Introduction
Prostate carcinoma is common among elderly men and
has a high incidence in Europe and America, being second in terms
of cancer-associated mortality (1).
In East Asia, the morbidity of prostate cancer is relatively low
but has demonstrated a rising trend due to the aging population,
dietary structure alterations and improvements in diagnosis
(2). Prostate carcinoma is currently
the focus of much research attention, and if identified early and
treated correctly patient quality of life and treatment efficacy
will be high (3).
According to previous studies, the use of Chinese
herbs to treat tumors is achieving increasing recognition (4–6). Previous
studies have reported that Traditional Chinese Medicine (TCM) has
effects on androgen-dependent and -independent prostate carcinoma
(7–9).
At present, there are seldom resources of TCM for the treatment of
prostate carcinoma and the results of clinical and experimental
studies are indispensable for further data regarding TCM therapy
for the treatment of this disease (10).
The natural compounds extracted by TCM have been
widely used to treat a number of diseases (11). Researchers have studied structural
modification and artificial synthesis using the natural structure
as a template to investigate the structure of these compounds
(12). At present, numerous natural
compounds utilized as medicines have demonstrated multiple chemical
properties (13). These natural
medicines are a homologous series of natural products composed from
important sources of natural compounds whose structures are
combined together for modern drug development (14). For example, pentacyclic triterpene
compounds are a type of plant secondary metabolite with clinical
antitumor research and development value, and the most in-depth
studies concerning these compounds have been performed on lupane,
oleanolic acid and ursolic acid (15). Ursolic acid is pentacyclic triterpene
compound that is widespread in nature, and is present in the leaves
and fruits of Ericaceae bearberry, leaves of
Scrophulariaceae paulownia tomentosa and Oleaceae
privet (16). Ursolic acid has
extensive biological activity, including cancer resistance,
protection from liver injury, antisepsis, anti-inflammation and
antiviral activity (13,17).
It has previously been reported that only
dephosphorylated cofilin can translocate into mitochondria to
induce apoptosis, while phosphorylation inhibits mitochondrial
translocation of cofilin and, thus, apoptosis (18). Phosphatase and tensin homolog (PTEN)
is the substrate of rho-associated protein kinase 1 (ROCK1) kinase,
which is involved in regulating cell survival and cell death.
Substantial evidence indicates that the activation of Ras homolog
gene family, member A (RhoA)/ROCK1 can increase PTEN activity,
thus, inhibiting the activation of Akt. Furthermore, ROCK1 can lead
to the dephosphorylation of cofilin by activating protein
phosphatase 1/2A, inducing cofilin mitochondrial translocation and
leading to mitochondrial damage (19). In addition, ROCK1 can induce the
translocation of dynamin-related protein 1 (Drp1) into mitochondria
by regulating the phosphorylation state of Drp1, resulting in
remodelling of the morphology of mitochondria (20).
In the present study, evidence was provided that
indicates that the anticancer effect of ursolic acid may activate
apoptosis of prostate cancer cells via ROCK/PTEN-mediated
mitochondrial translocation of cofilin-1.
Materials and methods
Reagents
RPMI-1640 medium and fetal bovine serum were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
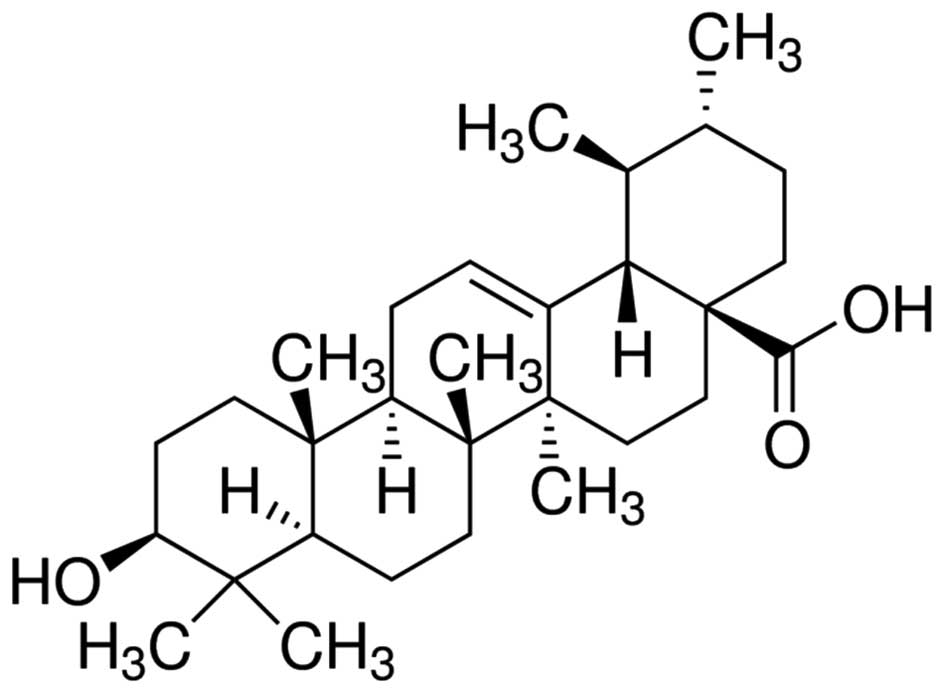
USA). Ursolic acid (with a purity of 90%) was purchased from
Sigma-Aldrich (St. Louis, MO, USA) and its chemical structure is
indicated in Fig. 1. Cell Counting
kit (CCK)-8 and bicinchoninic acid protein assays were purchased
from Sangon Biotech Co., Ltd. (Shanghai, China). Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) Apoptosis
Detection kit was obtained from BestBio (Shanghai, China).
