Introduction
Colorectal cancer is the third most common
malignancy and the fourth most lethal type of cancer in the
world (1). Recurrence and metastases
frequently occur in affected patients during the course of the
disease, and chemotherapy is the major management strategy.
Irinotecan is considered to be an essential component of first- and
second-line treatments for metastatic or recurrent colorectal
cancer, as 5-FU and leucovorin (FOLFIRI) ± molecular target drug,
although other regimens, such as FOLFOX and CapeOX ± molecular
target drug, are also considered good options (2). Current guidelines report that the
selection of a specific chemotherapy regimen at present is solely
based on the response to previous therapies or treatments in trials
(2). Therefore, treatment results for
patients with colorectal cancer have been far from satisfactory,
with a response rate of ~50% for irinotecan-based combinations
(2).
Certain predictive markers for the response of
colorectal cancer to chemotherapy have been identified, including
microsatellite instability, thymidylate synthase, dihydropyrimidine
dehydrogenase for 5-fluorouracil (5-FU), excision repair
cross-complementing protein 1 for oxaliplatin and mutations in
Kirsten rat sarcoma viral oncogene homolog, and B-Raf
proto-oncogene, serine/threonine kinase for panitumumab and
cetuximab (3,4). In addition, numerous studies have been
designed to indicate novel predictors of cellular response to
irinotecan in vitro, including topoisomerase-I and -II,
membrane transporter proteins, carboxylesterase,
glucuronosyltransferases and proteasome (5,6). However,
these predictors have not been proved to be effective in clinical
studies (7).
Multidrug resistance is a serious problem, and is
considered one of the major causes of chemotherapy failure.
Multidrug resistance is often associated with the overexpression of
adenosine triphosphate-binding cassette (ABC) transporter proteins,
including ABCB1, ABCC1, ABCC2 and ABCG2 (8). Expression of ABCG2 has been observed in
the epithelial cells of the intestine, colon, liver canaliculi,
renal tubules and placenta, where it eliminates anticancer drugs
and ingested toxins (9). The
association between overexpression of ABCG2, response to
chemotherapy and prognosis has been reported for leukemia (10) and various solid tumors, including
breast cancer (11), oral squamous
cell carcinoma (12), esophageal
cancer (13) and lung cancer
(14). Irinotecan and its active
metabolite, SN-38, are included among the transport substrates of
ABCG2 (15). ABCG2 is abundant in the
normal colon, and its expression is decreased in colorectal cancer.
The downregulation of ABCG2 expression may have a role in
tumorigenesis by enabling the accumulation of genotoxins and the
overproduction of nitric oxide (16).
The overexpression of ABCG2 protein in colon cancer cell lines has
been associated with increased levels of resistance to SN-38 in
vitro (17).
The aim of the present study was to assess whether
the immunohistochemical expression of ABCG2 may be a potential
predictor of the response to irinotecan-based treatment of patients
with colorectal cancer. The results of the present study indicated
that the increased expression of ABCG2 was associated with
resistance to SN-38 in colorectal cancer and a negative response to
irinotecan-based chemotherapy.
Materials and methods
Patients
The Ethics Committee of Shiga University of Medical
Science (Otsu, Japan) approved this study. Signed informed consent
was obtained prior to surgery from each of the 189 patients that
underwent a colorectal resection at the Department of Surgery,
Shiga University of Medical Science Hospital, between May 2004 and
May 2012. All patients were chemotherapy-naive. The resected tumors
were histologically confirmed as adenocarcinoma, and the
chemosensitivities of the tumors to SN-38 and 5-FU were measured
using the collagen gel droplet embedded culture drug sensitivity
test (CD-DST). Among the 189 patients enrolled in the study, 17
underwent irinotecan-based chemotherapy for ≥2 months. The cancer
statuses of all 17 patients were recurrent and unresectable. The
patients received no surgical or radiation interventions during the
period of chemotherapy. A total of 13 patients received the FOLFIRI
regimen; Irinotecan at a dose of 120 mg/m2 as a 2 h intravenous
(i.v.) infusion on day 1; Leucovorin was given at a dose of 400
mg/m2 as a 2-h i.v. infusion, followed by 5-FU 400 mg/m2 as an i.v.
bolus, and then, 2,400 mg/m2 as a 22-h continuous i.v. infusion, on
days 1 and 2, repeated every 2 weeks (18). A further 4 patients received the IRIS
regimen; Irinotecan at a dose of 150 mg/m2 as a 1.5-h i.v. infusion
on day 1, followed by S-1 100 mg/day for 14 days perorally,
repeated every 3 weeks. The Bevacizumab dose was 7.5 mg/kg and was
administered i.v. every 2 weeks, initially over 90 min. The best
responses across all time points, which were evaluated 2 months
following the initial administration of the irinotecan-based
regimen, were used for classification according to the Response
Evaluation Criteria In Solid Tumors guideline, version 1.1
(19), and assigned complete response
(CR), partial response (PR), stable disease (SD) or progressive
disease (PD).
CD-DST
CD-DST was used to evaluate the sensitivity of
cancer tissue to SN-38. Briefly, 5 mm cube of colorectal cancer
specimens obtained by surgery were minced by surgical knife, and
digested with collagenase, and the dispersed cancer cells were
incubated in a collagen gel coated flask. Only the viable cells
adhering to the collagen gel layer were collected and added to the
reconstructed type I collagen solution (Cellmatrix Type CD; Kurabo
Industries, Ltd., Osaka, Japan). SN-38 (0.03 µg/ml; LKT
Laboratories, Inc., St Paul, MN, USA) and 5-FU (1 µg/ml; Kyowa
Hakko Kirin Co., Ltd., Tokyo, Japan) were added to each well. The
plate was then incubated for 24 h at 37°C. Subsequent to the
removal of the medium containing the anticancer drug, each well was
incubated with PCM-2 medium (Kurabo Industries, Ltd.) for 7 days.
