Introduction
Pancreatic cancer is currently one of the leading
causes of cancer-associated mortality in industrialized countries,
with an incidence rate equaling its mortality rate (1). The number of newly diagnosed cases and
mortalities per 100,000 individuals are 12.4 and 10.9,
respectively, according to the National Cancer Institute (Bethesda,
MD, USA; http://seer.cancer.gov). Despite
research efforts, there has been limited progress regarding the
treatment of this disease; therefore, novel strategies to identify
new therapeutic agents are urgently required. The Hedgehog (Hh)
signaling pathway is critical for morphogenesis signaling and is
inappropriately activated in patients with pancreatic cancer
(2,3).
In addition, this pathway is associated with the growth and
metastasis of various types of tumors, including pancreatic cancer
(4,5).
Therefore, the Hh pathway serves as a therapeutic target, and
inhibitors of Hh signaling may function as novel therapeutic drugs
for the treatment of pancreatic cancer.
The Hedgehog (Hh) pathway regulates embryonic
organogenesis and tissue growth (6),
and is activated by Hh ligands, including Sonic Hh (Shh), which
binds to a 12-pass transmembrane spanning receptor known as Patched
(Ptch) (7). Binding of the Hh ligand
to Ptch relieves repression of a 7-pass transmembrane receptor
called Smoothened (Smo) (8). Released
Smo activates the signaling pathway, resulting in release of the
glioma-associated oncogene (Gli) transcription factor family
[including Gli homolog 1 (Gli1), Gli2 and Gli3], which translocate
to the nucleus and trigger expression of Gli target genes, such as
Gli1 and cyclin D1, for cell growth (9).
Inhibitors of Smo in the Hh pathway have been a
frequent focus of therapeutic drug development and have been
evaluated in preclinical models (10,11).
However, resistance to Smo inhibitors may develop clinically
(12,13). Smo inhibitors are ineffective in
tumors accompanied by overactivated Gl, downstream of Smo (14). Thus, Gli, which is downstream of Smo,
is an important target for repressing activated Hh signaling.
Several studies have reported that Gli1 and Gli2 are the primary
transcriptional effectors involved in tumor formation (15,16). In
addition, the importance of Gli1 in tumor progression and
development is well recognized in human cell culture systems. A
number of studies have demonstrated that targeting Gli1 may be a
promising cancer therapeutic strategy (17,18).
During the search for plant-derived inhibitors of
the Hh/Gli signaling pathway, the present study observed that a
sesquiterpene from Siegesbeckia glabrescens repressed
Gli-mediated transcriptional activity and suppressed proliferation
of human pancreatic cancer cells. S. glabrescens Makino
(Compositae, ‘Hi-Chum’ in Korea) has been used as an herbal
medicine to treat paralysis, inflammatory diseases, allergic
disorders and asthma. Extracts of S. glabrescens
exhibit antioxidative, antiallergic, anti-inflammatory (19) and anti-tumor activities (20). It was previously reported that a
germacranolide sesquiterpene lactone (GSL) from S.
glabrescens exhibited anti-inflammatory activity by