Cell culture
DU145 human prostate cancer cells were obtained from
the Affiliated Hospital of Qingdao University (Qingdao, China), and
cultured in RPMI-1640 medium supplemented with 10% fetal bovine
serum and antibiotics (100 g/ml streptomycin and 100 U/ml
penicillin) at 37°C in an atmosphere of 5% CO2.
Analysis of cell growth
DU145 cells were seeded into 96-well plates at a
density of 1×104 cells/well and cultured with complete medium
containing various concentrations of ursolic acid (0, 10, 20, 40
and 80 µM) for 24, 48 and 72 h. Following ursolic acid treatment,
CCK-8 reagent was added to the cells and incubated for 4 h at 37°C
in an atmosphere of 5% CO2. Subsequently, the absorbance
of each well was detected at 450 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA).
Flow cytometric analysis for cell
apoptosis
DU145 cells were seeded into 6-well plates at a
density of 1–2×106 cells/well and cultured with complete medium
containing various concentrations of ursolic acid (0, 10, 20 and 40
µM) for 48 h. DU145 cells were washed with cold phosphate-buffered
saline twice and resuspended using 500 µl of binding buffer.
Following resuspension, 5 µl of Annexin V-FITC and 10 µl of PI were
added and incubated for 10 min at 4°C in the dark. Flow cytometry
was performed using a FACSCalibur flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA).
Analysis of caspase-3 and caspase-9
activity
DU145 cells were seeded into 6-well plates at a
density of 1–2×106 cells/well and cultured with complete medium
containing various concentrations of ursolic acid (0, 10, 20 and 40
µM) for 48 h. Cells were collected and the total protein
concentration was determined using the bicinchoninic acid protein
assay. Proteins were blended with 100 µl of Caspase-Glo 3 or
Caspase-Glo 9 reagent (Promega Corporation, Madison, WI, USA) and
incubated for 2 h at room temperature. Luciferase activity was
measured using a TD 20/20 luminometer (Promega Corporation).
Western blot analysis
DU145 cells were seeded into 6-well plates at a
density of 1–2×106 cells/well and cultured with complete medium
containing various concentrations of ursolic acid (0, 10, 20 and 40
µM) for 48 h. DU145 cells were prepared using a ProteoJET
cytoplasmic protein extraction kit (Fermentas; Thermo Fisher
Scientific, Inc.) or a Mitochondrial Fractionation kit (Active
Motif, Shanghai, China). The mixed liquor was collected to
