Introduction
The Ewing's sarcoma family of tumors (ESFT) consists
of a group of neoplasms with a number of characteristics in common,
including the presence of small round cells, a shared
neuroectodermal origin, an immunohistochemical panel positive for
cluster of differentiation (CD)99 and the Ewing's sarcoma
(EWS)-friend leukemia integration 1 (FLI1) transcription factor
translocation t(11;22)(q24;q12), which occurs in 85% of cases
(1,2).
The EWS-E26 transformation-specific (ETS)-related gene (ERG)
translocation t(21;22)(q21;q22) is present in 5–10% of cases
(2). Other rarer translocations
described include the genes ETS variant (ETV)1, ETV4 or fifth
Ewing's sarcoma variant (FEV), forming t(7;22)(p22;q12),
t(17;22)(q12;q12) or t(2;22)(q35;q12), respectively (2,3). Certain
cases have translocations that involve the fused in sarcoma (FUS)
gene, such as FUS-ERG t(16;21)(p11;q24) or FUS-FEV t(2;16)(q35;p11)
(2).
Several tumors that had traditionally been
considered separate entities are now included in this family of
tumors, including skeletal Ewing's sarcoma, extraskeletal Ewing's
sarcoma, primitive neuroectodermal tumors (PNETs) and Askin's tumor
(1,2).
This classification allows rare tumors such as PNETs to benefit
from chemotherapy regimens traditionally reserved for skeletal
Ewing's sarcoma (1,2). Chemotherapy for these patients is based
on the vincristine, doxorubicin (Adriamycin®)and
cyclophosphamide alternating with ifosfamide and etoposide (VAC/IE)
regimen (4). Regimens used in
subsequent lines include cyclophosphamide/topotecan,
irinotecan/temozolomide and docetaxel/gemcitabine (1,2). However,
since patients with sarcomas are typically young and achieve good
clinical status with subsequent lines of chemotherapy (1,2), alternate
therapies must be identified if the disease progresses following
standard chemotherapy. The current report presents a case of a
patient with metastatic extraskeletal Ewing's sarcoma who responded
well to trabectedin.
Case report
A Caucasian 69-year-old female patient with a
history of high blood pressure, diabetes and dyslipidemia presented