downregulating inducible nitric oxide synthase and cyclooxygenase-2
expression in lipopolysaccharide-activated macrophages (19). Herein, the present study reports the
use of a GSL from S. glabrescens as an inhibitor of
Hh/Gli-mediated transcription.
Materials and methods
Plant material and isolation from S.
glabrescens
Part of a S. glabrescens plant (voucher
specimen no. SPH 05007 at the College of Pharmacy, Sookmyung
Women's University, Seoul, Korea) was obtained from wild at Wan-Do
(Jeollanam-do province, Korea) in 2005. The GSL isolation procedure
was performed as described previously (19). The GSL structure was confirmed to be
2-propenoic acid,
2-methyl-2,3,3a,4,5,8,9,10,11,11a,-decahydro-6,10-bis
(hydroxymethyl)-3-methylene-2-oxocyclodeca (b) furan-4-yl ester by
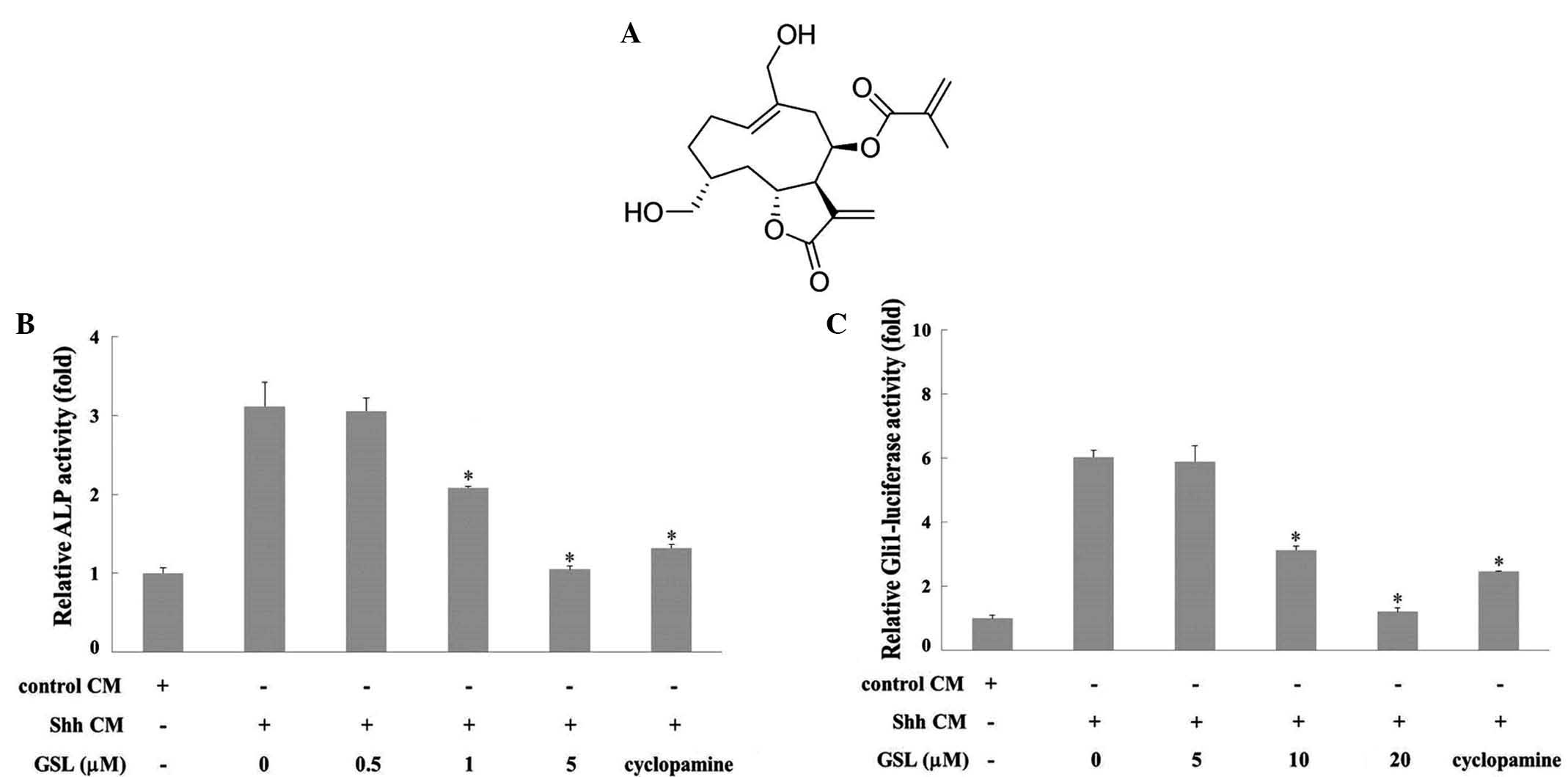
spectroscopic methods (Fig. 1A).
Cell lines, chemicals and
biochemicals
Human pancreatic cancer PANC-1 and AsPC-1 cell
lines, and mouse mesenchymal C3H10T1/2 stem cells (American Type
Culture Collection, Manassas, VA, USA) were cultured in Dulbecco's
modified Eagle's medium (DMEM) containing 10% fetal bovine serum
(FBS) and streptomycin/penicillin (Thermo Fisher Scientific, Inc.,
Waltham, MA, USA). GANT61 (purity, ≥98%), a synthetic Gli
inhibitor, was purchased from Sigma-Aldrich (St. Louis, MO, USA).
Cyclopamine (purity, ≥98%; Sigma-Aldrich), a representative
plant-derived Smo inhibitor in the Hh pathway, was used as a
positive control for the evaluation of the inhibitory properties of
the Hh pathway.
Preparation of Shh-conditioned media
(Shh-CM)
Hh signaling was induced by Shh-CM (21). The Shh expression construct was
transiently transfected into HEK293 cells to prepare the Shh-CM.
Shh-producing HEK293 cells were grown to 80% confluency in DMEM
containing 10% FBS. The medium was subsequently replaced with DMEM
containing 2% FBS, and allowed to grow for 5 days. The CM was
harvested and filtered through a 0.22 µm membrane.
Alkaline phosphatase (ALP) assay
C3H10T1/2 cells were seeded at 5×103
cells/well in a 96-well plate and allowed to attach for 4 h, which
was followed by the addition of Shh-CM and GSL. Following
incubation at 37°C for 96 h, 0.9% NaCl with 0.2% Triton X-100 was
added to the cells and lysed for 15 min. The cell lysates were
mixed with ALP substrate (4 mM p-nitrophenyl phosphate disodium)
and reaction buffer [200 mM Tris-HCl (pH 10.5), 0.4 M
2-amino-2-methylpropanol and 8 mM MgCl2] and incubated
in the dark at 37°C for 45 min. Absorbance was measured at 415 nm
using a microplate reader (Molecular Devices, LLC, Sunnyvale, CA,
USA). The assay was performed in triplicate.
Gli1/Gli-dependent luciferase reporter
assay
C3H10T1/2 cells were transiently transfected with
Gli1, Gli-dependent firefly luciferase and β-galactosidase
expression constructs (C3H10T1/2-Gli1-Luc cells) to assess
Gli1-mediated transcriptional activity. The transfected cells were
treated with GSL in the presence of either Shh-CM or HEK293 control
medium. Following 30 h of incubation at 37°C, Gli1-dependent
firefly luciferase and β-galactosidase activities were measured
using a microplate luminometer (Perkin Elmer, Inc., Waltham, MA,
USA).