determine the total protein concentration using the bicinchoninic
acid protein assay. Protein (50 µg) was loaded onto 10–12% SDS-PAGE
gels for electrophoresis, transferred onto polyvinylidene
difluoride (PVDF) membranes (0.22 mm) and blocked with
Tris-buffered saline containing 5% non-fat milk for 2 h at room
temperature. Subsequently, PVDF membranes were incubated with
primary antibodies against the following: Cytochrome c
(dilution, 1:2,000; #BBA2469; BestBio), ROCK (dilution, 1:1,000;
#BBA5547; BestBio), PTEN (dilution, 1:1,000; #BBA5274; BestBio) and
cofilin-1 (dilution, 1:2,000; #BBA2205; BestBio) overnight at 4°C.
PVDF membranes were subsequently incubated with horseradish
peroxidase-conjugated sheep anti-mouse immunoglobulin G (dilution,
1:1,000; #BB-2201-1; BestBio) at room temperature for 2 h and
visualized by enhanced chemiluminescence. Protein expression was
quantified using the ChemiDoc™ XRS system (Bio-Rad Laboratories,
Inc.).
Statistical analysis
All data are expressed as the mean ± standard
deviation. Analysis of variance was performed followed by the
Student-Newman-Keuls method for pairwise comparison. SPSS version
11.0 (SPSS, Inc., Chicago, IL, USA) was used to perform all
statistical analyses. P<0.05 was considered to indicate a
statistically significant difference.
Results
Anticancer effect of ursolic acid
treatment on cell growth in prostate cancer cells
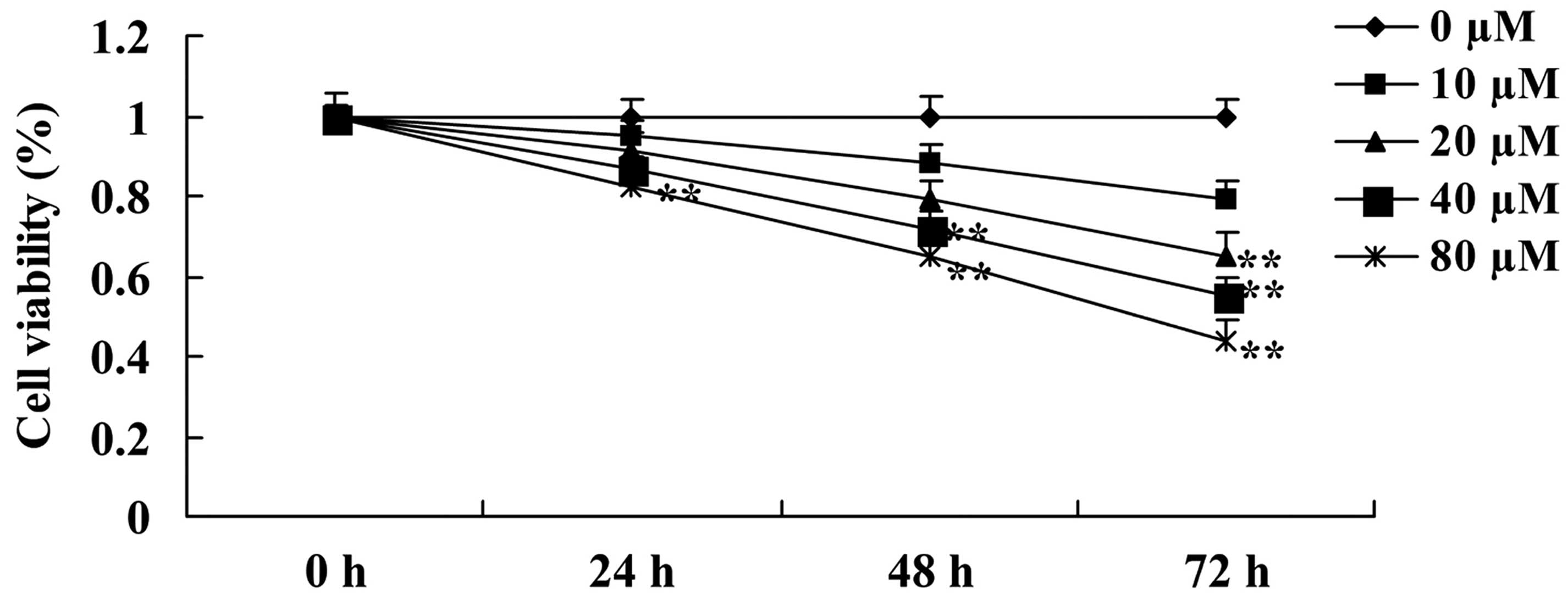
Initially, the present study investigated the
anticancer effect of ursolic acid treatment on the growth of DU145
cells. The results of the present study revealed the anticancer
effect of ursolic acid treatment was able to reduce the growth of
DU145 cells in a time- and dose-dependent manner (Fig. 2). Notably, when cells were treated
with 20 µM of ursolic acid for 72 h, 40 µM of ursolic acid for 48
and 72 h or 80 µM of ursolic acid for 24, 48 and 72 h, the growth
of DU145 cells was significantly decreased compared with treatment
with 0 µM of ursolic acid (Fig.
2).
Anticancer effect of ursolic acid
treatment on cell apoptosis in prostate cancer cells
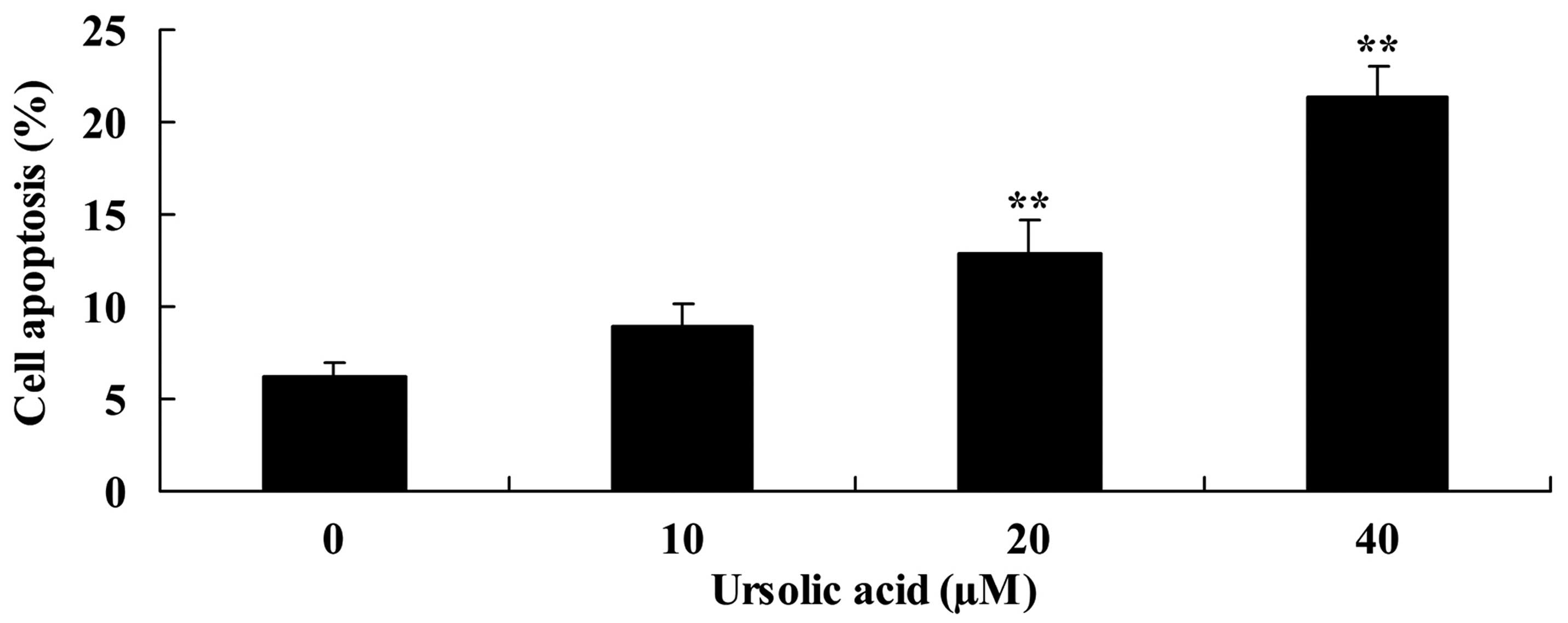
Subsequently, the present study investigated the
anticancer effect of ursolic acid treatment on apoptosis of DU145
cells. As shown in Fig. 3, treatment
with ursolic acid (20 and 40 µM) significantly increased apoptosis
of DU145 cells at 48 h in a dose-dependent manner, compared with
treatment with 0 µM of ursolic acid (Fig.
3).