to the University Hospital Miguel Servet (Zaragoza, Spain) in June
2011 for diagnosis and treatment of a suspicious soft tissue mass
on the chest wall. The patient neither smoked, had any other toxic
habits or had a family history of neoplasms. The patient had
previously consulted her primary care doctor 7 months earlier (in
November 2010) regarding a soft tissue mass on the chest wall;
however, no scans were performed at that time. Later, in June 2011,
during a pre-operative evaluation for knee surgery, multiple lung
nodules were observed on chest X-ray; thus, a full investigation
was conducted.
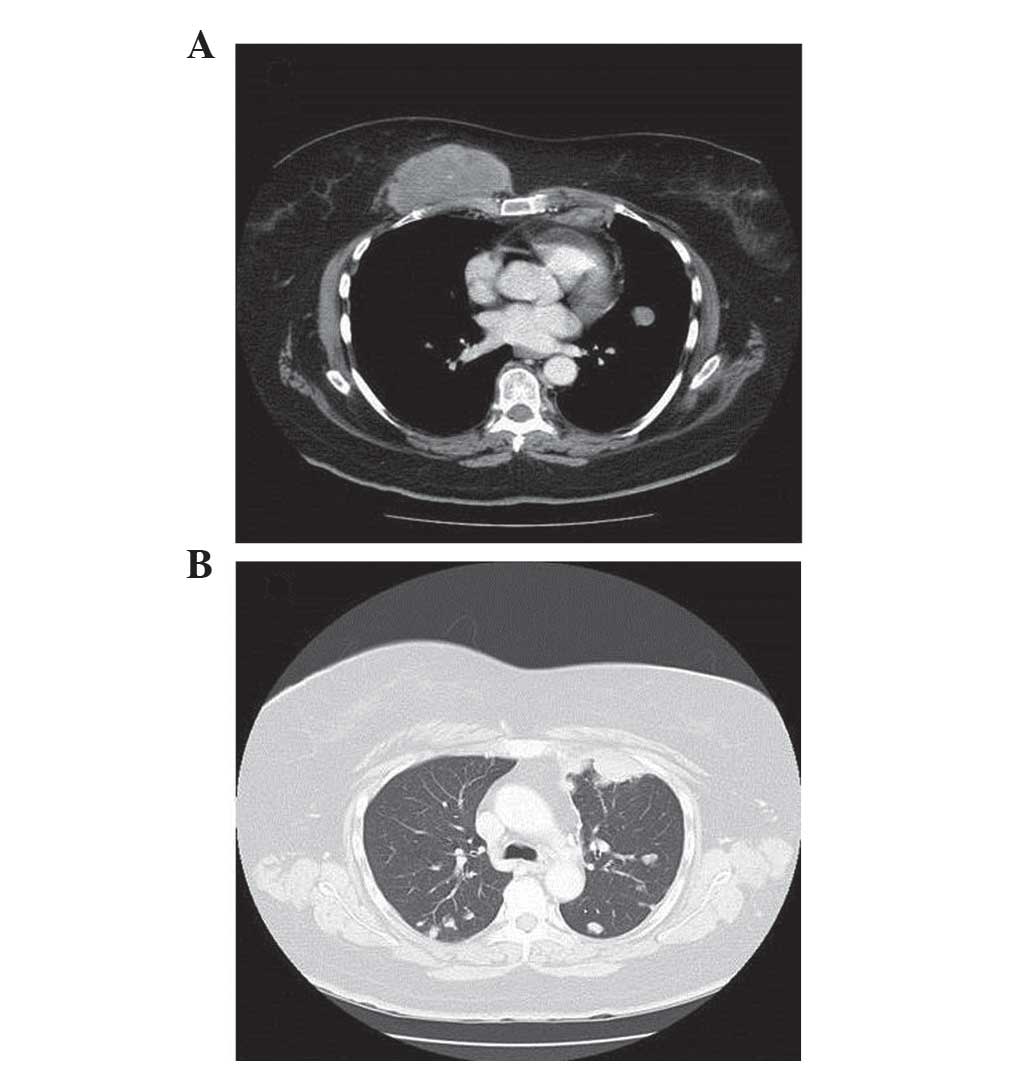
The computed tomography (CT) scan revealed a
9×8×7-cm mass on the anterior chest wall above the pectoral muscle,
two enlarged internal mammary lymph nodes measuring 12 and 16 mm,
respectively, a right axillary lymphadenopathy (14-mm) and multiple
bilateral lung metastases (Fig. 1).
The biopsy of the superficial mass was consistent with a
PNET/extraskeletal Ewing's sarcoma. Immunohistochemical staining
demonstrated that the tumor cells were positive for CD99
(anti-CD99; dilution, 1:20-1:200; catalog number 23079-1-AP;
ProteinTech Group, Inc., Chicago, IL, USA) and vimentin
(anti-vimentin; dilution 1:1,000; catalog number 18-0052;
Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA, USA).
Proliferative activity, detected by the Ki-67 index, was estimated
to be 20%. A molecular analysis was performed by fluorescence in
situ hybridization using a commercially available Vysis LSI
EWSR1 (22q12) Dual Colour, Break Apart Rearrangement Probe (Abbott
Molecular Inc., Des Plaines, IL, USA), which revealed that 8% of
the tumor cells were positive for the EWS-RNA-binding protein 1
translocation. The patient was then referred to the Department of
Oncology at the University Hospital Miguel Servet for assessment.
The patient was diagnosed with metastatic extraskeletal Ewing's
sarcoma. Chemotherapy was initiated with an alternating VAC/IE
regimen (vincristine, 2 mg/m2, Adriamycin®,
75 mg/m2 and cyclophosphamide, 1,200 mg/m2,
alternating with ifosfamide, 1,800 mg/m2 and etoposide,
100 mg/m2).