Plasmids of the Gli-dependent firefly luciferase
reporter construct and β-galactosidase expression construct were
transiently transfected into PANC-1 cells (PANC-1-Gli-Luc cells) to
assess Gli-mediated transcriptional activity in human pancreatic
cancer cells. These cells were treated with various concentrations
of GSL (0, 1, 5, 10 and 20 µM). Following 20 h of incubation at
37°C, cellular firefly luciferase and β-galactosidase activities
were measured with a microplate luminometer.
Cell proliferation assay
Cells were plated at a density of 3×103
cells/well in a 96-well plate. The cells were incubated with
various concentrations of GSL (0, 5, 10 and 20 µM) for 3 days,
treated with MTT (5 mg/ml) solution for 4 h, and lysed with
dimethyl sulfoxide. Absorbance was read at 540 nm using a
microplate reader. The vehicle-treated group was considered as 100%
of cell proliferation for each cell line.
Western blot analysis
PANC-1 and AsPC-1 cells were treated with various
concentrations of GSL (0, 1, 5, 10 and 20 µM) for 20 h, harvested
and lysed gently with cell lysis buffer (Cell Signaling Technology,
Inc., Danvers, MA, USA). The cell lysates were centrifuged at
10,000 × g for 20 min at 4°C and the supernatants were collected,
which were subjected to 10% sodium dodecyl sulfated-polyacrylamide
gel electrophoresis and transferred to polyvinylidene difluoride
membranes. Anti-Gli1 (cat. no. 2553), cyclin D1 (cat. no. 2922),
phospho-Akt (p-Akt; cat. no. 9271), and total Akt (cat. no. 9272)
rabbit polyclonal antibodies (dilution, 1:1,000; Cell Signaling
Technology, Inc.) were used for the immunoblot analysis. The bands
were detected by LAS3000 (Fujifilm Holdings Corporation, Tokyo,
Japan) upon incubation with enhanced chemiluminescence reagents (GE
Healthcare Life Sciences, Uppsala, Sweden).
Statistical analysis
Data are presented as the mean ± standard deviation.
P-values were determined with the unpaired Student's t-test,
which was used to evaluate significant differences between the
control and the test group. P<0.05 was considered to indicate a
statistically significant difference. All analyses were performed
using SPSS 19.0 software (SPSS Inc., Chicago, IL, USA).
Results
Effects of GSL on ALP activity and
Hh/Gli-mediated transcriptional activity in mouse mesenchymal
C3H10T1/2 stem cells
ALP activity was evaluated in Shh-CM-triggered mouse
mesenchymal C3H10T1/2 stem cells to investigate the effects of GSL
on the Hh signaling pathway. The Hh signaling pathway is involved
in osteoblast differentiation accompanying activation of ALP as a
marker of osteoblast differentiation (22). C3H10T1/2 cells were treated with GSL
and/or Shh-CM for 96 h. Cyclopamine, a Smo inhibitor (23), was used as a positive control. As
presented in Fig. 1B, GSL decreased
ALP activity in Shh-CM-treated C3H10T1/2 cells in a dose-dependent
manner compared with the vehicle, which exhibited a high level of
ALP activity. These results indicate that GSL inhibits the
differentiation of Shh-induced C3H10T1/2 cells into ALP-positive
osteoblasts by interfering with Hh signaling. The present study
subsequently investigated whether GSL inhibits Shh-induced
Gli1-mediated transcription. The activity of Gli1-mediated
transcription was measured as luciferase activity in
C3H10T1/2-Gli1-Luc cells. Cyclopamine was used as the positive
control. As presented in Fig. 1C, GSL
treatment inhibited Shh-CM-induced luciferase activity in a
dose-dependent manner. β-galactosidase activities were not affected
by GSL up to 20 µM, indicating no cytotoxic effects. These results
suggest that GSL modulates the Hh/Gli signaling pathway by
suppressing Shh-CM-induced Gli1-mediated transcriptional
activity.