Anticancer effect of ursolic acid
treatment on caspase-3 and caspase-9 activity in prostate cancer
cells
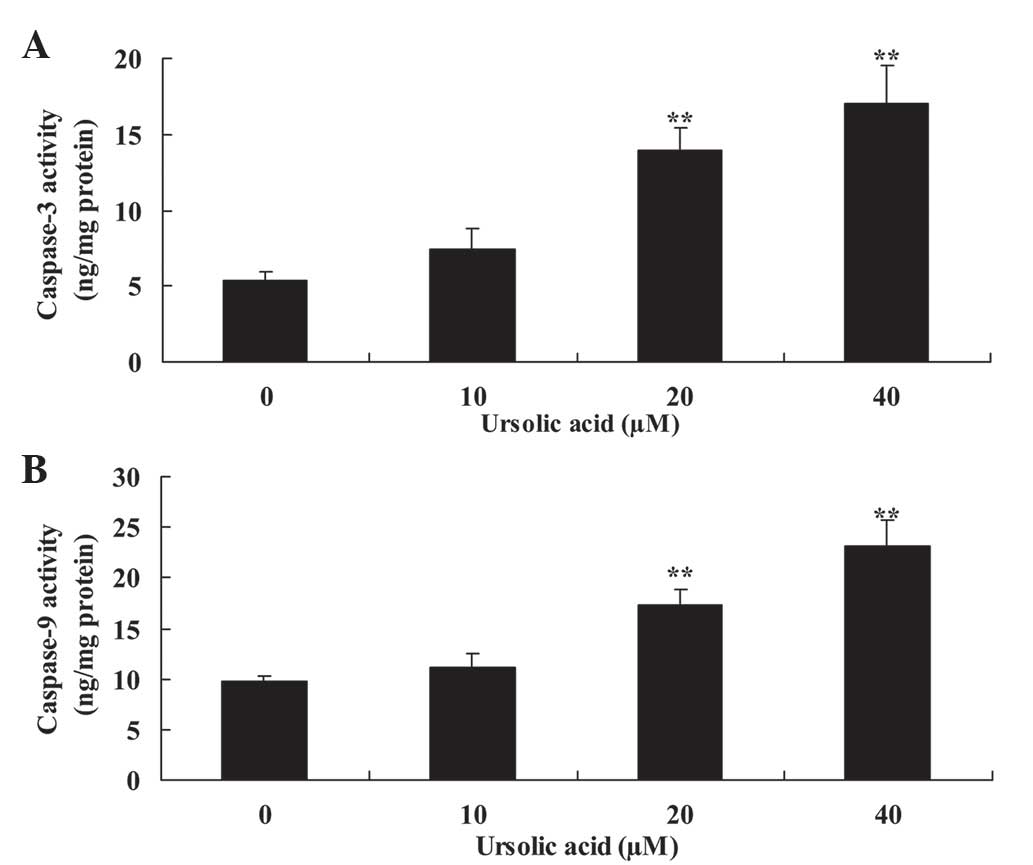
Subsequently, the present study investigated the
anticancer effect of ursolic acid treatment on caspase-3 and
caspase-9 activity of DU145 cells. Compared with treatment with 0
µM of ursolic acid, treatment with ursolic acid (20 and 40 µM)
significantly induced caspase-3 and caspase-9 activity of DU145
cells at 48 h in a dose-dependent manner (Fig. 4).
Anticancer effect of ursolic acid
treatment on cytochrome c protein expression in prostate cancer
cells
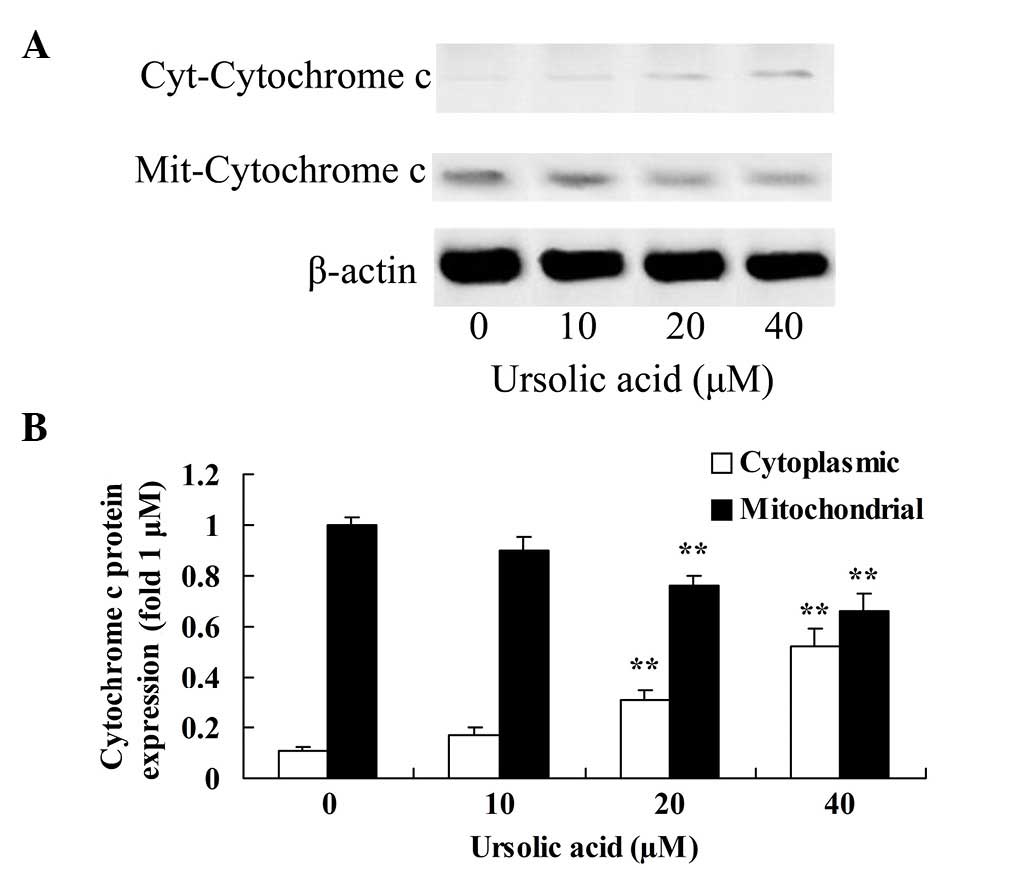
To investigate the underlying anticancer mechanism
of ursolic acid on prostate cancer cells, cytochrome c
protein expression in the cytoplasm and mitochondria was measured
using western blotting. The results of the present study indicated
that treatment with ≥20 µM ursolic acid for 48 h significantly
activated cytochrome c protein expression in the cytoplasm
of DU145 cells and suppressed cytochrome c protein
expression in the mitochondria of DU145 cells, compared with
treatment with 0 µM of ursolic acid (Fig.