Following four VAC/IE cycles, in November 2011,
stable disease in the chest wall mass and lungs was observed on CT
scan of the chest. Given this poor response to the VAC/IE regimen
and the patient's advanced age, it was decided to administrate
palliative chemotherapy with a combination of gemcitabine (1,800
mg/m2) and dacarbazine (500 mg/m2) every 2
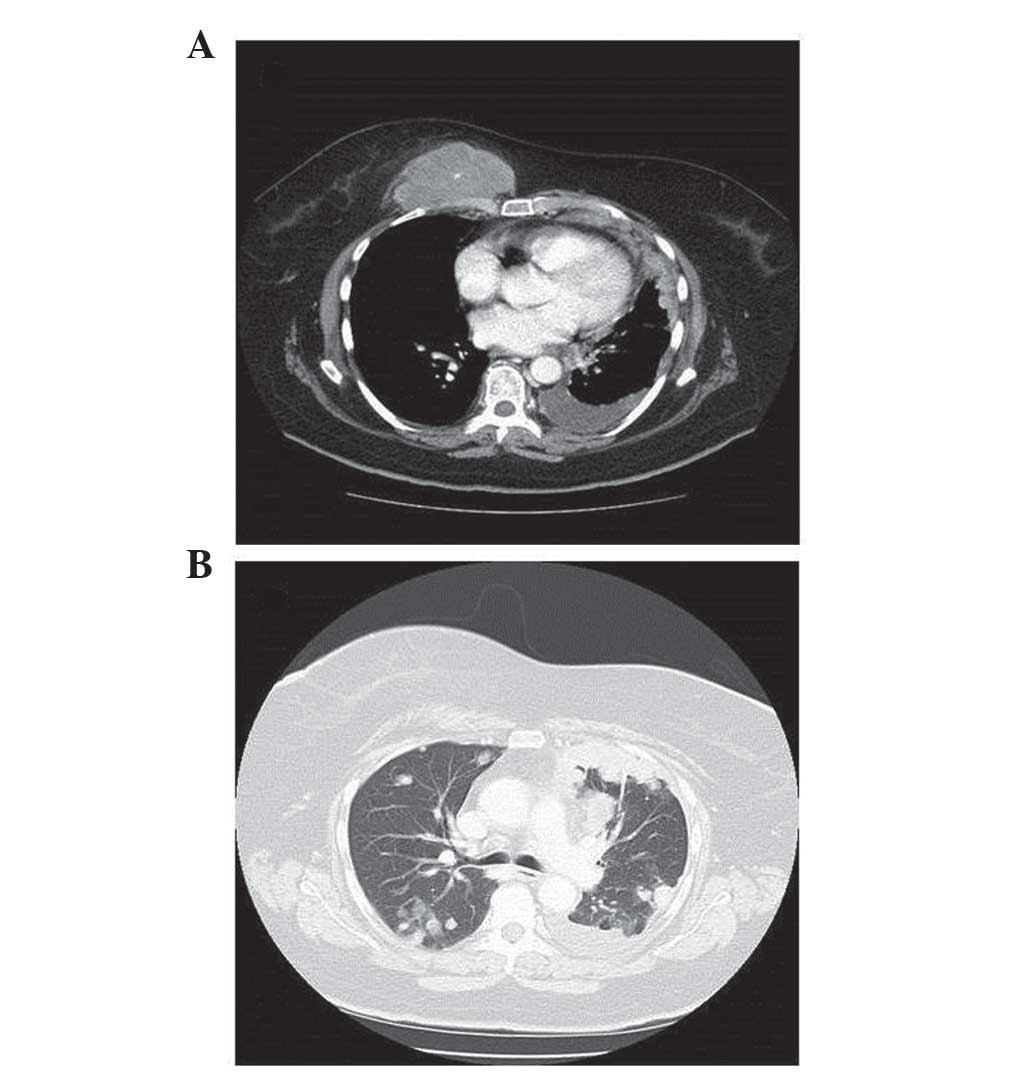
weeks. However, in February 2012, following 8 cycles of treatment,
a CT scan revealed that the lung cancer had progressed in spite of
therapy (Fig. 2).
