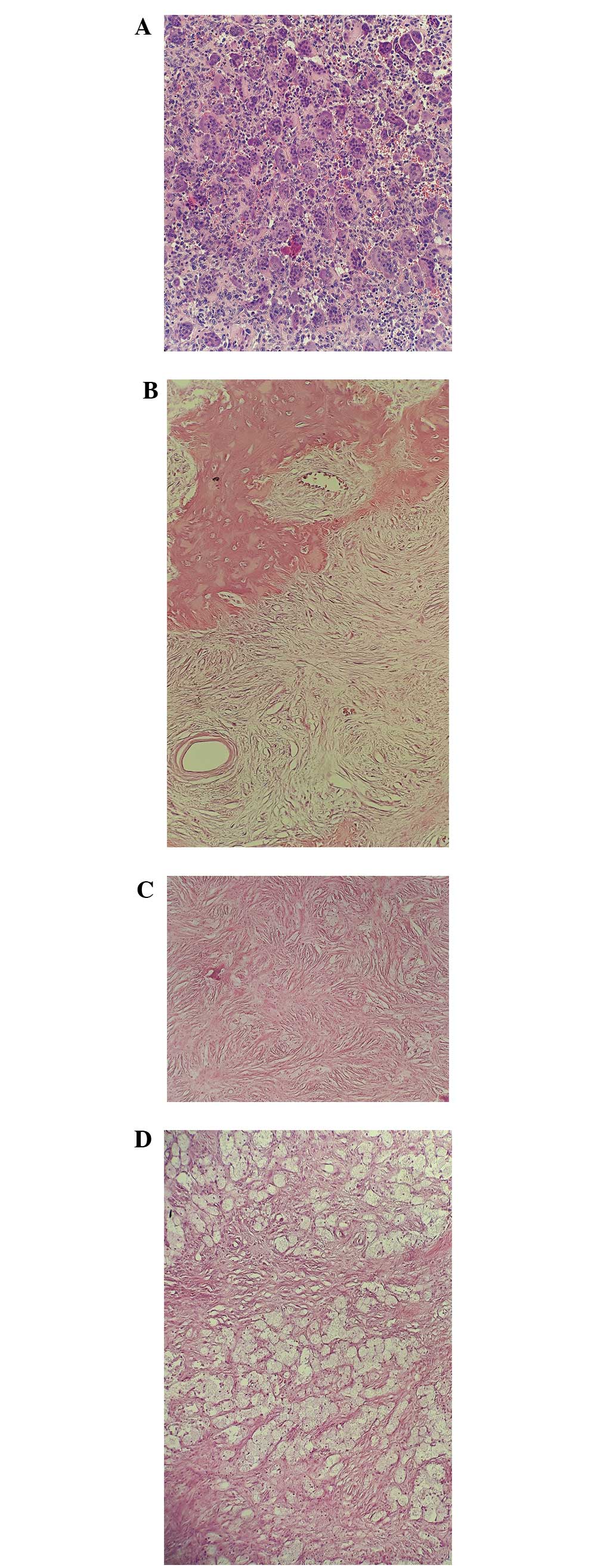
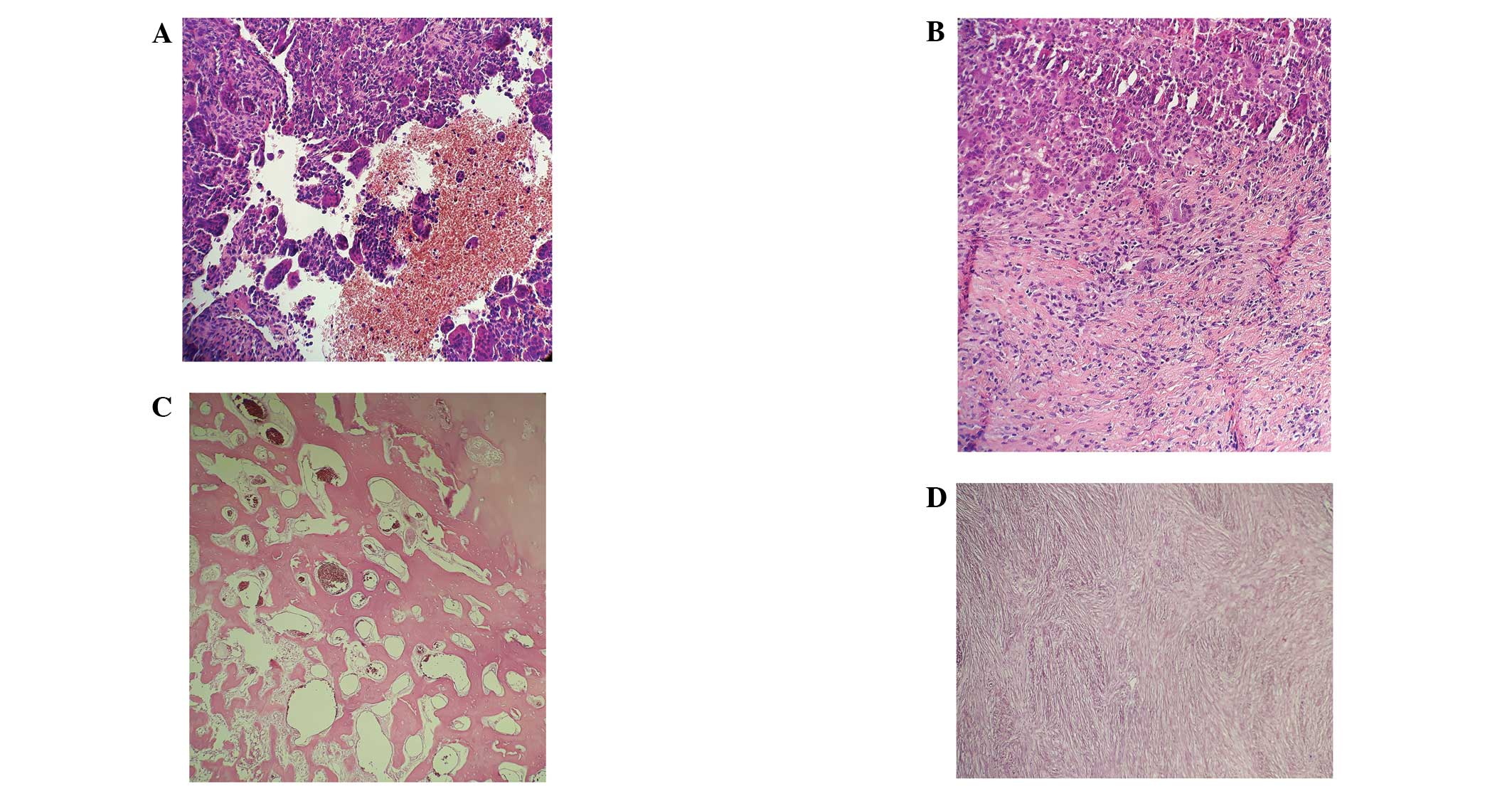