Effects of GSL on Gli-mediated
transcriptional activity in human pancreatic cancer cells
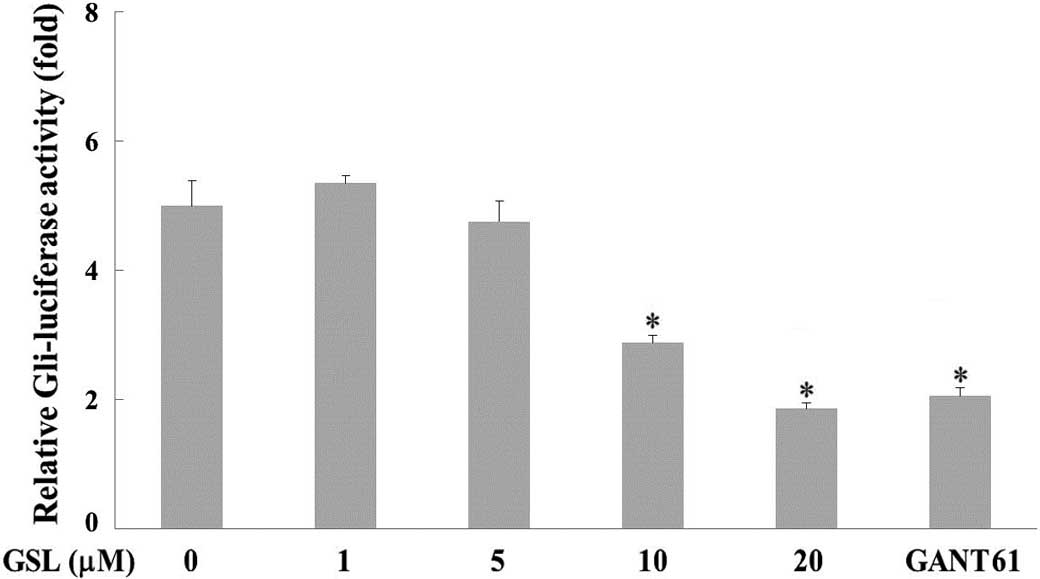
Gli-mediated transcriptional activity was determined
in PANC-1 cells to elucidate the effect of GSL on Shh signaling in
human pancreatic cancer. PANC-1 cells, which express aberrantly
activated Gli1 (2), were transiently
transfected with Gli-dependent firefly and β-galactosidase
reporters (PANC-1-Gli-Luc cells). GSL treatment suppressed
luciferase activity in a dose-dependent manner compared with the
vehicle treatment, which exhibited high luciferase activity
(Fig. 2). GANT61, an inhibitor of
Gli-mediated transcription (24), was
used as the positive control. The GSL or vehicle treatments did not
affect β-galactosidase activities, indicating that GSL inhibits
Gli-mediated transcriptional activity in human pancreatic cancer
cells.
Effects of GSL on the proliferation of
human pancreatic cancer cells
Next, the current study investigated whether GSL
affects Gli-mediated proliferation in human pancreatic cells
(PANC-1 and AsPC-1 cells) with an increased Hh signal. C3H10T1/2
cells were used as controls with no dependence on the Hh signal.
GANT61 was used the positive control Gli-inhibitor that inhibits
cell proliferation. As presented in Table
I, GSL dose-dependently suppressed proliferation of the
pancreatic cancer cells, with 50% inhibitory concentration
(IC50) values of 6.9 and 5.1 µM in PANC-1 and AsPC-1
cells, respectively. However, GSL exhibited a weak inhibitory
effect on the proliferation of C3H10T1/2 control cells, with an
IC50 value of 35.7 µM.
 | Table I.Inhibitory effect (%) of GSL on the
proliferation of PANC-1, AsPC-1 and C3H10T1/2 cells. |
Table I.
Inhibitory effect (%) of GSL on the
proliferation of PANC-1, AsPC-1 and C3H10T1/2 cells.
|
| Concentration of
GSL, µM |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Cells | 5 | 10 | 20 | GANT61a | IC50
values, µM |
|---|
| PANC-1 | 48.8±2.1 | 62.4±3.2 | 70.0±4.1 | 82.2±1.7 |
6.9 |
| AsPC-1 | 49.9±0.8 | 62.1±2.5 | 73.1±2.3 | 79.7±3.3 |
5.1 |
| C3H10T1/2 | 17.0±1.5 | 27.8±1.1 | 35.3±2.5 | 60.8±2.8 | 35.7 |
Effects of GSL on Gli-related protein
levels in human pancreatic cancer cells
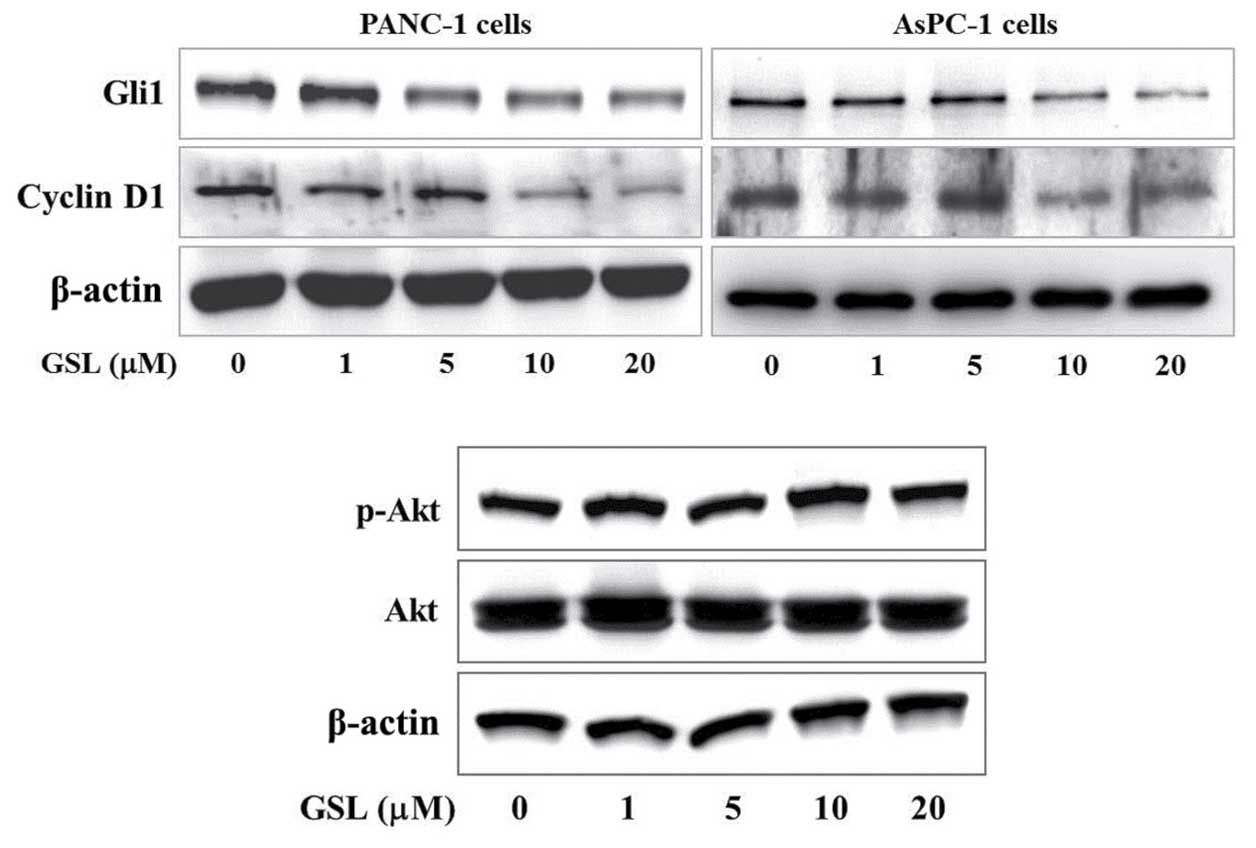
The present study examined the effect of GSL on
Gli-mediated protein expression, including Gli1 and cyclin D1, as
key regulators of tumor cell growth. Immunoblot analysis
demonstrated that GSL reduced the expression of Gli1 and cyclin D1
proteins in a concentration-dependent manner compared with that of
the vehicle treated PANC-1 and AsPC-1 cells (Fig. 3). It has been previously reported that
phosphatidylinositol 3-kinase (PI3K)/Akt signaling interferes with
Gli signaling without inhibiting Smo (25). Thus, the current study examined the
effect of GSL on PI3K/Akt signaling, and it was observed that GSL
had no effect on p-Akt/total Akt levels in the PANC-1 cells
(Fig. 3). These results indicate that
GSL suppressed proliferation of pancreatic cancer cells by
downregulating Gli1 and cyclin D1 expression through repression of
