Introduction
Lung adenocarcinoma is the most frequent
histological type of lung cancer, and the rates of adenocarcinoma
are increasing in the majority of countries (1). An important step in tumor progression is
the acquisition of invasive properties by tumor cells. Within lung
adenocarcinoma, certain scholars suggested that invasiveness may
represent the developmental process of tumor from adenocarcinoma
in situ (AIS) and minimally invasive adenocarcinoma (MIA) to
lepidic predominant adenocarcinoma (LPA) (2). The theoretic transition of AIS/MIA to
LPA as a model was thus proposed in our study.
Recent studies have shown that the tumor
microenvironment and the neoplastic cells act in concert to promote
the growth and progression of the tumor mass (3,4). In the
stroma of several tumor types, a critical role has been reported
for tumor-associated macrophages (TAMs), which exist in the tumor
microenvironment (5). Although there
is a growing body of preclinical and clinical evidence associating
TAMs with poor prognosis of cancer patients (5), their roles in the progression of lung
adenocarcinoma remain mostly unknown. A characterized progression
that epithelial-derived tumor cells undergo is termed the
epithelial-mesenchymal transition (EMT), which involves loss of
polarity and adhesion, increased mobility and invasiveness, and
acquisition of an invasive mesenchymal phenotype (6). However, the role of TAMs and their
association with EMT in the progression from AIS/MIA to LPA are
still unclear.
The present study investigated the clinical
differences between AIS/MIA and LPA. We hypothesized that the tumor
microenvironment serves a role in such differences. The present
study intended to directly detect the expression of the TAMs marker
cluster of differentiation (CD) 68 and a number of potential
chemokines that are associated with invasion and progression of
cancer cells, and to explore their prognostic value in lung
adenocarcinoma, as well as to further study the association between
TAMs and EMT. It was of great significance to further clarify the
biological function of TAMs in the development of lung
adenocarcinoma, and thereby open up a new avenue for the
comprehensive treatment of cancer.
Materials and methods
Tissue samples
A cohort of 285 consecutive patients who received
complete pulmonary resection and systematic lymph node dissection
for stage I–IIIA lung adenocarcinoma (37 cases of AIS/MIA, 127
cases of LPA and 121 cases of other cancer types) at Tianjin
Medical University Cancer Institute and Hospital (Tianjin, China)
from September 2004 to September 2008 were included in the present
retrospective study. Inclusion criteria were as follows: i) No
reception of neoadjuvant therapy; ii) no presence of metastatic
diseases pre-operatively; iii) complete clinicopathological and
follow-up data available; and iv) survival of >1 month after
surgery. The histology of all cases was re-assessed by two
independent pathologists (Professor B.S. Sun and Dr Z.F. Zhang)
according to the latest pathological classification (2). Tumor staging was based on the
International Association for the Study of Lung Cancer
tumor-node-metastasis (TNM) 7th classification system (7). All patients were followed up until
September 1, 2013. The mean follow-up time was 42 months (ranging
from 2 to 100 months). Patients who were still alive after the last
follow-up and who were lost to follow-up were censored in the
present study. The research ethics committee of Tianjin Cancer
Institute and Hospital (Tianjin, China) provided ethical approval
for the study of human subjects, and all patients provided written
informed consent.
Tissue microarray and
immunohistochemistry or immunofluorescent double staining
For tissue microarray construction, two experienced
pathologists (Professor B.S. Sun and Dr Z.F. Zhang) reviewed the
hematoxylin and eosin-stained sections from each paraffin-embedded,
formalin-fixed block. The most representative areas of the tumor
regions were carefully selected and sampled for tissue microarray
collector blocks.
Immunohistochemistry was performed with mouse
anti-human CD68 monoclonal antibody at 1:5 dilution (clone KP1;
Abcam, Cambridge, UK), rabbit anti-human colony-stimulating factor
(CSF)-1 polyclonal antibody at 1:100 dilution (BA0750; Wuhan Boster
Biological Technology, Ltd., Wuhan, China), anti-interleukin (IL)-6
at 1:100 dilution (BS0781R; BIOSS, Beijing, China), anti-matrix
metalloproteinase (MMP)-2 at 1:400 dilution (ab37150; Abcam),
anti-E-cadherin at 1:200 dilution (sc-7,870; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA) and anti-Snail at 1:25
dilution (AP2054a; Abgent, Inc., San Diego, CA, USA).
Immunofluorescent double staining was used to detect CD68 (1:10
dilution; clone KP1; Abcam) and E-cadherin (1:300 dilution;
sc-7870, Santa Cruz Biotechnology, Inc.). After deparaffinization,
rehydration and heat-induced antigen retrieval, tissues were
immersed in methanol containing 3% hydrogen peroxide for 20 min.