5).
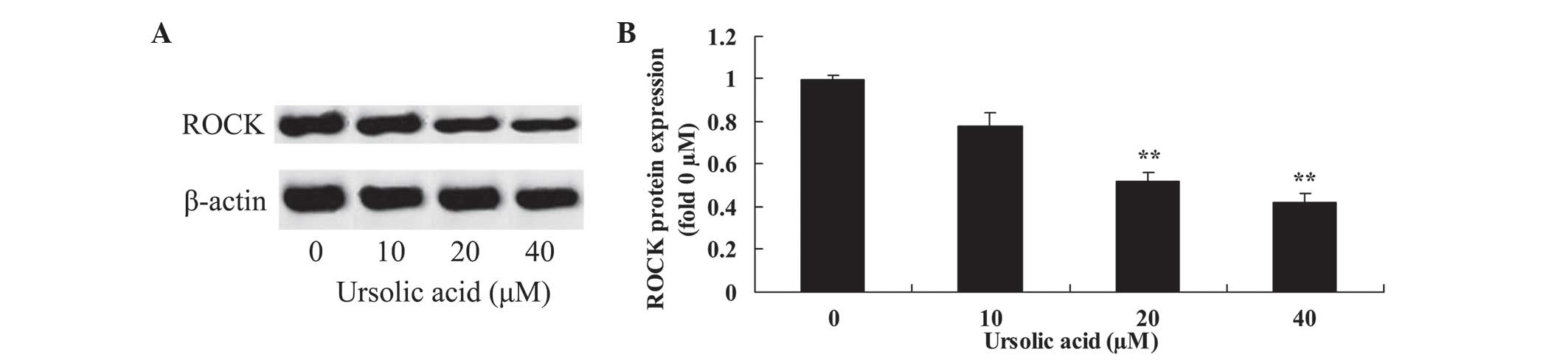
Anticancer effect of ursolic acid
treatment on ROCK protein expression in prostate cancer cells
To observe the underlying mechanism of ursolic acid
action on prostate cancer cells, ROCK protein expression was
measured using western blotting. The results of the present study
revealed that treatment with 20 and 40 µM ursolic acid for 48 h
significantly suppressed ROCK protein expression in DU145 cells,
compared with treatment with 0 µM of ursolic acid (Fig. 6).
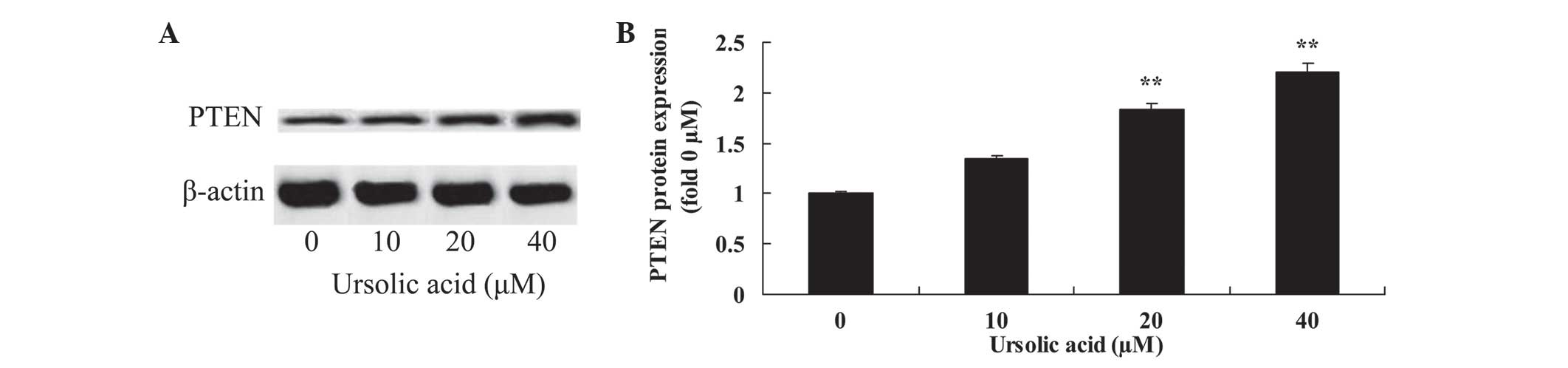
Anticancer effect of ursolic acid
treatment on PTEN protein expression in prostate cancer cells
To investigate the underlying mechanism of ursolic
acid action on prostate cancer cells, PTEN protein expression was
measured using western blotting. Following 48 h of ursolic acid (20
and 40 µM) treatment, PTEN protein expression was significantly
enhanced in DU145 cells, compared with treatment with 0 µM of
ursolic acid (Fig. 7).