Gli-mediated transcription and did not disrupt PI3K/Akt
signaling.
Discussion
The Hh pathway is responsible for regulating and
coordinating cellular growth and development in the embryo, and is
implicated in the formation and development of tumors, including
pancreatic cancer (26,27). The Hh pathway is activated by binding
of Shh to Ptch, which activates Smo and subsequently the Gli family
(28). The Gli family has three
mammalian homologs; Gli1 and Gli2 function as activators of Hh
signaling to induce the expression of target genes, including Gli1
and cyclin D, whilst Gli3 is a repressor of Hh signaling (29,30). Gli1
and Gli2 regulate the expression of target genes related to cell
growth and survival; however, Gli2 may function as an effective
transcription factor in the absence of Gli1 (31).
Recent evidence suggests a critical role of Hh
signaling in tumorigenesis through mutations in Ptch or Smo, which
lead to hyperactivation of Gli in certain tumor types, including
pancreatic cancer (32). Constitutive
activation of Gli is associated with tumor formation and growth
(33–35). Although Smo antagonists have been
studied to treat Hh-directed cancer (36), therapeutic efficiencies were extremely
low in preclinical and clinical models of tumors accompanying
activation of Smo-downstream components, including Gli (37,38).
Therefore, targeting the Hh pathway at the level of Gli rather than
at the level of Smo may be a better strategy for treating cancer
associated with an uncontrolled Hh pathway.
In the present study, it was observed that GSL from
S. glabrescens inhibited the Hh/Gli signaling pathway in
C3H10T1/2 cells (Fig. 1). Mesenchymal
stem cells differentiate into osteoblasts following Hh signal
activation, and high ALP activity may be measured as a marker of
osteoblast differentiation (39).
Mesenchymal C3H10T1/2 stem cells were treated with Shh-CM to induce
osteoblast differentiation through activation of the Hh pathway.
GSL inhibited Shh-induced ALP activity in the C3H10T1/2 cells by
suppressing the Hh signaling pathway. Luciferase activity was
measured in C3H10T1/2-Gli1-Luc cells to evaluate the inhibitory
potential of GSL on Shh-induced Gli-mediated transcription. While
Shh-CM increased luciferase activity by inducing Gli1
transcription, GSL inhibited luciferase activity in a
dose-dependent manner. These results indicate that GSL inhibits the
Hh/Gli signaling pathway, consistent with the ALP activity
result.
The majority of inhibitors of the Hh signaling
pathway target Smo; however, other mechanisms of Hh signal-directed
cancer have been reported, including Ptch mutations or
overexpression of the Shh ligand or Gli in pancreatic cancer
(40,41). The present study examined the
inhibitory potential of GSL on Gli-mediated transcription in human
pancreatic cancer PANC-1 cells, which overexpress Gli and are Smo
insensitive (2,42). The inhibition of Gli-mediated
transcription activity suggests that GSL suppresses downstream of
Gli to inhibit cancer cell proliferation or gene expression.