Neutral red was then added to stain the colonies in the collagen
gel droplets, which were next fixed with formalin. The in
vitro chemosensitivity effect was expressed as a ratio of the
total colony volume of the treated group (T) to that of the control
group (C) (T/C ratio) (20). A T/C
ratio of ≤60% was regarded as sensitive.
Immunohistochemical staining
All specimens were archived as formalin-fixed and
paraffin-embedded tissues. Sections (3-µm thick) were cut and
immunostained using the Ventana Discovery XT staining system
(Ventana Medical Systems, Inc., Tucson, AZ, USA). Normal and
cancerous tissues from the same patient were mounted onto the same
slide to ensure identical conditions. Slides were then incubated
with a primary anti-ABCG2 antibody (clone BXP-21 mouse anti-human
monoclonal antibody; dilution, 1:500; catalog no. MAB4146; Merck
Millipore, Darmstadt, Germany) at 37°C for 32 min, followed by
incubation with Discovery™ Universal Secondary Antibody, a
biotinylated immunoglobulin (lg) cocktail of goat anti-mouse lgG,
goat anti-mouse lgM, goat anti-rabbit lgG and protein block (ready
for use; catalog no. 760-4205; Ventana Medical Systems, Inc.). The
immunological reaction was visualized with 3,3′-diaminobenzidine
chromogen (DAB Map Kit; Ventana Medical Systems, Inc.), followed by
counterstaining with hematoxylin. Sections were dehydrated and
cover slips were mounted.
Stained slides were examined independently by two
researchers from the Departments of Surgery and Pathology of Shiga
University of Medical Science (Otsu, Japan). The staining intensity
of positive cell membranes was classified as negative (no
staining), 0; weak, 1; moderate, 2; or intense (as strong as in
normal colonocytes), 3. The proportion of total positive cancer
cells with membranous positivity was scored as follows: <5%, 0;
5–25%, 1; 26–50%, 2; 51–75%, 3; or >75%, 4.
ABCG2 expression was determined by multiplication of
the values for intensity and proportion, and was classified as low-
or high-expression for scores of 0–8 or 9–12, respectively
(21). For heterogeneous signals, the
results were based on the most intensely stained group of cells.
The negative control was processed by replacing the primary
antibody against ABCG2 with phosphate-buffered saline.
Statistical analysis
SPSS software, version 17.0 (SPSS, Inc., Chicago,
IL, USA) and Stata software version 10.1 (StataCorp LP, College
Station, TX, USA) were used for statistical analyses. In order to
compare the differences across stratified groups, the t-test
or Wilcoxon test was performed for continuous variables, and the χ2
test or Fisher's exact test was performed for categorical
variables, as appropriate. The univariate and multivariate logistic
regression or Pearson's χ2 statistic were used to analyze the
effect of clinicopathological factors and ABCG2 expression on SN-38
sensitivity, or the discrepancy between these variables,
respectively; variables with P<0.25 were selected as candidates
for inclusion in the multivariate model (22). A 95% confidence interval (CI) for
prevalance ratio (PR) were calculated with standard errors
estimated by the Wald test. Overall survival (OS) was defined as
the time between the date of initial administration of the
irinotecan-based regimen and mortality or final follow-up.
Progression-free survival (PFS) was defined as the time between the
date of initial administration of the irinotecan-based regimen and
recurrence or final follow-up. The survival curves were calculated
according to the Kaplan-Meier method, and differences between
curves were assessed using the generalized Wilcoxon test. P<0.05
was considered to indicate a statistically significant
difference.
Results
Expression of ABCG2 in colorectal
cancer tissues
In the normal colon mucosa samples, the expression
of ABCG2 was increased along the brush border membranes of normal
colonocytes (Fig. 1A and B). High
expression in the tumor was defined as non-intense expression or
low proportion of ABCG2 positivity (Fig.
1C and D) and the tumor was defined as intense expression in
50% or more of the cancer cells, and low expression (Fig. 1E and F) The patients were classified
into low-expression (76 patients; 40%) or high-expression (113
patients; 60%) groups, of which the median immunohistochemistry
scores were 4.21±0.25 and 10.55±0.15, respectively. A
three-dimensional distribution of the proportion of positive cancer
cells and signal intensities are shown in Fig. 1G.