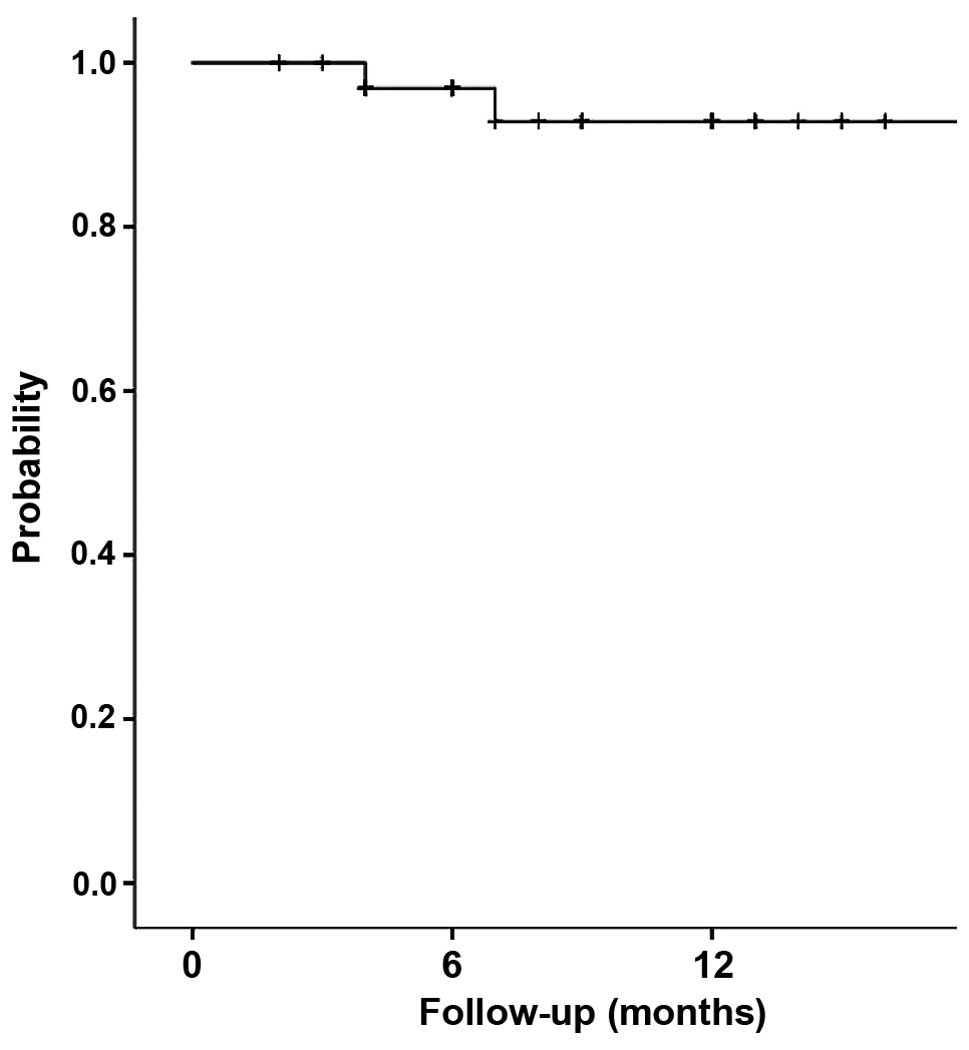
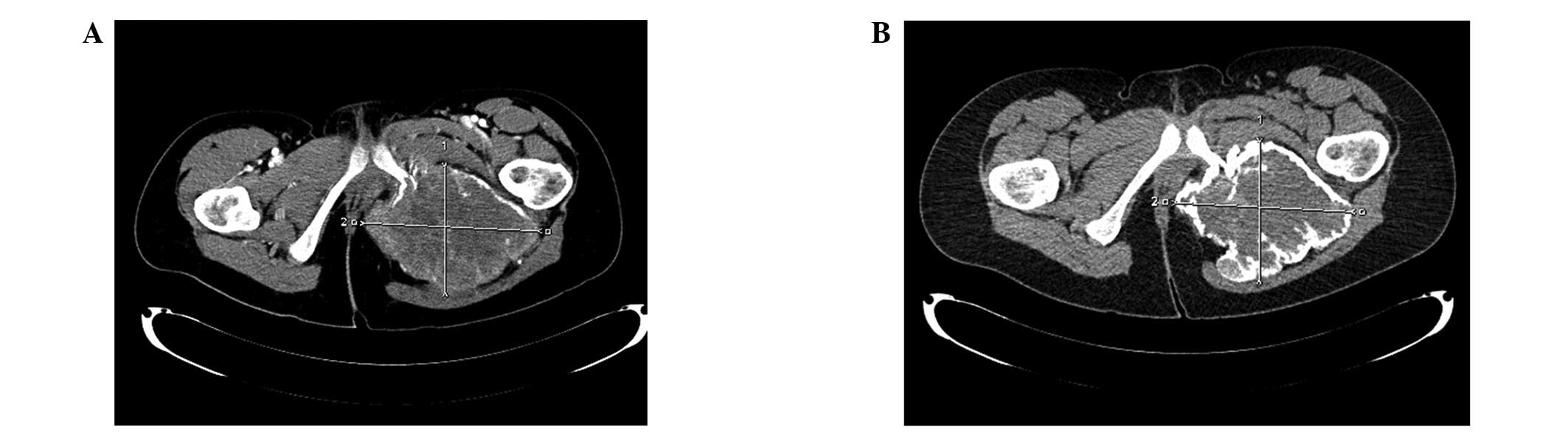
|
1
|
Amanatullah DF, Clark TR, Lopez MJ, Borys
D and Tamurian RM: Giant cell tumor of bone. Orthopedics.