Then, the sections were incubated with the above primary antibodies
overnight at 4°C. Following 30 min of incubation with the
corresponding horseradish peroxidase-labeled secondary antibody
(1:200 dilution; PV-6000; Beijing Zhongshan Golden Bridge
Biotechnology Co. Ltd., Beijing, China) or fluorochrome-conjugated
secondary antibody [Alexa Fluor 594-conjugated anti-rabbit
immunoglobulin G (IgG) (1:400 dilution; ZF-0516; Zhongshan Golden
Bridge Biotechnology Co. Ltd.) or Alexa Fluor 488-conjugated
anti-mouse IgG (1:400 dilution; ZF-0512; Zhongshan Golden Bridge
Biotechnology Co. Ltd.) at room temperature, the
immunohistochemical sections were developed in a
3,3′-diaminobenzidine solution under microscopic observation and
counterstained with hematoxylin, and the immunofluorescent sections
were then stained with DAPI to visualize the nuclei. Negative
control slides with the primary antibodies omitted were included in
all assays.
Two pathologists (Professor B.S. Sun and Dr Z.F.
Zhang) independently evaluated the staining of all anonymized
samples. Five different areas at ×400 magnification from each
sample were systematically evaluated. The mean value of five scores
was considered representative of one tumor. For CSF-1 and IL-6
staining in tumor cells, the sum of staining intensity (0=negative;
1=weak; 2=intermediate; and 3=strong) and percentage of positive
tumor cells (0=none or <5; 1=5–25; 2=25–50; and 3>50%) was
calculated (8). The same staining
extent evaluation was used to assess MMP-2, E-cadherin and Snail
immunoreactivity. Scores between 0 and 2 were regarded as negative,
while and scores between 3 and 6 were considered as positive. The
number of CD68+ TAMs was analyzed as previously described (9). Briefly, the areas counted corresponded
to the tumor stroma. The alveolar spaces in the tumor parts with
lepidic pattern were deducted. According to the median number of
stromal TAMs per high-power field (hpf) at ×400 magnification,
tissue samples were divided into three grades: (−) = No
infiltration; (+) = infiltration level below the median; and (++) =
infiltration level above the median.
Statistical analysis
All statistical analyses were carried out using SPSS
version 16.0 statistical software (SPSS, Inc., Chicago, IL, USA).
The median extent of infiltration in tumor stroma was used as the
cut-off point for assigning tumor samples. The association between
ranked data and clinical parameters was analyzed by χ2
test. Overall survival (OS) was defined as the interval between
surgery and mortality or last observation. Disease-free survival
(DFS) was measured from the date of resection until detection of
recurrent tumor or last follow-up assessment. OS and DFS were
analyzed using the Kaplan-Meier method and the log-rank test, and
multivariate analysis was tested with the Cox proportional hazard
model. P<0.05 was considered to indicate a statistically
significant difference.
Results
Patient characteristics
For the purposes of the study, patients were
excluded due to meet the aforementioned criteria: Patients received
neoadjuvant therapy (n=3) or succumbed within 1 month after surgery
(n=2), thus leaving 285 cases for analysis (Table I). The median age of the patients at
the time of surgery was 60 years (range, 36–79 years). Of all
patients, 47.0% were men, 47.0% were former or current smokers, and
44.2% had stage I disease. Patients were classified into three
categories according to histological type, with 37 cases of AIS/MIA
(13.0%), 127 cases of LPA (44.6%) and 121 cases of other cancer
types (42.4%). Five patients were excluded from the study, since
their pathological types did not belong to AIS/MIA or LPA, but to
other types of adenocarcinoma.
 | Table I.Association between adenocarcinoma
subtype, TAMs marker CD68 expression and clinicopathological
factors. |
Table I.
Association between adenocarcinoma
subtype, TAMs marker CD68 expression and clinicopathological
factors.