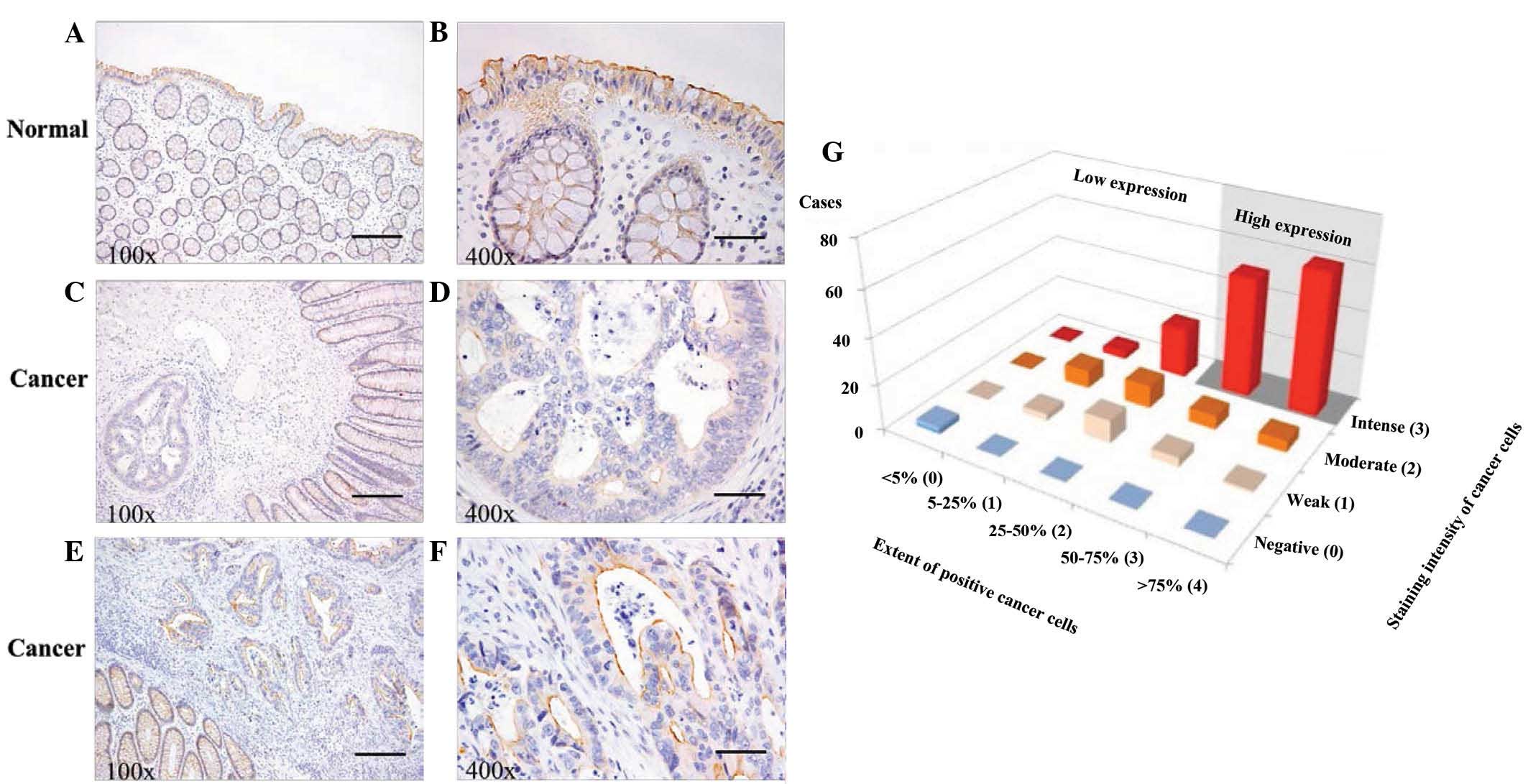 | Figure 1.ABCG2 expression was evaluated by
immunohistochemistry in normal colon and colorectal cancer tissues.
(A and B) ABCG2 protein signal (brown) was strongest along the
brush border membranes of normal colonocytes (patient with colon
cancer). (C and D) ABCG2 expression in cancerous gland (left side)
is weaker than that of normal ground surrounding the tumor (right
side). (Low expression group, colon cancer). (E and F) Intense
ABCG2 expression in the cancerous glands (right upper side) is
similar to normal colonocytes around the tumor (left lower side)
(High expression group, rectal cancer). Scale bar, 200 µm and
magnification, ×100 in panels A, C and E. Scale bar, 50 µm and
magnification, ×400 in panels B, D and F. (G) ABCG2 expression was
quantified by multiplication of the scores for intensity and
proportion, and patients were classified into low- or
high-expression groups, according to scores of 0–8 or 9–12,
respectively. ABCG2, adenosine triphosphate-binding cassette
sub-family G (WHITE) member 2 (Junior blood group). |
Association between SN-38 response,
ABCG2 expression and clinicopathological factors in patients with
colorectal cancer
The clinicopathological factors of the 189 patients
are presented in Table I. Briefly,
119 patients possessed colon cancer and 70 possessed rectal cancer.
The ages of the patients ranged between 33 and 88 years (median, 65
years). Moderate differentiation (72%) and stage 3 or 4 (54%)
tumors were identified in the majority of patients. ABCG2
expression in rectal cancer was significantly increased, compared
with expression in colon cancer (P=0.019). The median SN-38 T/C
ratio was significantly increased in the high-expression group,
compared with the low-expression group (P<0.001). No significant
association between the expression levels of ABCG2 and any other
clinicopathological factors studied was observed, including age,
gender, histological type, tumor invasion, lymph node metastasis,
distant metastasis, lymphatic invasion, venous invasion and stage
of the tumor.
 | Table I.Clinicopathological factors and
protein expression levels of adenosine triphosphate-binding
cassette sub-family G (WHITE) member 2 (Junior blood group) in 189
patients with colorectal cancer. |
Table I.
Clinicopathological factors and
protein expression levels of adenosine triphosphate-binding
cassette sub-family G (WHITE) member 2 (Junior blood group) in 189
patients with colorectal cancer.