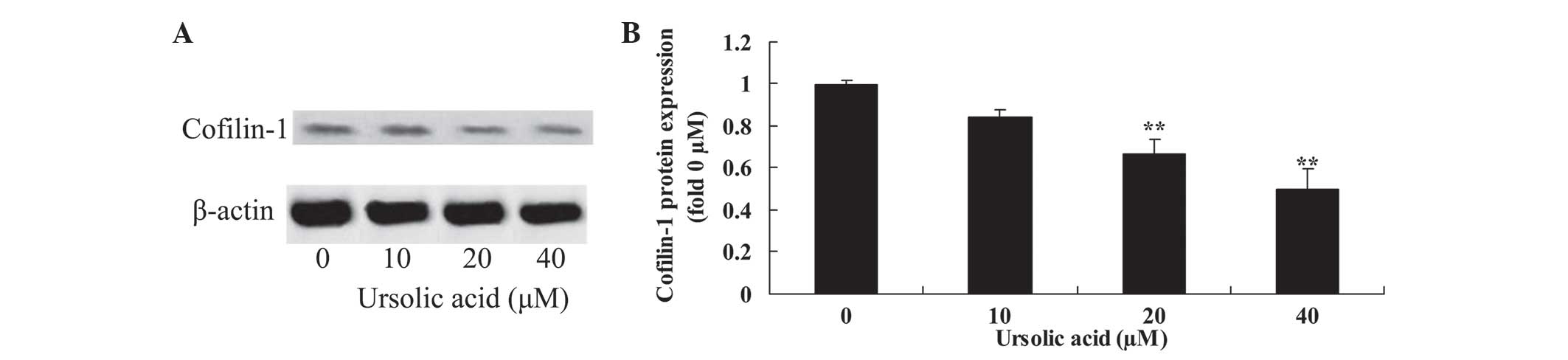
Anticancer effect of ursolic acid
treatment on cofilin-1 protein expression in prostate cancer
cells
To investigate the underlying mechanism of ursolic
acid action on prostate cancer cells, cofilin-1 protein expression
was measured using western blotting. The results of the present
study demonstrated that treatment with ursolic acid (20 and 40 µM)
significantly reduced cofilin-1 protein expression in DU145 cells,
compared with treatment with 0 µM of ursolic acid (Fig. 8).
Discussion
Prostate carcinoma is common among elderly men and
its morbidity in European and American developed countries is high,
with up to 650,000 new cases each year (1). In 2008, there were 900,000 new cases of
prostate carcinoma globally; therefore, prostate cancer has become
the second most serious cancer in terms of its threat to men's
health (21). The morbidity of
prostate cancer is lower in East Asia; however, due to the aging
population, dietary structure alterations and improvements in
diagnosis, the morbidity is also rising in countries in this region
(2). The present study observed that
ursolic acid significantly suppressed cell growth, and induced
apoptosis and caspase-3 and caspase-9 activity in DU145 cells.
Previous studies have revealed that ursolic acid is able to
suppress cell proliferation and induce apoptosis of APC-mutated
colon cancer cells (22), U937 cells
(23) and colon cancer-initiating
cells (24).
A current focus of cancer research concerns the
development of medicines to promote cell apoptosis. Cytochrome
c is an essential component of the respiratory chain and has
a significant role in the oxidation and reduction of cells
(25). Utilized as a cell
respiration-activating enzyme in the clinic, cytochrome c is
an ancillary drug used to treat cancer (26). Furthermore, cytochrome c
additionally has a significant role in apoptosis (27). A previous study reported the
extraction of 3 substances associated with cell apoptosis,
including cytochrome c (28).
It was observed that cells in adrenal cortex tumors of mice
underwent apoptosis following treatment with cytochrome c
(27). In the present study, ursolic
acid significantly activated cytochrome c protein expression
in the cytoplasm and suppressed cytochrome c protein
expression in mitochondria of DU145 cells. Achiwa et al
(29) suggested that ursolic acid
induces apoptosis in SNG-II endometrial cancer cells through
reduction of mitochondrial cytochrome c release. Li et
al (25) reported that ursolic
acid induced apoptosis of SGC-7901 gastric cancer cells through
cytochrome c.
Prostate carcinoma cells may express RhoA protein,
and the expression of RhoA protein in prostate carcinoma is higher
than that in benign prostatic hyperplasia (BPH) (30). Therefore it has been speculated that
the formation of prostate carcinoma may be associated with
overexpression of RhoA (30,31). The present study observed that
prostate cells expressed ROCK/PTEN protein, and the expression of
ROCK/PTEN protein in prostate carcinoma is also higher than that in
BPH. Furthermore, the expression level of RhoA and ROCK/PTEN
protein demonstrates significant positive correlation (30,31). These
findings indicated that the ROCK/PTEN signal transduction pathway
may participate in the occurrence of prostate carcinoma, and the
expression level of ROCK/PTEN protein may be regulated and
controlled by RhoA (32). The present
study observed that ursolic acid significantly downregulated the
ROCK/PTEN signal transduction pathway in DU145 cells. Li et
al (25) reported that ursolic
acid induced apoptosis of SGC-7901 gastric cancer cells through
suppression of ROCK/PTEN.