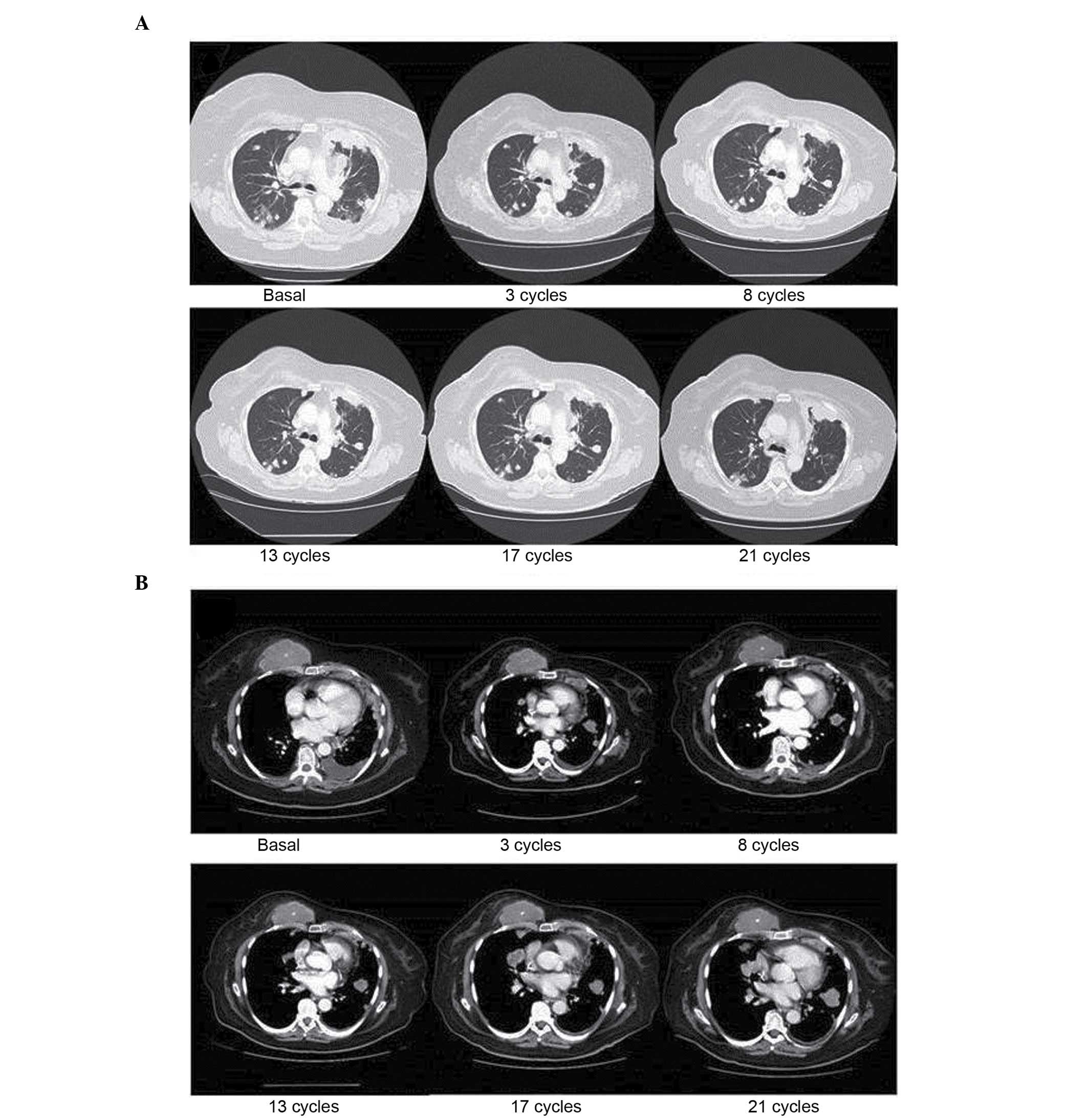
In March 2012, treatment with trabectedin (1.5
mg/m2 as a 24-h continuous infusion every 3 weeks) was
initiated. The patient received a total of 23 cycles between March
2012 and August 2013, with all radiological examinations revealing
stable disease of the chest wall mass and the lung metastases
(Fig. 3). In terms of toxicity, the
patient exhibited only grade 1 asthenia.
From mid 2013, the patient experienced severe lower
back pain and was diagnosed with severe spondyloarthropathy at
L5-S1 with S1 compression of degenerative origin, which was
unrelated to the sarcoma. The Department of Trauma at the
University Hospital Miguel Servet did not consider the patient
eligible for surgery due to her history of cancer. Despite the use
of the three-step analgesic ladder for the management of pain with
escalating opioid dosages, the patient's condition progressively
worsened with serious functional limitation and confinement to bed
due to her lower back pain. In September 2013, the patient was
admitted to hospital, contracted hospital-acquired pneumonia and
succumbed to the disease. Imaging tests performed during this
admission revealed no signs of tumor progression. Therefore, the
patient had stable disease, both clinically and radiographically,
for 18 months from the commencement of treatment with
trabectedin.
Discussion
Ewing's sarcoma belongs to a diverse group of
small-round-cell tumors characterized by the same chromosomal
translocation, t(11;22) (1). ESFT
most commonly affects children, adolescents and young adults
(1). There are no treatment
guidelines for older patients with Ewing's sarcoma. Based in our
own experience with older patients, it may be very difficult to
maintain the full dosages of the drugs that the most aggressive
chemotherapy regimens recommend in this population, as elderly
patients may be at higher risk of toxicity than the young ones due
to factors unrelated to treatment.