| Characteristics | All patients, n
(%) | Low-expression group,
n (%) | High-expression
group, n (%) | P-value |
|---|
| Total | 189 (100) | 76 (40) | 113 (60) |
|
| Age, years |
|
|
|
|
|
Median | 65 | 64 | 66 | 0.271a |
|
Range | 33–88 | 33–88 | 43–86 |
|
| Gender |
|
|
|
|
|
Female | 77
(41) | 29 (38) | 48 (42) | 0.659b |
| Male | 112 (59) | 47 (62) | 65 (58) |
|
| Tumor location |
|
|
|
|
|
Colon | 119 (63) | 56 (74) | 63 (56) | 0.019b |
|
Rectum | 70
(37) | 20 (26) | 50 (44) |
|
| Differentiation |
|
|
|
|
| Well | 39
(21) | 15 (20) | 24 (21) | 0.733b |
|
Moderate | 136 (72) | 54 (71) | 82 (73) |
|
| Poor | 14 (7) | 7 (9) | 7 (6) |
|
| Stage grouping |
|
|
|
|
| Duke's
A | 87
(46) | 35 (46) | 52 (46) | 1.000b |
| Duke's
B, C | 102 (54) | 41 (54) | 61 (54) |
|
| Tumor depth |
|
|
|
|
|
pT1,2 | 35
(19) | 9
(12) | 26 (23) | 0.081b |
|
pT3,4 | 154 (81) | 67 (88) | 87 (77) |
|
| Lymph node
metastasis |
|
|
|
|
| N0 | 94
(50) | 38 (50) | 56 (50) | 1.000b |
|
N1,2 | 95
(50) | 38 (50) | 57 (50) |
|
| Distant
metastasis |
|
|
|
|
| M0 | 156 (83) | 60 (79) | 96 (85) | 0.383b |
| M1 | 33
(17) | 16 (21) | 17 (15) |
|
| Lymphatic
invasion |
|
|
|
|
|
Ly0,1 | 133 (70) | 50 (66) | 83 (73) | 0.333b |
|
Ly2,3 | 56
(30) | 26 (34) | 30 (27) |
|
| Venous
invasion |
|
|
|
|
|
V0,1 | 126 (67) | 49 (64) | 77 (68) | 0.714b |
|
V2,3 | 63
(33) | 27 (36) | 36 (32) |
|
| SN-38 effect,
T/C |
|
|
|
|
|
Median | 66 | 55 | 73 |
<0.001a |
| Range | 21–100 | 21–100 | 32–100 |
|
|
Sensitive, T/C <60 | 77
(41) | 56 (74) | 21 (19) |
<0.001b |
|
Resistant, T/C ≥60 | 112 (59) | 20 (26) | 92 (81) |
|
Associations between sensitivity to SN-38 and
clinicopathological factors were analyzed as categorical variables
in a univariate analysis (Table II).
Patients with increased expression of ABCG2 were significantly more
resistant to SN-38, compared with patients with low expression of
ABCG2 (P<0.001). The sensitivity of increased expression of
ABCG2 to predict the low response to SN-38 by CD-DST was 82%, and
the specificity was 73%. Other factors, including age, gender,
differentiation, stage grouping, tumor depth, lymph node
metastasis, distant metastasis, lymphatic invasion and venous
invasion, did not associate with sensitivity to SN-38. Two selected
variables, namely location of the tumor and expression of ABCG2,
were analyzed in a multivariate regression model. Patients with
increased expression of ABCG2 were the strongest indicators of
resistance to SN-38 [prevalence ratio (PR), 11.77; 95% confidence
interval (CI), 5.83–23.76; P<0.001).
 | Table II.Multivariate analysis of
clinicopathological factors and SN-38 sensitivity in 189 patients
with colorectal cancer. |
Table II.
Multivariate analysis of
clinicopathological factors and SN-38 sensitivity in 189 patients
with colorectal cancer.
|
|
|
|
| Multivariate
analysis |
|---|
|
|
|
|
|
|
|---|
| Factors | Sensitive, n
(%) | Resistant, n
(%) | Univariate analysis
P-valuea | Adjusted PR (95%
CI) |
P-valueb |
|---|
| Total | 77 (41) | 112 (59) |
|
|
|
| Age, years |
|
|
|
|
|
|
<65 | 40 (52) | 59 (53) | 1.000 |
|
|
|
≥65 | 37 (48) | 53 (47) |
|
|
|
| Gender |
|
|
|
|
|
|
Female | 35 (45) | 42 (38) | 0.346 |
|
|
|
Male | 42 (55) | 70 (63) |
|
|
|
| Tumor location |
|
|
|
|
|
|
Colon | 55 (71) | 64 (57) | 0.065 | Ref | 0.446 |
|
Rectum | 22 (29) | 48 (43) |
| 1.33
(0.63–2.77) |
|
|
Differentiation |
|
|
|
|
|
|
Well | 16 (21) | 23 (21) | 0.756 |
|
|
|
Moderate | 54 (70) | 82 (73) |
|
|
|
|
Poor | 7 (9) | 7 (6) |
|
|
|
| Stage grouping |
|
|
|
|
|
| Duke's
A | 36 (47) | 51 (46) | 0.987 |
|
|
| Duke's
B,C | 41 (53) | 61 (54) |
|
|
|
| Tumor depth |
|
|
|
|
|
|
pT1,2 | 11 (14) | 24 (21) | 0.093 |
|
|
|
pT3,4 | 66 (86) | 88 (79) |
|
|
|
| Lymph node
metastasis |
|
|
|
|
|
| N0 | 40 (52) | 54 (48) | 0.722 |
|
|
|
N1,2 | 37 (48) | 58 (52) |
|
|
|
| Distant
metastasis |
|
|
|
|
|
| M0 | 64 (83) | 92 (82) | 1.000 |
|
|
| M1 | 13 (17) | 20 (18) |
|
|
|
| Lymphatic
invasion |
|
|
|
|
|
|
Ly0,1 | 53 (69) | 80 (71) | 0.824 |
|
|
|
Ly2,3 | 24 (31) | 32 (29) |
|
|
|
| Venous
invasion |
|
|
|
|
|
|
V0,1 | 53 (69) | 73 (65) | 0.714 |
|
|
|
V2,3 | 24 (31) | 39 (35) |
|
|
|
| ABCG2
expression |
|
|
|
|
|
|
Low | 56 (73) | 20 (18) | <0.001 | Ref | <0.001 |
|
High | 21 (27) | 92 (82) |
| 11.77
(5.83–23.76) |
|
ABCG2 expression and clinical response
to irinotecan-based chemotherapy
Eligibility criteria for tumor response to
irinotecan-based regimens were identified in 17 patients, of which,
5 (29%) were classified as PR and 12 (71%) as non-responders,
including 11 SD and 1 PD. The patient and tumor characteristics for
responders and non-responders are shown in Table III. There were no significant
differences between the two groups, with the exception of the
expression levels of ABCG2 and the effect of SN-38 by CD-DST.