Cofilin-1 is a eukaryotic actin-binding protein with
a low molecular mass. The cofilin-1 gene is located at llq13
(32). In addition, cofilin-1 has a
large amount of biological activities, including participating in
cell apoptosis, cytoplasmic division and affecting phalloidin
(33). It has been reported that
cofilin-1 protein will translocate into the mitochondria from the
cytoplasm; subsequently, cytochrome c will be released from
mitochondria and combine with Apaf-1 to activate caspase-9, so that
the caspase cascade will be activated and lead to apoptosis
(19). In addition, the corresponding
mechanism of action of cofilin-1 may be associated with the
promotion of cyclase-associated protein 1 (25). Furthermore, when cofilin-1 protein
translocates into the mitochondria from the cytoplasm, it activates
cytochrome c release and activation of the caspase cascade,
indirectly inducing cell apoptosis (25). The results of the present study
demonstrated that ursolic acid significantly inhibited cofilin-1
protein expression in DU145 cells. Previous studies have reported
that ursolic acid induces apoptosis of SGC-7901 and BGC-823 gastric
cancer cells through suppression of mitochondrial translocation of
cofilin-1 (25,34).
In conclusion, the results of the present study
suggest that ursolic acid suppresses cell growth, induces apoptosis
and increases caspase-3 and caspase-9 activity in DU145 cells.
Ursolic acid treatment affects cytochrome c protein
expression in the cytoplasm and mitochondria, leading to
suppression of ROCK/PTEN signaling and mitochondrial translocation
of cofilin-1 in prostate cancer cells. Therefore, ursolic acid may
present a novel treatment strategy for prostate cancer by targeting
ROCK/PTEN and mitochondrial translocation of cofilin-1, leading to
activation of apoptosis.
References
|
1
|
Dossus L, Kaaks R, Canzian F, Albanes D,
Berndt SI, Boeing H, Buring J, Chanock SJ, Clavel-Chapelon F,
Feigelson HS, et al: PTGS2 and IL6 genetic variation and risk of
breast and prostate cancer: Results from the Breast and Prostate
Cancer Cohort Consortium (BPC3). Carcinogenesis. 31:455–461. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jiang J, Yu L, Huang X, Chen X, Li D,
Zhang Y, Tang L and Zhao S: Identification of two novel human
dynein light chain genes, DNLC2A and DNLC2B, and their expression
changes in hepatocellular carcinoma tissues from 68 Chinese
patients. Gene. 281:103–113. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Che JP, Li W, Yan Y, Liu M, Wang GC, Li
QY, Yang B, Yao XD and Zheng JH: Expression and clinical
significance of the nin one binding protein and p38 MAPK in
prostate carcinoma. Int J Clin Exp Pathol. 6:2300–2311.
2013.PubMed/NCBI
|
|
4
|
Gao L, Wang XD, Niu YY, Duan DD, Yang X,
Hao J, Zhu CH, Chen D, Wang KX, Qin XM and Wu XZ: Molecular targets
of Chinese herbs: A clinical study of hepatoma based on network
pharmacology. Sci Rep. 6:249442016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Yen HR, Chen YY, Huang TP, Chang TT, Tsao
JY, Chen BC and Sun MF: Prescription patterns of Chinese herbal
products for patients with uterine fibroid in Taiwan: A nationwide
population-based study. J Ethnopharmacol. 171:223–230. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Su M, Wu X, Chung HY, Li Y and Ye W:
Antiproliferative activities of five Chinese medicinal herbs and
active compounds in Elephantopus scaber. Nat Prod Commun.
4:1025–1030. 2009.PubMed/NCBI
|
|
7
|
Hsieh TC, Lu X, Guo J, Xiong W, Kunicki J,
Darzynkiewicz Z and Wu JM: Effects of herbal preparation Equiguard
on hormone-responsive and hormone-refractory prostate carcinoma
cells: Mechanistic studies. Int J Oncol. 20:681–689.
2002.PubMed/NCBI
|
|
8
|
Gui Y, Qiu X, Xu Y, Li D and Wang L:
Bu-Shen-Ning-Xin decoction suppresses osteoclastogenesis via
increasing dehydroepiandrosterone to prevent postmenopausal
osteoporosis. Biosci Trends. 9:169–181. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jenny M, Wondrak A, Zvetkova E, Nguyen
Thi, Ngoc Tram, Phi PT, Schennach H, Culig Z, Ueberall F and Fuchsl
D: Crinum Latifolium leave extracts suppress immune activation
cascades in peripheral blood mononuclear cells and proliferation of
prostate tumor cells. Sci Pharm. 79:323–335. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lin YH, Chen KK and Chiu JH: Prevalence,
patterns and costs of Chinese medicine use among prostate cancer
patients: A population-based study in Taiwan. Integr Cancer Ther.
9:16–23. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang WB, Wang GJ and Fuxe K: Classic and
modern meridian studies: A review of low hydraulic resistance
channels along meridians and their relevance for therapeutic
effects in Traditional Chinese Medicine. Evid Based Complement
Alternat Med. 2015:4109792015. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Li N, Ma Z, Li M, Xing Y and Hou Y:
Natural potential therapeutic agents of neurodegenerative diseases
from the traditional herbal medicine Chinese dragon's blood. J
Ethnopharmacol. 152:508–521. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen J, Wong HS and Ko KM: Ursolic
acid-enriched herba cynomorii extract induces mitochondrial
uncoupling and glutathione redox cycling through mitochondrial
reactive oxygen species generation: Protection against menadione
cytotoxicity in h9c2 cells. Molecules. 19:1576–1591. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liao Q, Yang W, Jia Y, Chen X, Gao Q and
Bi K: LC-MS determination and pharmacokinetic studies of ursolic
acid in rat plasma after administration of the traditional chinese
medicinal preparation Lu-Ying extract. Yakugaku Zasshi.
125:509–515. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gao N, Cheng S, Budhraja A, Gao Z, Chen J,
Liu EH, Huang C, Chen D, Yang Z, Liu Q, et al: Ursolic acid induces
apoptosis in human leukaemia cells and exhibits anti-leukaemic
activity in nude mice through the PKB pathway. Br J Pharmacol.