Following a poor response to the traditional
first-line treatment regimen of alternating VAC/IE, the present
patient was administered combination therapy with gemcitabine and
dacarbazine every 2 weeks. Studies in paediatric populations have
demonstrated that combination therapy with gemcitabine and
docetaxel exerts antitumor activity against advanced sarcoma
(primarily Ewing's sarcoma) while maintaining a good quality of
life (5,6). Therefore, this combination is usually
recommended as the second-line treatment for patients with Ewing's
sarcoma; however, this regimen was ruled out in the present case
due to the patient's age and health status (7). Instead, a combination of gemcitabine and
dacarbazine appeared to be a suitable regimen for the patient,
based on the palliative approach undertaken and her advanced
age.
The efficacy of the gemcitabine and dacarbazine
regimen in soft tissue sarcomas was assessed in a phase II clinical
trial by García-Del-Muro et al (8) The trial compared the combination of
gemcitabine (180 mg/m2) followed by dacarbazine (500
mg/m2) every 2 weeks with dacarbazine alone (1,200
mg/m2) every 3 weeks. The combination regimen
demonstrated a greater progression-free survival (PFS) rate than
dacarbazine alone, with a similar toxicity profile (8). However, in the present case, the patient
did not benefit from this treatment regimen, as lung progression
occurred following 8 cycles of treatment. It was then decided to
commence treatment with trabectedin, with dosages administered
according to the Summary of Product Characteristics (www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000773/WC500045832.pdf).
Trabectedin (also known as Ecteinascidin 743) is a
drug of marine origin obtained from Ecteinascidia turbinat a
(9). By binding to the minor groove
of DNA, trabectedin can induce damage by altering the normal
function of DNA repair and transcription processes, thus inhibiting
cell proliferation and differentiation and leading to cell death
(9). This drug has demonstrated
efficacy against soft tissue sarcomas in patients pre-treated with
standard chemotherapy in several phase II trials (10–13). Based
on the results of these studies, trabectedin was approved by the
European Medicines Agency (London, UK) for the treatment of adult
patients with advanced-stage soft tissue sarcomas in whom treatment
with anthracyclines and ifosfamide has failed, or for those who are
not candidates for these therapies (10–13).
Although the efficacy is based on findings mainly for liposarcoma
and leiomyosarcoma patients and does not include patients with
Ewing's sarcoma or PNET, its approval has been extended to other
subtypes of sarcoma (10–13), which enables the use of trabectedin as
a treatment in other rare cancer histologies.
Trabectedin has also exhibited efficacy in
translocation-related sarcomas (TRSs), including synovial sarcoma,
alveolar soft part sarcoma and endometrial stromal sarcoma
(14–17). A recent phase II trial in a Japanese
population evaluated the efficacy and safety of trabectedin
compared with second-line or later supportive treatment in TRS
(18). A total of 76 patients with
extraskeletal Ewing's sarcoma, myxoid liposarcoma or synovial
sarcoma were randomized. The analysis of the results revealed
higher rates of PFS and overall survival (OS) that were
statistically significant in favour of treatment with trabectedin,
both in the overall population (median PFS, 5.6 vs. 0.9 months;
hazard ratio, 0.07; P<0.0001) and in the analysis by subtype
(18).
There are limited reported data regarding the
efficacy of trabectedin in the treatment of extraskeletal Ewing's
sarcoma/PNET. It has been demonstrated in vitro that
trabectedin is active against cell lines that express the EWS-FLI1
translocation (19). A number of
phase I clinical trials have reported promising results (20–22), even
achieving complete responses in 2 cases [10 months in the study by
Lau et al (20) and 16 months
in the study by Chu et al (21)]. In spite of these findings, a phase II
clinical trial reported that trabectedin conferred no treatment
benefit in this tumor type (Table I)
(23).
 | Table I.Phase I and II clinical trials of T in
Ewing's sarcoma/PNET. |
Table I.