Increased expression of ABCG2 was observed in 11 of 12
non-responders, whereas 4 of 5 responders exhibited decreased
expression of ABCG2. The sensitivity of increased ABCG2 expression
to predict the resistance to irinotecan-based chemotherapy was 92%,
and the specificity was 80%. The results of CD-DST indicated
sensitivity to SN-38 in 4 of 5 responders (80%), compared with 2 of
12 non-responders (17%); the difference between which was
significant (P=0.028).
 | Table III.Clinicopathological characteristics
and response to irinotecan-based chemotherapy of 17 patients with
colorectal cancer. |
Table III.
Clinicopathological characteristics
and response to irinotecan-based chemotherapy of 17 patients with
colorectal cancer.
|
Characteristics | All patients, n
(%) | Responders (n=5), n
(%) | Non-responders
(n=12), n (%) | P-value |
|---|
| Age, years |
|
|
|
|
|
Median | 60 | 67 | 58 | 0.246a |
|
Range | 33–77 | 56–76 | 33–77 |
|
| Gender |
|
|
|
|
|
Female | 6
(35) | 1
(20) | 5
(42) | 0.600b |
|
Male | 11 (65) | 4
(80) | 7
(58) |
|
| Tumor location |
|
|
|
|
|
Colon | 8
(47) | 3
(60) | 5
(42) | 0.620b |
|
Rectum | 9
(53) | 2
(40) | 7
(58) |
|
|
Differentiation |
|
|
|
|
|
Well | 0
(0) | 0 (0) | 0 (0) | 0.515b |
|
Moderate | 15 (88) | 4
(80) | 11 (92) |
|
|
Poor | 2
(12) | 1
(20) | 1 (8) |
|
| Stage grouping |
|
|
|
|
| Duke's
A | 1
(6) | 0 (0) | 1 (8) | 1.000b |
| Duke's
B | 4
(24) | 1 (20) | 3 (25) |
|
| Duke's
C | 12
(70) | 4 (80) | 8 (57) |
|
| No. of metastatic
sites |
|
|
|
|
| 1 | 7
(41) | 2
(40) | 5
(42) | 1.000b |
| 2 | 8
(47) | 3
(60) | 5
(42) |
|
| 3 | 2
(12) | 0 (0) | 2
(17) |
|
| Line of
chemotherapy |
|
|
|
|
|
1st | 4
(23) | 1
(20) | 3
(25) | 0.744b |
|
2nd | 6
(35) | 2
(40) | 4
(33) |
|
|
3rd | 4
(23) | 2
(40) | 2
(17) |
|
|
Other | 3
(18) | 0 (0) | 3
(25) |
|
| Chemotherapy
regimen |
|
|
|
|
|
FOLFIRI | 13 (76) | 4
(80) | 9
(75) | 1.000b |
|
IRIS | 4
(23) | 1
(20) | 3
(25) |
|
| Bevacizumab |
|
|
|
|
|
Without | 8
(47) | 3
(60) | 5
(42) | 0.620b |
|
With | 9
(53) | 2
(40) | 7
(58) |
|
| ABCG2
expression |
|
|
|
|
|
Low | 5
(29) | 4
(80) | 1 (8) | 0.010b |
|
High | 12 (71) | 1
(20) | 11 (92) |
|
| SN-38 effect,
T/C |
|
|
|
|
|
Median | 69 | 59 | 73 | 0.113a |
|
Range | 31–100 | 31–87 | 32–100 |
|
|
Sensitive, T/C <60 | 6
(35) | 4
(80) | 2
(17) | 0.028b |
|
Resistant, T/C ≥60 | 11 (65) | 1
(20) | 10 (83) |
|
| 5-FU effect,
T/C |
|
|
|
|
|
Median | 71 | 64 | 74 | 0.342a |
|
Range | 32–100 | 32–81 | 42–100 |
|
|
Sensitive, T/C <60 | 3
(18) | 1
(20) | 2
(17) | 1.000b |
|
Resistant, T/C ≥60 | 14 (82) | 4
(80) | 10 (83) |
|
The association between treatment characteristics
and PFS is shown in Table IV. The
median PFS of the responder group was significantly longer,
compared with the non-responder group (372 vs. 104 days; P=0.013).