37:112–120. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Jagiello-Wieczorek E, Pieńkowski A and
Rutkowski P: Denosumab for treating giant cell tumor of bone.
Expert Opinion on Orphan Drugs. 3:1219–1229. 2015. View Article : Google Scholar
|
|
3
|
Thomas DM and Skubitz KM: Giant cell tumor
of bone. Curr Opin Oncol. 21:338–344. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Skubitz KM: Giant cell tumor of bone:
Current treatment options. Curr Treat Options Oncol. 15:507–518.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Campanacci M, Baldini N, Boriani S and
Sudanese A: Giant-cell tumor of bone. J Bone Joint Surg Am.
69:106–114. 1987. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Dominkus M, Ruggieri P, Bertoni F,
Briccoli A, Picci P, Rocca M and Mercuri M: Histologically verified
lung metastases in benign giant cell tumours - 14 cases from a
single institution. Int Orthop. 30:499–504. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
van der Heijden L, Dijkstra PD, van de
Sande MA, Kroep JR, Nout RA, van Rijswijk CS, Bovée JV, Hogendoorn
PC and Gelderblom H: The clinical approach toward giant cell tumor
of bone. Oncologist. 19:550–561. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fletcher DM: WHO Classification of Tumours
of Soft Tissue and Bone. 4th. IARC Press; Lyon: 2013
|
|
9
|
Greenspan A, Jundt G and Remagen W:
Differential Diagnosis in Orthopaedic Oncology. 2nd. Lippincott
Williams & Wilkins; Philadelphia, PA: 2007, PubMed/NCBI
|
|
10
|
Biermann JS: Updates in the treatment of
bone cancer. J Natl Compr Canc Netw. 11:(Suppl 5). S681–S683.
2013.
|
|
11
|
Athanassou NA, Bensal M, Forsyth R, et al:
Giant cell tumor of boneWHO Classification of Tumours of Soft
Tissue and Bone. Fletcher CDM, Bridge JA, Hogendoorn PCW and
Mertens F: IARC Press; Lyon: pp. 321–324. 2013
|
|
12
|
Hemingway F, Taylor R, Knowles HJ and
Athanasou NA: RANKL-independent human osteoclast formation with
APRIL, BAFF, NGF, IGF I and IGF II. Bone. 48:938–944. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Wu PF, Tang JY and Li KH: RANK pathway in
giant cell tumor of bone: Pathogenesis and therapeutic aspects.
Tumour Biol. 36:495–501. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Chakarun CJ, Forrester DM, Gottsegen CJ,
Patel DB, White EA and Matcuk GR Jr: Giant cell tumor of bone:
Review, mimics, and new developments in treatment. Radiographics.
33:197–211. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Thomas DM: RANKL, denosumab, and giant
cell tumor of bone. Curr Opin Oncol. 24:397–403. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Roux S, Amazit L, Meduri G,
Guiochon-Mantel A, Milgrom E and Mariette X: RANK (receptor
activator of nuclear factor kappa B) and RANK ligand are expressed
in giant cell tumors of bone. Am J Clin Pathol. 117:210–216. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Morgan T, Atkins GJ, Trivett MK, Johnson
SA, Kansara M, Schlicht SL, Slavin JL, Simmons P, Dickinson I,
Powell G, et al: Molecular profiling of giant cell tumor of bone
and the osteoclastic localization of ligand for receptor activator
of nuclear factor kappaB. Am J Pathol. 167:117–128. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liao TS, Yurgelun MB, Chang SS, Zhang HZ,
Murakami K, Blaine TA, Parisien MV, Kim W, Winchester RJ and Lee
FY: Recruitment of osteoclast precursors by stromal cell derived
factor-1 (SDF-1) in giant cell tumor of bone. J Orthop Res.
23:203–209. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Clézardin P: The role of
RANK/RANKL/osteoprotegerin (OPG) triad in cancer-induced bone
diseases: Physiopathology and clinical implications. Bull Cancer.