Furthermore, the current study observed that GLS
suppressed Gli-mediated proliferation of the human pancreatic
cancer PANC-1 and AsPC-1 cells. PANC-1 cells are reported to be
cyclopamine-insensitive (42),
whereas AsPC-1 cells are cyclopamine-sensitive and Hh
signaling-dependent cancer cells, as they overproduce Shh (43). As presented in Table I, the anti-proliferative potential of
GSL against pancreatic cancer cells is stronger than that against
the control C3H10T1/2 cells.
Several plant-derived modulators of the Hh/Gli
signaling pathway have been reported, including cyclopamine
(steroidal alkaloid) as a Smo antagonist, and staurosporinone
(bisindole alkaloid) and zerumbone (sesquiterpene) as inhibitors of
Gli-mediated transcription (44).
Hosoya et al (44) reported
that the α, β-unsaturated carbonyl group in zerumbone is important
for inhibiting Gli-mediated transcription, but zerumbone has
similar cytotoxicity in PANC-1 and C3H10T1/2 cells. In the present
study, GSL was 5-fold more toxic against the PANC-1 and AsPC-1
cells than it was against the C3H10T1/2 cells. The structural
requirements for cell-type selective toxicity of GSL in Hh/Gli
signaling requires clarification in further investigations.
Gli1 and cyclin D1 expression is dependent on
Gli-mediated transcription (45).
Gli1, a target gene of the Gli transcription factor, regulates
transcription of the Hh responsive genes by itself (46). Gli1 is upregulated in the majority of
pancreatic cancer tissues and its level of expression is positively
correlated with Hh signaling (47).
Cyclin D1, another Gli target gene, functions as a cell cycle
regulator and is important in carcinogenesis (48,49). In
the present study, basal levels of Gli1 and cyclin D1 were elevated
in PANC-1 and AsPC-1 cells. GSL downregulated Gli1 and cyclin D1
protein levels in a dose-dependent manner (Fig. 3). These results are in accordance with
the decline of Gli-mediated transcriptional activity and
proliferation of pancreatic cancer cells. Furthermore, p-Akt/total
Akt protein levels were not affected by GSL treatment in the PANC-1
cells (Fig. 3), suggesting that GSL
has no effect on the PI3K/Akt signaling pathway, which may be
involved specifically in regulating Hh/Gli signaling.
In conclusion, the current study identified a GSL
derived from S. glabrescens that functions as a modulator of
the Hh/Gli signaling pathway. This compound exerted
anti-proliferative effects against human pancreatic cancer PANC-1
and AsPC-1 cells, at least partially, by downregulating
Gli-mediated transcriptional activity, and Gli1 and cyclin D1
expression. GSL from S. glabrescens may be a valuable
candidate for the development of novel therapeutic agents against
Hh/Gli-dependent forms of cancer.
Acknowledgements
The present study was supported by grants from the
National Research Foundation of Korea funded by the Korean
Government (MSIP) (nos. 2011-0030074 and 2012R1A1A3013645).
Glossary
Abbreviations
Abbreviations:
|
Hh
|
Hedgehog
|
|
Shh
|
Sonic hedgehog
|
|
Ptch
|
12-pass transmembrane spanning
receptor patched
|
|
Smo
|
7-pass transmembrane receptor
Smoothened
|
|
Gli
|
glioma-associated oncogene
|
|
Shh CM
|
Sonic hedgehog conditioned medium
|
References
|
1
|
Bosetti C, Bertuccio P, Negri E, La
Vecchia C, Zeegers MP and Boffetta P: Pancreatic cancer: Overview
of descriptive epidemiology. Mol Carcinog. 51:3–13. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Guo J, Gao J, Li Z, Gong Y, Man X, Jin J
and Wu H: Adenovirus vector-mediated Gli1 siRNA induces growth
inhibition and apoptosis in human pancreatic cancer with
Smo-dependent or Smo-independent Hh pathway activation in vitro and
in vivo. Cancer Lett. 339:185–194. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Hao K, Tian XD, Qin CF, Xie XH and Yang
YM: Hedgehog signaling pathway regulates human pancreatic cancer
cell proliferation and metastasis. Oncol Rep. 29:1124–1132.
2013.PubMed/NCBI
|
|
4
|
Morton JP, Mongeau ME, Klimstra DS, Morris
JP, Lee YC, Kawaguchi Y, Wright CV, Hebrok M and Lewis BC: Sonic
hedgehog acts at multiple stages during pancreatic tumorigenesis.
Proc Natl Acad Sci USA. 104:5103–5108. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kelleher FC: Hedgehog signaling and
therapeutics in pancreatic cancer. Carcinogenesis. 32:445–451.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ingham PW and McMahon AP: Hedgehog
signaling in animal development: Paradigms and principles. Genes
Dev. 15:3059–3087. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Stone DM, Hynes M, Armanini M, Swanson TA,
Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et
al: The tumour-suppressor gene patched encodes a candidate receptor
for Sonic hedgehog. Nature. 384:129–134. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Akiyama H, Shigeno C, Hiraki Y, Shukunami
C, Kohno H, Akagi M, Konishi J and Nakamura T: Cloning of a mouse
smoothened cDNA and expression patterns of hedgehog signalling
molecules during chondrogenesis and cartilage differentiation in
clonal mouse EC cells, ATDC5. Biochem Biophys Res Commun.