The median PFS was significantly longer in the patients with
low-expression of ABCG2 than those with high-expression of ABCG2
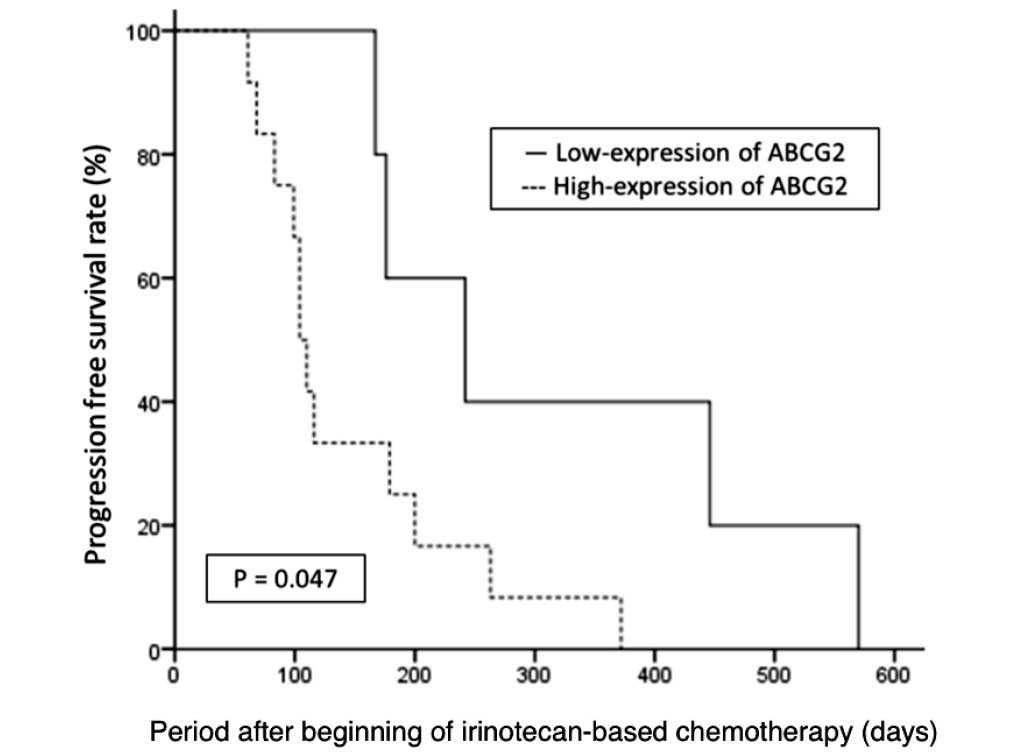
(242 vs. 104 days; P=0.047, Fig. 2).
The median PFS of the 17 patients sensitive to SN-38 tended to be
longer than the median PFS of resistant patients; however, this
result was not statistically significant (242 vs. 110 days;
P=0.061). Other factors, including the line or content of the
chemotherapy and sensitivity to 5-FU, did not affect the median
PFS. The median OS of the 17 patients was 554 days. The median OS
of the patients with increased and decreased ABCG2 expression were
449 days and 554 days, respectively, which showed no significant
difference (P=0.505).
 | Table IV.Treatment characteristics and
association with PFS following irinotecan-based chemotherapy in 17
patients with colorectal cancer. |
Table IV.
Treatment characteristics and
association with PFS following irinotecan-based chemotherapy in 17
patients with colorectal cancer.
|
| PFS following
irinotecan-based chemotherapy |
|---|
|
|
|
|---|
|
Characteristics | Patients, n
(%) | Median PFS,
days | Generalized
Wilcoxon |
|---|
| Line of
chemotherapy |
|
|
|
|
1st | 4(23) | 176 | 0.267 |
|
2nd | 6(35) | 104 |
|
|
3rd | 4
(23) | 242 |
|
|
Other | 3
(18) | 104 |
|
| Chemotherapy
regimen |
|
|
|
|
FOLFIRI | 13 (76) | 167 | 0.773 |
|
IRIS | 4
(23) | 104 |
|
| Bevacizumab |
|
|
|
|
Without | 8
(47) | 104 | 0.631 |
|
With | 9
(53) | 176 |
|
| ABCG2
expression |
|
|
|
|
Low | 5
(29) | 242 | 0.047 |
|
High | 12 (71) | 104 |
|
| SN-38 effect,
T/C |
|
|
|
|
Sensitive, T/C <60 | 6
(35) | 242 | 0.061 |
|
Resistant, T/C ≥60 | 11 (65) | 110 |
|
| 5-FU effect,
T/C |
|
|
|
|
Sensitive, T/C <60 | 3
(18) | 200 | 0.381 |
|
Resistant, T/C ≥60 | 14 (82) | 116 |
|
| Response |
|
|
|
|
Responder | 5
(29) | 372 | 0.013 |
|
Non-responder | 12 (71) | 104 |
|
Discussion
The resistance of cancer cells with ABCG2
overexpression to SN-38 is most likely due to the efflux
transportation of SN-38 and SN-38 glucuronide out of the cells
(23). ABCG2 has been the subject of
numerous studies on leukemia and several solid tumors; however,
there are numerous conflicting reports regarding the association
between ABCG2 expression and the outcome of chemotherapy or
survival (24,25). The present study investigated whether
ABCG2 expression is associated with SN-38 resistance in human
colorectal cancer, and demonstrated that the increased expression
of ABCG2 may predict resistance to SN-38 treatment, with a
sensitivity of 82%, and the lack of response to irinotecan-based
chemotherapy, with a sensitivity of 92%. Patients with primary
tumors that demonstrated increased ABCG2 expression were at an
increased (11.77-fold) risk of a negative response to
irinotecan-based chemotherapy (P<0.001).
Deitrich et al (26) showed that the downregulation of ABCG2
led to the accumulation of carcinogens, including
2-amino-1-methyl-6-phenylimidazo [4,5-b] pyridine, in the
colorectal adenomas of mice and humans, and suggested that this may
promote the adenoma-carcinoma sequence. Gupta et al
(16) reported that the expression of
ABCG2 mRNA and protein was abundant in the normal colon and
decreased in colon cancer tissue. However, the possibility of
alterations in ABCG2 expression during the progression of a
carcinoma remained to be clarified. The present study demonstrated
that ~60% of patients that possessed tumors belonged to the
high-ABCG2-expression group.
A few studies have investigated the expression of
ABCG2 mRNA in human colorectal cancer, but to the best of our
knowledge, none have reported associations with the effects of
chemotherapy, including irinotecan (17,27).