98:837–846. 2011.(In French). PubMed/NCBI
|
|
20
|
Forsyth RG, De Boeck G, Baelde JJ,
Taminiau AH, Uyttendaele D, Roels H, Praet MM and Hogendoorn PC:
CD33+ CD14- phenotype is characteristic of multinuclear
osteoclast-like cells in giant cell tumor of bone. J Bone Miner
Res. 24:70–77. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Behjati S, Tarpey PS, Presneau N, Scheipl
S, Pillay N, Van Loo P, Wedge DC, Cooke SL, Gundem G, Davies H, et
al: Distinct H3F3A and H3F3B driver mutations define
chondroblastoma and giant cell tumor of bone. Nat Genet.
45:1479–1482. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Huvos AG: Bone TumorsDiagnosis, treatment
and prognosis. 2nd. W.B. Saunders; Philadelphia, PA: 1990
|
|
23
|
Enneking WF: Staging musculoskeletal
tumorsMusculoskeletal Tumor Surgery. Churchill Livingstone; New
York, NY: pp. 87–88. 1983
|
|
24
|
Nielsen GP and Rosenberg AE: Diagnostic
Pathology: Bone. Amirsys Lippincott Williams & Wilkins;
Philadelphia, PA: 2012
|
|
25
|
Dufresne A, Derbel O, Cassier P, Vaz G,
Decouvelaere AV and Blay JY: Giant-cell tumor of bone, anti-RANKL
therapy. Bonekey Rep. 1:1492012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Shehadeh A, Noveau J, Malawer M and
Henshaw R: Late complications and survival of endoprosthetic
reconstruction after resection of bone tumors. Clin Orthop Relat
Res. 468:2885–2895. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Jeys LM, Suneja R, Chami G, Grimer RJ,
Carter SR and Tillman RM: Impending fractures in giant cell tumors
of the distal femur: Incidence and outcome. Int Orthop. 30:135–138.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Tse LF, Wong KC, Kumta SM, Huang L, Chow
TC and Griffith JF: Bisphosphonates reduce local recurrence in
extremity giant cell tumor of bone: A case-control study. Bone.
42:68–73. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Branstetter DG, Nelson SD, Manivel JC,
Blay JY, Chawla S, Thomas DM, Jun S and Jacobs I: Denosumab induces
tumor reduction and bone formation in patients with giant-cell
tumor of bone. Clin Cancer Res. 18:4415–4424. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Steensma MR, Tyler WK, Shaber AG, Goldring
SR, Ross FP, Williams BO, Healey JH and Purdue PE: Targeting the
giant cell tumor stromal cell: Functional characterization and a
novel therapeutic strategy. PLoS One. 8:e691012013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Thomas D, Henshaw R, Skubitz K, Chawla S,
Staddon A, Blay JY, Roudier M, Smith J, Ye Z, Sohn W, et al:
Denosumab in patients with giant-cell tumour of bone: An
open-label, phase 2 study. Lancet Oncol. 11:275–280. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
32
|
López-Pousa A, Martín Broto J, Garrido T
and Vázquez J: Giant cell tumour of bone: New treatments in
development. Clin Transl Oncol. 17:419–430. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chawla S, Henshaw R, Seeger L, Choy E,
Blay JY, Ferrari S, Kroep J, Grimer R, Reichardt P, Rutkowski P, et
al: Safety and efficacy of denosumab for adults and skeletally
mature adolescents with giant cell tumour of bone: Interim analysis
of an open-label, parallel-group, phase 2 study. Lancet Oncol.
14:901–908. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
National Institutes of Health, . Common
Terminology Criteria for Adverse Events (CTCAE) Version 4.0.
http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf
|
|
35
|
Rutkowski P, Ferrari S, Grimer RJ, Stalley
PD, Dijkstra SP, Pienkowski A, Vaz G, Wunder JS, Seeger LL, Feng A,
et al: Surgical downstaging in an open-label phase ii trial of
denosumab in patients with giant cell tumor of bone. Ann Surg
Oncol. 22:2860–2868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Bukata SV, Sudan M, Mendanha W, et al:
Considerations for long-term maintenance treatment with denosumab
for stable inoperable Giant Cell Tumor: Making a case for spacing
of doses after initial response. Connective Tissue Oncology
Society: Abstract 047. 2015.
|