235:142–147. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kasper M, Regl G, Frischauf AM and Aberger
F: GLI transcription factors: Mediators of oncogenic hedgehog
signaling. Eur J Cancer. 42:437–445. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Ailles L and Siu LL: Targeting the
Hedgehog pathway in cancer: Can the spines be smoothened? Clin
Cancer Res. 17:2071–2073. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Tang JY and Marghoob AA: Emerging
treatments and signaling pathway inhibitors. Semin Cutan Med Surg.
30(Suppl 4): S14–S18. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Schnidar H, Eberl M, Klingler S,
Mangelberger D, Kasper M, Hauser-Kronberger C, Regl G, Kroismayr R,
Moriggl R, Sibilia M and Aberger F: Epidermal growth factor
receptor signaling synergizes with hedgehog/GLI in oncogenic
transformation via activation of the MEK/ERK/JUN pathway. Cancer
Res. 69:1284–1292. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
LoRusso PM, Rudin CM, Reddy JC, Tibes R,
Weiss GJ, Borad MJ, Hann CL, Brahmer JR, Chang I, Darbonne WC, et
al: Phase I trial of hedgehog pathway inhibitor vismodegib
(GDC-0449) in patients with refractory, locally advanced or
metastatic solid tumors. Clin Cancer Res. 17:2502–2511. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Mazumdar T, Devecchio J, Agyeman A, Shi T
and Houghton JA: Blocking Hedgehog survival signaling at the level
of the GLI genes induces DNA damage and extensive cell death in
human colon carcinoma cells. Cancer Res. 71:5904–5914. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Kimura H, Stephen D, Joyner A and Curran
T: Gli1 is important for medulloblastoma formation in Ptc1+/- mice.
Oncogene. 24:4026–4036. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Thiyagarajan S, Bhatia N, Reagan-Shaw S,
Cozma D, Thomas-Tikhonenko A, Ahmad N and Spiegelman VS: Role of
GLI2 transcription factor in growth and tumorigenicity of prostate
cells. Cancer Res. 67:10642–10646. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Lauth M, Bergström A, Shimokawa T and
Toftgård R: Inhibition of GLI-mediated transcription and tumor cell
growth by small-molecule antagonists. Proc Natl Acad Sci USA.
104:8455–8460. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Zahreddine HA, Culjkovic-Kraljacic B,
Assouline S, Gendron P, Romeo AA, Morris SJ, Cormack G, Jaquith JB,
Cerchietti L, Cocolakis E, et al: The sonic hedgehog factor GLI1
imparts drug resistance through inducible glucuronidation. Nature.
511:90–93. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Li H, Kim JY, Hyeon J, Lee HJ and Ryu JH:
In vitro antiinflammatory activity of a new sesquiterpene lactone
isolated from Siegesbeckia glabrescens. Phytother Res.
25:1323–1327. 2011.PubMed/NCBI
|
|
20
|
Cho YR, Choi SW and Seo DW: The in vitro
antitumor activity of Siegesbekia glabrescens against ovarian
cancer through suppression of receptor tyrosine kinase expression
and the signaling pathways. Oncol Rep. 30:221–226. 2013.PubMed/NCBI
|
|
21
|
Chen JK, Taipale J, Young KE, Maiti T and
Beachy PA: Small molecule modulation of Smoothened activity. Proc
Natl Acad Sci USA. 99:14071–14076. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakamura TT, Aikawa M, Iwamoto-Enomoto M,
Iwamoto M, Higuchi Y, Pacifici M, Kinto N, Yamaguchi A, Noji S,
Kurisu K and Matsuya T: Induction of osteogenic differentiation by
hedgehog proteins. Biochem Biophys Res Commun. 237:465–469. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Lee ST, Welch KD, Panter KE, Gardner DR,
Garrossian M and Chang CW: Cyclopamine: From cyclops lambs to
cancer treatment. J Agric Food Chem. 62:7355–7362. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Stanton BZ and Peng LF: Small-molecule
modulators of the Sonic hedgehog signaling pathway. Mol Biosyst.
6:44–54. 2009. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Riobó NA, Lu K, Ai X, Haines GM and
Emerson CP Jr: Phosphoinositide 3-kinase and akt are essential for
Sonic hedgehog signaling. Proc Natl Acad Sci USA. 103:4505–4510.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Lauth M and Toftgård R: Hedgehog signaling
and pancreatic tumor development. Adv Cancer Res. 110:1–17. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Xie J, Bartels CM, Barton SW and Gu D:
Targeting hedgehog signaling in cancer: Research and clinical
developments. Onco Targets Ther. 6:1425–1435. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Park HL, Bai C, Platt KA, Matise MP,
Beeghly A, Hui CC, Nakashima M and Joyner AL: Mouse Gli1 mutants
are viable but have defects in SHH signaling in combination with a
Gli2 mutation. Development. 127:1593–1605. 2000.PubMed/NCBI
|
|
29
|
Katoh Y and Katoh M: Hedgehog signaling
pathway and gastrointestinal stem cell signaling network (review).