Associations between the increased expression of ABCG2 with lymph
node metastasis and the clinical stage of breast cancer (28), and with poor differentiation in glioma
cells (29), have been reported.
However, the present study did not indicate any significant
associations with clinicopathological factors, with the exception
of the precise location of the primary tumor. The expression level
of ABCG2 in rectal cancer was significantly increased compared with
in colon cancer (P=0.019). In addition, the number of patients
whose tumors were resistant to SN-38 was increased for rectal
cancer compared with colon cancer, although this was not
statistically significant (P=0.065). No evidence was identified
that explained this result; therefore, future investigation is
required in order to understand this phenomenon.
Topoisomerase I mutations (30), ABCG2 overexpression (17) in cancer cell lines and gene expression
profiles (31) in human colorectal
cancer tissue have been suggested to be involved in the development
of resistance to irinotecan. The expression of topoisomerase I has
been investigated as a predictive factor for patient response to
irinotecan in vitro (32), but
no effects on response, time to progression or OS have been
identified in clinical studies (33).
In the present study, despite having only 17 eligible patients, the
increased expression of ABCG2 was significantly associated with
resistance to irinotecan-based chemotherapy (P=0.01) and a shorter
PFS (P=0.047). These data suggest that ABCG2 may be useful as a
biomarker to predict chemoresistance to SN-38 of primary colorectal
cancer tissues. Patients with recurrent or metastatic colorectal
cancer that possess primary tumors with increased ABCG2 expression
may, therefore, avoid irinotecan-based chemotherapy, enabling
treatment with other regimens instead. Similarly, patients with
decreased ABCG2 expression may possibly receive more benefits from
irinotecan-based regimens compared patients with increased
expression. The median OS of the patients with low-ABCG2-expression
(n=5) was not significantly different compared with those with
high-expression (n=12) (P=0.505). This may be due to the majority
of patients that were judged as PD receiving additional lines of
chemotherapy, following the irinotecan-based chemotherapy.
ABCG2 immunohistochemical staining of primary
colorectal cancer tissues is easy to perform, and may provide
information regarding the chemosensitivity of patients to
irinotecan. Prospective studies with increased numbers of patients
are required to confirm this hypothesis. An inhibitor of ABCG2 may
possibly be used as an additional agent with irinotecan-based
regimens in patients defined as irinotecan-resistant due to the
increased expression of ABCG2. In conclusion, the increased
expression of ABCG2 may be involved in SN-38 resistance in
colorectal cancer and may be a useful predictive biomarker for use
in patients that are under consideration for treatment with
irinotecan-based chemotherapy.
Acknowledgements
The authors would like to thank Ms. Ikuko Arikawa,
Ms. Ai Kenmochi and Ms. Miho Yamamoto from the Department of
Surgery, Shiga University of Medical Science (Otsu, Japan), for
their expert technical assistance in immunohistochemical staining
of ABCG2.
References
|
1
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Benson AB, Bekaii-Saab T, Chan E, Chen YJ,
Choti MA, Cooper HS, Engstrom PF, Enzinger PC, Fakih MG, Fenton MJ,
et al: National Comprehensive Cancer Network: Localized colon
cancer, version 3.2013: Featured updates to the NCCN Guidelines. J
Natl Compr Canc Netw. 11:519–528. 2013.PubMed/NCBI
|
|
3
|
Pritchard CC and Grady WM: Colorectal
cancer molecular biology moves into clinical practice. Gut.
60:116–129. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Winder T and Lenz HJ: Molecular predictive
and prognostic markers in colon cancer. Cancer Treat Rev.
36:550–556. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Vanhoefer U, Harstrick A, Achterrath W,
Cao S, Seeber S and Rustum YM: Irinotecan in the treatment of
colorectal cancer: Clinical overview. J Clin Oncol. 19:1501–1518.
2001.PubMed/NCBI
|
|
6
|
Xu Y and Villalona-Calero MA: Irinotecan:
Mechanisms of tumor resistance and novel strategies for modulating
its activity. Ann Oncol. 13:1841–1851. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Allen WL, Coyle VM and Johnston PG:
Predicting the outcome of chemotherapy for colorectal cancer. Curr
Opin Pharmacol. 6:332–336. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Leslie EM, Deeley RG and Cole SP:
Multidrug resistance proteins: Role of P-glycoprotein, MRP1, MRP2,
and BCRP (ABCG2) in tissue defense. Toxicol Appl Pharmacol.
204:216–237. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Han B and Zhang JT: Multidrug resistance
in cancer chemotherapy and xenobiotic protection mediated by the
half ATP-binding cassette transporter ABCG2. Curr Med Chem
Anticancer Agents. 4:31–42. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Benderra Z, Faussat AM, Sayada L, Perrot
JY, Tang R, Chaoui D, Morjani H, Marzac C, Marie JP and Legrand O:
MRP3, BCRP, and P-glycoprotein activities are prognostic factors in
adult acute myeloid leukemia. Clin Cancer Res. 11:7764–7772. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Burger H, Foekens JA, Look MP, Meijer-van
Gelder ME, Klijn JG, Wiemer EA, Stoter G and Nooter K: RNA
expression of breast cancer resistance protein, lung
resistance-related protein, multidrug resistance-associated
proteins 1 and 2, and multidrug resistance gene 1 in breast cancer:
Correlation with chemotherapeutic response. Clin Cancer Res.
9:827–836. 2003.PubMed/NCBI
|
|
12
|
Friedrich RE, Punke C and Reymann A:
Expression of multi-drug resistance genes (mdr1, mrp1, bcrp) in
primary oral squamous cell carcinoma. In Vivo. 18:133–147.