Phase I and II clinical trials of T in
Ewing's sarcoma/PNET.
| First author
(ref.) | Year | Phase | Dose(infusion
duration) | Patients with Ewing's
sarcoma/PNET, n | CR | PR | SD | PD |
|---|
| Lau (20) | 2005 | I | T: 1,100–1,300
ng/m2 (3 h) q3w | 3 (children) | 1 | 0 | 1 | 1 |
| Papadopoulos
(21) | 2006 | I | Paclitaxel + T:
525–775 ng/m2 (3 h) q2w | 1 (adult) | 1 | 0 | 0 | 0 |
| Dileo (22) | 2007 | I | Cisplatin + T: 0.6
mg/m2 (3 h) q3w | 29 (adults) | 0 | 6 | 4 | 19 |
| Baruchel (23) | 2012 | II | T: 1.5
mg/m2 (24 h) q3w |
8
(children) | 0 | 0 | 1 | 7 |
Recently, Grohar et al (24) highlighted that Ewing's sarcoma is
sensitive to trabectedin, and suggested that the drug's apparent
lack of clinical efficacy in this subtype of sarcoma could be due
to its pharmacokinetics (24). In a
phase II study (23), the level of
trabectedin in the blood was 3.26±2.95 nmol/l, which was lower than
the 5–10 nmol/l level that had demonstrated in vitro
activity (24). By contrast, in the
phase I study by Lau et al (20), the levels of trabectedin reached
6.02±3.28 ng/ml in patients on 1,100 ng/m2 trabectedin
and 10.52±5.00 ng/ml in patients on 1,300 ng/m2
trabectedin.
The study by Grohar et al (24) also analyzed the effect of combining
trabectedin with SN38, an active metabolite of irinotecan. In this
in vitro model, trabectedin blocked EWS-FLI1 and secondarily
suppressed the production of the Werner syndrome adenosine
triphosphate-dependent helicase protein, which is important in
telomere maintenance, thereby sensitizing the cell to the toxic
effect that camptothecin derivatives such as irinotecan exert on
DNA. Thus, the combination of trabectedin and SN38 had a
synergistic effect, requiring a lower concentration of trabectedin
to have an antitumor effect.
In 2013, Cesne et al (25) published a review of five phase II
studies in which 350 patients with soft tissue sarcoma were treated
with trabectedin. The review evaluated the effect of age on the
efficacy and safety of trabectedin. Patients were stratified into
one cohort under 60 years of age (n=267) and another over 60 years
of age (n=83). The retrospective analysis revealed no significant
differences in PFS or OS between the two age groups, and no
evidence of cumulative toxicity was observed. Trabectedin has an
acceptable and manageable safety profile, and its antitumor
activity in elderly patients with soft tissue sarcoma is similar to
that observed in the general population (25). Based on these findings, trabectedin
was recommended as part of palliative care for the patient
described in the present case report.
The current case report highlights the clinical
outcome of a patient with extraskeletal Ewing's sarcoma treated
with trabectedin who achieved a PFS longer than any PFS reported
thus far in the literature, following progression with first- and
second-line chemotherapy. This is one of the few cases
demonstrating that trabectedin has a clinical benefit in this type
of tumor, and also confirms its excellent tolerability profile. The
PFS could have been even longer upon treatment with trabectedin,
since the patient succumbed to causes unrelated to the tumor. It
should be emphasized that the patient received treatment as a 24-h
continuous infusion, and although this regimen did not demonstrate
efficacy in the phase II clinical trial by Baruchel et al
(23), it can be hypothesized that
this dosing regimen may lead to therapeutic levels in the tumor, as
has been observed in vitro (23).
The authors consider that it is important to
continue developing novel drugs for patients with ESFT following
progression upon standard chemotherapy. Trabectedin may represent
an alternative for patients with these tumors, although further
studies are required in order to determine the efficacy of the
treatment as monotherapy and combined with other drugs, including
irinotecan, based on the potential synergy of these two drugs. It
may also be important to identify which tumor subtypes, specific
translocations and patient profiles will benefit the most from
trabectedin.
In summary, the current report presents a case of a
patient with metastatic, pre-treated Ewing's sarcoma achieving
disease stabilization with trabectedin. Based on these findings and
the observed tolerability profile, trabectedin represents an
alternative treatment for patients with ESFT.
Acknowledgements
The authors would like to thank Miss Therese
Chapman, who provided English editing and styling for submission on
behalf of Springer Healthcare Ltd. (Chester, UK). This medical
writing assistance was funded by PharmaMar S.A. (Madrid,
Spain).
Glossary
Abbreviations
Abbreviations:
|
CT
|
computed tomography
|
|
ESFT
|
Ewing's sarcoma family of tumors
|
|
EWS
|
Ewing's sarcoma
|
|
FLI1
|
friend leukemia integration 1
|
|
OS
|
overall survival
|
|
PFS
|
progression-free survival
|
|
PNET
|
primitive neuroectodermal tumors
|
|
TRSs
|
translocation-related sarcomas
|
|
VAC/IE
|
vincristine, doxorubicin
(Adriamycin®) plus cyclophosphamide alternating with
ifosfamide and etoposide
|
References
|
1
|
Delattre O, Zucman J, Melot T, Garau XS,
Zucker JM, Lenoir GM, Ambros PF, Sheer D, TurcCarel C, Triche TJ,
et al: The Ewing family of tumors-a subgroup of small-round-cell
tumors defined by specific chimeric transcripts. N Engl J Med.