Int J Mol Med. 18:1019–1023. 2006.PubMed/NCBI
|
|
30
|
Hu MC, Mo R, Bhella S, Wilson CW, Chuang
PT, Hui CC and Rosenblum ND: GLI3-dependent transcriptional
repression of Gli1, Gli2 and kidney patterning genes disrupts renal
morphogenesis. Development. 133:569–578. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bai CB and Joyner AL: Gli1 can rescue the
in vivo function of Gli2. Development. 128:5161–5172.
2001.PubMed/NCBI
|
|
32
|
Di Magno L, Coni S, Di Marcotullio L and
Canettieri G: Digging a hole under Hedgehog: Downstream inhibition
as an emerging anticancer strategy. Biochim Biophys Acta.
1856:62–72. 2015.PubMed/NCBI
|
|
33
|
Reifenberger J, Wolter M, Knobbe CB,
Köhler B, Schönicke A, Scharwächter C, Kumar K, Blaschke B, Ruzicka
T and Reifenberger G: Somatic mutations in the PTCH, SMOH, SUFUH
and TP53 genes in sporadic basal cell carcinomas. Br J Dermatol.
152:43–51. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Bahra M, Kamphues C, Boas-Knoop S, Lippert
S, Esendik U, Schüller U, Hartmann W, Waha A, Neuhaus P, Heppner F,
et al: Combination of hedgehog signaling blockage and chemotherapy
leads to tumor reduction in pancreatic adenocarcinomas. Pancreas.
41:222–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Wang XD, Inzunza H, Chang H, Qi Z, Hu B,
Malone D and Cogswell J: Mutations in the hedgehog pathway genes
SMO and PTCH1 in human gastric tumors. PLoS One. 8:e544152013.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bisht S, Brossart P, Maitra A and Feldmann
G: Agents targeting the hedgehog pathway for pancreatic cancer
treatment. Curr Opin Investig Drugs. 11:1387–1398. 2010.PubMed/NCBI
|
|
37
|
Lauth M and Toftgård R: Non-canonical
activation of GLI transcription factors: Implications for targeted
anti-cancertherapy. Cell Cycle. 6:2458–2463. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Lee Y, Kawagoe R, Sasai K, Li Y, Russell
HR, Curran T and McKinnon PJ: Loss of suppressor-of-fused function
promotes tumorigenesis. Oncogene. 26:6442–6447. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kim WK, Meliton V, Bourquard N, Hahn TJ
and Parhami F: Hedgehog signaling and osteogenic differentiation in
multipotent bone marrow stromal cells are inhibited by oxidative
stress. J Cell Biochem. 111:1199–1209. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Fendrich V, Oh E, Bang S, Karikari C,
Ottenhof N, Bisht S, Lauth M, Brossart P, Katsanis N, Maitra A and
Feldmann G: Ectopic overexpression of Sonic Hedgehog (Shh) induces
stromal expansion and metaplasia in the adult murine pancreas.
Neoplasia. 13:923–930. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Bahra M, Kamphues C, Boas-Knoop S, Lippert
S, Esendik U, Schüller U, Hartmann W, Waha A, Neuhaus P, Heppner F,
et al: Combination of hedgehog signaling blockage and chemotherapy
leads to tumor reduction in pancreatic adenocarcinomas. Pancreas.
41:222–229. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Thayer SP, di Magliano MP, Heiser PW,
Nielsen CM, Roberts DJ, Lauwers GY, Qi YP, Gysin S, Fernández-del
Castillo C, Yajnik V, et al: Hedgehog is an early and late mediator
of pancreatic cancer tumorigenesis. Nature. 425:851–856. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Hu WG, Liu T, Xiong JX and Wang CY:
Blockade of sonic hedgehog signal pathway enhances
antiproliferative effect of EGFR inhibitor in pancreatic cancer
cells. Acta Pharmacol Sin. 28:1224–1230. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Hosoya T, Arai MA, Koyano T, Kowithayakorn
T and Ishibashi M: Naturally occurring small-molecule inhibitors of
hedgehog/GLI-mediated transcription. Chembiochem. 9:1082–1092.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Hyman JM, Firestone AJ, Heine VM, Zhao Y,
Ocasio CA, Han K, Sun M, Rack PG, Sinha S, Wu JJ, et al:
Small-molecule inhibitors reveal multiple strategies for hedgehog
pathway blockade. Proc Natl Acad Sci USA. 106:14132–14137. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Katoh Y and Katoh M: Hedgehog target
genes: Mechanisms of carcinogenesis induced by aberrant hedgehog
signaling activation. Curr Mol Med. 9:873–886. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Xu X, Su B, Xie C, Wei S, Zhou Y, Liu H,
Dai W, Cheng P, Wang F, Xu X and Guo C: Sonic hedgehog-Gli1
signaling pathway regulates the epithelial mesenchymal transition
(EMT) by mediating a new target gene, S100A4, in pancreatic cancer
cells. PLoS One. 9:e96442014.
|
|
48
|
Gill PS and Rosenblum ND: Control of
murine kidney development by sonic hedgehog and its GLI effectors.
Cell Cycle. 5:1426–1430. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
49
|
Onishi H and Katano M: Hedgehog signaling
pathway as a new therapeutic target in pancreatic cancer. World J
Gastroenterol. 20:2335–2342. 2014. View Article : Google Scholar : PubMed/NCBI
|