2004.PubMed/NCBI
|
|
13
|
Tsunoda S, Okumura T, Ito T, Kondo K,
Ortiz C, Tanaka E, Watanabe G, Itami A, Sakai Y and Shimada Y:
ABCG2 expression is an independent unfavorable prognostic factor in
esophageal squamous cell carcinoma. Oncology. 71:251–258. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kim YH, Ishii G, Goto K, Ota S, Kubota K,
Murata Y, Mishima M, Saijo N, Nishiwaki Y and Ochiai A: Expression
of breast cancer resistance protein is associated with a poor
clinical outcome in patients with small-cell lung cancer. Lung
Cancer. 65:105–111. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Robey RW, To KK, Polgar O, Dohse M, Fetsch
P, Dean M and Bates SE: ABCG2: A perspective. Adv Drug Deliv Rev.
61:3–13. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Gupta N, Martin PM, Miyauchi S, Ananth S,
Herdman AV, Martindale RG, Podolsky R and Ganapathy V:
Down-regulation of BCRP/ABCG2 in colorectal and cervical cancer.
Biochem Biophys Res Commun. 343:571–577. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Candeil L, Gourdier I, Peyron D, Vezzio N,
Copois V, Bibeau F, Orsetti B, Scheffer GL, Ychou M, Khan QA, et
al: ABCG2 overexpression in colon cancer cells resistant to SN-38
and in irinotecan-treated metastases. Int J Cancer. 109:848–854.
2004. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Muro K, Boku N, Shimada Y, Tsuji A,
Sameshima S, Baba H, Satoh T, Denda T, Ina K, Nishina T, et al:
Irinotecan plus S-1 (IRIS) versus fluorouracil and folinic acid
plus irinotecan (FOLFIRI) as second-line chemotherapy for
metastatic colorectal cancer: A randomised phase 2/3
non-inferiority study (FIRIS study). Lancet Oncol. 11:853–860.
2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet embedded culture
and image analysis for clinical usefulness. Recent Results Cancer
Res. 161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Sinicrope FA, Ruan SB, Cleary KR, Stephens
LC, Lee JJ and Levin B: Bcl-2 and p53 oncoprotein expression during
colorectal tumorigenesis. Cancer Res. 55:237–241. 1995.PubMed/NCBI
|
|
22
|
Hosmer DW and Lemeshow S: Applied Logistic
Regression. Shewhart WA and Wilks SS: 2nd. John Wiley & Sons,
Inc.; New York: pp. 31–46. 2000
|
|
23
|
Kawabata S, Oka M, Shiozawa K, Tsukamoto
K, Nakatomi K, Soda H, Fukuda M, Ikegami Y, Sugahara K, Yamada Y,
et al: Breast cancer resistance protein directly confers SN-38
resistance of lung cancer cells. Biochem Biophys Res Commun.
280:1216–1223. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Robey RW, Ierano C, Zhan Z and Bates SE:
The challenge of exploiting ABCG2 in the clinic. Curr Pharm
Biotechnol. 12:595–608. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Mo W and Zhang JT: Human ABCG2: Structure,
function and its role in multidrug resistance. Int J Biochem Mol
Biol. 3:1–27. 2012.PubMed/NCBI
|
|
26
|
Dietrich CG, Vehr AK, Martin IV, Gassler
N, Rath T, Roeb E, Schmitt J, Trautwein C and Geier A:
Downregulation of breast cancer resistance protein in colon
adenomas reduces cellular xenobiotic resistance and leads to
accumulation of a food-derived carcinogen. Int J Cancer.
129:546–552. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Glasgow SC, Yu J, Carvalho LP, Shannon WD,
Fleshman JW and McLeod HL: Unfavourable expression of pharmacologic
markers in mucinous colorectal cancer. Br J Cancer. 92:259–264.
2005.PubMed/NCBI
|
|
28
|
Xiang L, Su P, Xia S, Liu Z, Wang Y, Gao P
and Zhou G: ABCG2 is associated with HER-2 expression, lymph node
metastasis and clinical stage in breast invasive ductal carcinoma.
Diagn Pathol. 6:902011. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Jin Y, Bin ZQ, Qiang H, Liang C, Hua C,
Jun D, Dong WA and Qing L: ABCG2 is related with the grade of
glioma and resistance to mitoxantone, a chemotherapeutic drug for
glioma. J Cancer Res Clin Oncol. 135:1369–1376. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gongora C, VezzioVie N, Tuduri S, Denis V,
Causse A, Auzanneau C, CollodBeroud G, Coquelle A, Pasero P,
Pourquier P, et al: New Topoisomerase I mutations are associated
with resistance to camptothecin. Mol Cancer. 10:642011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
DelRio M, Molina F, BascoulMollevi C,
Copois V, Bibeau F, Chalbos P, Bareil C, Kramar A, Salvetat N,
Fraslon C, et al: Gene expression signature in advanced colorectal
cancer patients select drugs and response for the use of
leucovorin, fluorouracil, and irinotecan. J Clin Oncol. 25:773–780.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Lansiaux A, BrasGoncalves RA, Rosty C,
LaurentPuig P, Poupon MF and Bailly C: Topoisomerase I-DNA covalent
complexes in human colorectal cancer xenografts with different p53
and microsatellite instability status: Relation with their
sensitivity to CTP-11. Anticancer Res. 21:471–476. 2001.PubMed/NCBI
|
|
33
|
Paradiso A, Xu J, Mangia A, Chiriatti A,
Simone G, Zito A, Montemurro S, Giuliani F, Maiello E and Colucci
G: Topoisomerase-I, thymidylate synthase primary tumour expression
and clinical efficacy of 5-FU/CPT-11 chemotherapy in advanced
colorectal cancer patients. Int J Cancer. 111:252–258. 2004.
View Article : Google Scholar : PubMed/NCBI
|