331:294–299. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ng TL, O'Sullivan MJ, Pallen CJ, Hayes M,
Clarkson PW, Winstanley M, Sorensen PH, Nielsen TO and Horsman DE:
Ewing sarcoma with novel translocation t(2;16) producing an
in-frame fusion of FUS and FEV. J Mol Diagn. 9:459–463. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Urano F, Umezawa A, Yabe H, Hong W,
Yoshida K, Fujinaga K and Hata J: Molecular analysis of Ewing's
sarcoma: Another fusion gene, EWS-E1AF, available for diagnosis.
Jpn J Cancer Res. 89:703–711. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Grier HE, Krailo MD, Tarbell NJ, Link MP,
Fryer CJ, Pritchard DJ, Gebhardt MC, Dickman PS, Perlman EJ, Meyers
PA, et al: Addition of ifosfamide and etoposide to standard
chemotherapy for Ewing's sarcoma and primitive neuroectodermal
tumor of bone. N Engl J Med. 348:694–701. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mora J, Cruz CO, Parareda A and de Torres
C: Treatment of relapsed/refractory pediatric sarcomas with
gemcitabine and docetaxel. J Pediatr Hematol Oncol. 31:723–729.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rapkin L, Qayed M, Brill P, Martin M,
Clark D, George BA, Olson TA, WasilewskiMasker K, Alazraki A and
Katzenstein HM: Gemcitabine and docetaxel (GEMDOX) for the
treatment of relapsed and refractory pediatric sarcomas. Pediatr
Blood Cancer. 59:854–858. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fox E, Patel S, Wathen JK, Schuetze S,
Chawla S, Harmon D, Reinke D, Chugh R, Benjamin RS and Helman LJ:
Phase II study of sequential gemcitabine followed by docetaxel for
recurrent Ewing sarcoma, osteosarcoma, or unresectable or locally
recurrent chondrosarcoma: Results of Sarcoma Alliance for Research
Through Collaboration Study 003. Oncologist. 17:3212012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
García-del-Muro X, López-Pousa A, Maurel
J, Martín J, Martínez-Trufero J, Casado A, Gómez-España A, Fra J,
Cruz J, Poveda A, et al: Randomized phase II study comparing
gemcitabine plus dacarbazine versus dacarbazine alone in patients
with previously treated soft tissue sarcoma: A Spanish Group for
Research on Sarcomas study. J Clin Oncol. 29:2528–2533. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
D'Incalci M and Galmarini CM: A review of
trabectedin (ET-743): A unique mechanism of action. Mol Cancer
Ther. 9:2157–2163. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
GarciaCarbonero R, Supko JG, Manola J,
Seiden MV, Harmon D, Ryan DP, Quigley MT, Merriam P, Canniff J,
Goss G, et al: Phase II and pharmacokinetic study of ecteinascidin
743 in patients with progressive sarcomas of soft tissues
refractory to chemotherapy. J Clin Oncol. 22:1480–1490. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
LeCesne A, Blay JY, Judson I, van Oosterom
A, Verweij J, Radford J, Lorigan P, Rodenhuis S, Ray-Coquard I,
Bonvalot S, et al: Phase II study of ET-743 in advanced soft tissue
sarcomas: A European Organisation for the Research and Treatment of
Cancer (EORTC) soft tissue and bone sarcoma group trial. J Clin
Oncol. 23:576–584. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Yovine A, Riofrio M, Blay JY, Brain E,
Alexandre J, Kahatt C, Taamma A, Jimeno J, Martin C, Salhi Y, et
al: Phase II study of ecteinascidin-743 in advanced pretreated soft
tissue sarcoma patients. J Clin Oncol. 22:890–899. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Demetri GD, Chawla SP, von Mehren M, Ritch
P, Baker LH, Blay JY, Hande KR, Keohan ML, Samuels BL, Schuetze S,
et al: Efficacy and safety of trabectedin in patients with advanced
or metastatic liposarcoma or leiomyosarcoma after failure of prior
anthracyclines and ifosfamide: Results of a randomized phase II
study of two different schedules. J Clin Oncol. 27:4188–4196. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
LeCesne A, Cresta S, Maki RG, Blay JY,
Verweij J, Poveda A, Casali PG, Balaña C, Schöffski P, Grosso F, et
al: A retrospective analysis of antitumor activity with trabectedin
in translocation-related sarcomas. Eur J Cancer. 48:3036–3044.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Pink D, BertzLepel J, Busemann C, Bitz U
and Reichardt P: Efficacy of trabectedin in patients with advanced
or metastatic alveolar soft-part sarcoma. Onkologie. 35:249–252.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Grosso F, Dileo P, Sanfilippo R,
Stacchiotti S, Bertulli R, Piovesan C, Jimeno J, D'Incalci M,
Gescher A and Casali PG: Steroid premedication markedly reduces
liver and bone marrow toxicity of trabectedin in advanced sarcoma.
