application for early detection and follow-up
of MDR phenotype. Anticancer Res. 27:4171–4178. 2007.PubMed/NCBI
|
|
25
|
Beachy PA, Karhadkar SS and Berman DM:
Tissue repair and stem cell renewal in carcinogenesis. Nature.
432:324–331. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Ravandi F, Burnett AK, Agura ED and
Kantarjian HM: Progress in the treatment of acute myeloid leukemia.
Cancer. 110:1900–1910. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Woodward WA and Sulman EP: Cancer stem
cells: Markers or biomarkers? Cancer Metastasis Rev. 27:459–470.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Hay DC, Sutherland L, Clark J and Burdon
T: Oct-4 knockdown induces similar patterns of endoderm and
trophoblast differentiation markers in human and mouse embryonic
stem cells. Stem Cells. 22:225–235. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Boyer LA, Lee TI, Cole MF, Johnstone SE,
Levine SS, Zucker JP, Guenther MG, Kumar RM, Murray HL, Jenner RG,
et al: Core transcriptional regulatory circuitry in human embryonic
stem cells. Cell. 122:947–956. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Wei H, Zhao MQ, Dong W, Yang Y and Li JS:
Expression of c-kit protein and mutational status of the c-kit gene
in osteosarcoma and their clinicopathological significance. J Int
Med Res. 36:1008–1014. 2008. View Article : Google Scholar : PubMed/NCBI
|