165:1813–1826. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yie Y, Zhao S, Tang Q, Zheng F, Wu J, Yang
L, Deng S and Hann SS: Ursolic acid inhibited growth of
hepatocellular carcinoma HepG2 cells through AMPKα-mediated
reduction of DNA methyltransferase 1. Mol Cell Biochem. 402:63–74.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Huang L, Chen T, Ye Z and Chen G: Use of
liquid chromatography-atmospheric pressure chemical ionization-ion
trap mass spectrometry for identification of oleanolic acid and
ursolic acid in Anoectochilus roxburghii (wall.) Lindl. J Mass
Spectrom. 42:910–917. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Chang CY, Leu JD and Lee YJ: The actin
depolymerizing factor (ADF)/cofilin signaling pathway and DNA
damage responses in cancer. Int J Mol Sci. 16:4095–4120. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li GB, Cheng Q, Liu L, Zhou T, Shan CY, Hu
XY, Zhou J, Liu EH, Li P and Gao N: Mitochondrial translocation of
cofilin is required for allyl isothiocyanate-mediated cell death
via ROCK1/PTEN/PI3K signaling pathway. Cell Commun Signal.
11:502013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Vitolo MI, Boggs AE, Whipple RA, Yoon JR,
Thompson K, Matrone MA, Cho EH, Balzer EM and Martin SS: Loss of
PTEN induces microtentacles through PI3K-independent activation of
cofilin. Oncogene. 32:2200–2210. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Klotz L, Boccon-Gibod L, Shore ND, Andreou
C, Persson BE, Cantor P, Jensen JK, Olesen TK and Schröder FH: The
efficacy and safety of degarelix: A 12-month, comparative,
randomized, open-label, parallel-group phase III study in patients
with prostate cancer. BJU Int. 102:1531–1538. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kim JH, Kim YH, Song GY, Kim DE, Jeong YJ,
Liu KH, Chung YH and Oh S: Ursolic acid and its natural derivative
corosolic acid suppress the proliferation of APC-mutated colon
cancer cells through promotion of β-catenin degradation. Food Chem
Toxicol. 67:87–95. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Deng L, Zhang R, Tang F, Li C, Xing YY and
Xi T: Ursolic acid induces U937 cells differentiation by PI3K/Akt
pathway activation. Chin J Nat Med. 12:15–19. 2014.PubMed/NCBI
|
|
24
|
Wang W, Zhao C, Jou D, Lü J, Zhang C, Lin
L and Lin J: Ursolic acid inhibits the growth of colon
cancer-initiating cells by targeting STAT3. Anticancer Res.
33:4279–4284. 2013.PubMed/NCBI
|
|
25
|
Li R, Wang X, Zhang XH, Chen HH and Liu
YD: Ursolic acid promotes apoptosis of SGC-7901 gastric cancer
cells through ROCK/PTEN mediated mitochondrial translocation of
cofilin-1. Asian Pac J Cancer Prev. 15:9593–9597. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yang YZ, Fan TT, Gao F, Fu J and Liu Q:
Exogenous cytochrome c inhibits the expression of transforming
growth factor-β1 in a mouse model of sepsis-induced myocardial
dysfunction via the SMAD1/5/8 signaling pathway. Mol Med Rep.
12:2189–2196. 2015.PubMed/NCBI
|
|
27
|
Faizi M, Salimi A, Rasoulzadeh M,
Naserzadeh P and Pourahmad J: Schizophrenia induces oxidative
stress and cytochrome C release in isolated rat brain mitochondria:
A possible pathway for induction of apoptosis and
neurodegeneration. Iran J Pharm Res. 13(Suppl): S93–S100. 2014.
|
|
28
|
Katoch B, Sebastian S, Sahdev S, Padh H,
Hasnain SE and Begum R: Programmed cell death and its clinical
implications. Indian J Exp Biol. 40:513–524. 2002.PubMed/NCBI
|
|
29
|
Achiwa Y, Hasegawa K, Komiya T and Udagawa
Y: Ursolic acid induces Bax-dependent apoptosis through the
caspase-3 pathway in endometrial cancer SNG-II cells. Oncol Rep.
13:51–57. 2005.PubMed/NCBI
|
|
30
|
Mikelis CM, Simaan M, Ando K, Fukuhara S,
Sakurai A, Amornphimoltham P, Masedunskas A, Weigert R, Chavakis T,
Adams RH, et al: RhoA and ROCK mediate histamine-induced vascular
leakage and anaphylactic shock. Nat Commun. 6:67252015. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Li G, Liu L, Shan C, Cheng Q, Budhraja A,
Zhou T, Cui H and Gao N: RhoA/ROCK/PTEN signaling is involved in
AT-101-mediated apoptosis in human leukemia cells in vitro and in
vivo. Cell Death Dis. 5:e9982014. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Wang Y, Kuramitsu Y, Ueno T, Suzuki N,
Yoshino S, Iizuka N, Zhang X, Oka M and Nakamura K: Differential
expression of up-regulated cofilin-1 and down-regulated cofilin-2
characteristic of pancreatic cancer tissues. Oncol Rep.
26:1595–1599. 2011.PubMed/NCBI
|
|
33
|
Yan H, Yang K, Xiao H, Zou YJ, Zhang WB
and Liu HY: Over-expression of cofilin-1 and phosphoglycerate
kinase 1 in astrocytomas involved in pathogenesis of
radioresistance. CNS Neurosci Ther. 18:729–736. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Tang Q, Ji Q, Tang Y, Chen T, Pan G, Hu S,
Bao Y, Peng W and Yin P: Mitochondrial translocation of cofilin-1
promotes apoptosis of gastric cancer BGC-823 cells induced by
ursolic acid. Tumour Biol. 35:2451–2459. 2014. View Article : Google Scholar : PubMed/NCBI
|