Eur J Cancer. 42:1484–1490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sanfilippo R, Dileo P, Blay JY,
Constantinidou A, Le Cesne A, Benson C, Vizzini L, Contu M, Daldi
GG, Dei Tos AP and Casali PG: Trabectedin in advanced synovial
sarcomas: A multicenter retrospective study from four European
institutions and the Italian Rare Cancer Network. Anticancer Drugs.
26:678–681. 2015.PubMed/NCBI
|
|
18
|
Takahashi S, Araki N, Sugiura H, Ueda T,
Takahashi M, Morioka H, Yonemoto T, Hiraga H, Hiruma T, Kunisada T,
et al: A randomized phase II study comparing trabectedin (T) and
best supportive care (BSC) in patients (pts) with
translocation-related sarcomas (TRS). J Clin Oncol (ASCO Annual
Meeting abstracts). 32:((Suppl; abstr 10524)). 5s2014.
|
|
19
|
Grohar PJ, Griffin LB, Yeung C, Chen QR,
Pommier Y, Khanna C, Khan J and Helman LJ: Ecteinascidin 743
interferes with the activity of EWS-FLI1 in Ewing sarcoma cells.
Neoplasia. 13:145–153. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lau L, Supko JG, Blaney S, Hershon L,
Seibel N, Krailo M, Qu W, Malkin D, Jimeno J, Bernstein M, et al: A
phase I and pharmacokinetic study of ecteinascidin-743 (Yondelis)
in children with refractory solid tumors. A Children's Oncology
Group study. Clin Cancer Res. 11:672–677. 2005.PubMed/NCBI
|
|
21
|
Chu Q, Mita A, Forouzesh B, Tolcher AW,
Schwartz G, Nieto A, SotoMatos A, Alfaro V, Lebedinsky C and
Rowinsky EK: Phase I and pharmacokinetic study of sequential
paclitaxel and trabectedin every 2 weeks in patients with advanced
solid tumors. Clin Cancer Res. 16:2656–2665. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dileo P, Grosso F, Casanova M, Jimeno J,
Marsoni S, Sanfilippo R, Podda M, Ferrari S, Bertulli R and Casali
P: Trabectedin (T) in metastatic Ewing's family tumors (EFT)
patients (pts) progressing after standard chemotherapy. Journal of
Clinical Oncology, 2007. In: ASCO Annual Meeting Proceedings
(Post-Meeting Edition). 25(18S): (June 20 Supplement). pp.
100402007
|
|
23
|
Baruchel S, Pappo A, Krailo M, Baker KS,
Wu B, Villaluna D, LeeScott M, Adamson PC and Blaney SM: A phase II
trial of trabectedin in children with recurrent rhabdomyosarcoma,
Ewing sarcoma and non-rhabdomyosarcoma soft tissue sarcomas: A
report from the Children's Oncology Group. Eur J Cancer.
48:579–585. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Grohar PJ, Segars LE, Yeung C, Pommier Y,
D'Incalci M, Mendoza A and Helman LJ: Dual targeting of EWS-FLI1
activity and the associated DNA damage response with trabectedin
and SN38 synergistically inhibits Ewing sarcoma cell growth. Clin
Cancer Res. 20:1190–1203. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Cesne AL, Judson I, Maki R, Grosso F,
Schuetze S, Mehren MV, Chawla SP, Demetri GD, Nieto A, Tanovic A
and Blay JY: Trabectedin is a feasible treatment for soft tissue
sarcoma patients regardless of patient age: A retrospective pooled
analysis of five phase II trials. Br J Cancer. 109:1717–1724. 2013.
View Article : Google Scholar : PubMed/NCBI
|