|
| Adenocarcinoma
subtype |
| TAMs marker CD68
expression |
|
|---|
|
|
|
|
|
|
|---|
| Clinicopathological
parameters | AIS/MIA | LPA | Total (%) | P-value | − | + | ++ | Total (%) | P-value |
|---|
| Gender |
|
|
| 0.093 |
|
|
|
| 0.264 |
| Male | 20 | 49 | 69 (42.1) |
| 20 | 36a | 78a | 134 (47.0) |
|
|
Female | 17 | 78 | 95 (57.9) |
| 34 | 37 | 80b | 151 (53.0) |
|
| Age |
|
|
| 0.341 |
|
|
|
| 0.537 |
| >60
years | 19 | 54 | 73 (44.5) |
| 21 | 32a | 75c | 128 (44.9) |
|
| ≤60
years | 18 | 73 | 91 (55.5) |
| 33 | 41 | 83c | 157 (55.1) |
|
| Smoking status |
|
|
| 0.140 |
|
|
|
| 0.134 |
| Never
smoked | 18 | 79 | 97 (59.1) |
| 33 | 32 | 86a | 151 (53.0) |
|
|
Smoker | 19 | 48 | 67 (40.9) |
| 21 | 41a | 72b | 134 (47.0) |
|
| P-T stage |
|
|
| <0.001 |
|
|
|
| 0.617 |
| T1 | 37 | 61 | 98 (59.8) |
| 25 | 32 | 66c | 123 (43.2) |
|
| T2 | 0 | 40 | 40 (24.4) |
| 20 | 26 | 50 | 96 (33.7) |
|
| T3 | 0 | 26 | 26 (15.8) |
| 9 | 15a | 42c | 66 (23.1) |
|
| P-TNM stage |
|
|
| 0.030 |
|
|
|
| 0.440 |
| I | 18 | 58 | 76 (46.3) |
| 26 | 34 | 66c | 126 (44.2) |
|
| II | 14 | 27 | 41 (25.0) |
| 12 | 10 | 37 | 59 (20.7) |
|
|
IIIA | 5 | 42 | 47 (28.7) |
| 16 | 29a | 55c | 100 (35.1) |
|
| Number of involved
nodal stations |
|
|
| <0.001 |
|
|
|
| 0.125 |
| ≤1 | 37 | 88 | 125 (76.2) |
| 40 | 54 | 99c | 193 (67.7) |
|
|
>1 | 0 | 39 | 39 (23.8) |
| 14 | 19a | 59c | 92 (32.3) |
|
| Adjuvant
chemotherapy |
|
|
| 0.122 |
|
|
|
| 0.235 |
|
Yes | 19 | 83 | 102 (62.2) |
| 28 | 48a | 100b | 176 (61.8) |
|
| No | 18 | 44 | 62 (37.8) |
| 26 | 25 | 58a | 109 (38.2) |
|
| Adjuvant
radiotherapy |
|
|
| 0.403 |
|
|
|
| 0.879 |
|
Yes | 8 | 20 | 28 (17.1) |
| 7 | 11a | 25 | 43 (15.1) |
|
| No | 29 | 107 | 136 (82.9) |
| 47 | 62 | 133d | 242 (84.9) |
|
| MMP-2
expression |
|
|
| 0.057 |
|
|
|
| 0.009 |
| − | 22 | 53 | 75 (45.7) |
| 27 | 35a | 49 | 111 (38.9) |
|
| + | 15 | 74 | 89 (54.3) |
| 27 | 38 | 109d | 174 (61.1) |
|
| E-cadherin
expression |
|
|
| 0.038 |
|
|
|
| 0.038 |
| − | 15 | 76 | 91 (55.5) |
| 19 | 31 | 85c | 135 (47.4) |
|
| + | 22 | 51 | 73 (44.5) |
| 35 | 42a | 73 | 150 (52.6) |
|
| Snail
expression |
|
|
| 0.010 |
|
|
|
| 0.004 |
| − | 16 | 28 | 44 (26.8) |
| 24 | 26 | 35 | 85
(29.8) |
|
| + | 21 | 99 | 120 (73.2) |
| 30 |
47a | 123d | 200 (70.2) |
|
| CD68
expression |
|
|
| 0.012 |
|
|
|
| − |
| − | 11 | 17 | 28 (17.1) |
| − | − | − | − |
|
| + | 12 | 29 | 41 (25.0) |
| − | − | − | − |
|
| ++ | 14 | 81 | 95 (57.9) |
| − | − | − | − |
|
Immunohistochemistry staining results,
and difference between AIS/MIA and LPA
CD68 was stained mostly in the cytoplasm of TAMs,
but not in tumor cells, lymphocytes, plasmocytes or fibroblasts
(Fig. 1A-D). Expression of IL-6
(Fig. 1E and F) and CSF-1 (Fig. 1G and H) was observed in the cytoplasm
of tumor cells, with sporadic positive staining in inflammatory
cells such as lymphocytes, macrophages and plasmocytes. E-cadherin
positive staining was located in the cytomembrane (Fig. 1I and J), and the expression of Snail
in the majority of tumor cells displayed a nuclear staining pattern
(Fig. 1K). MMP-2 expression was also
mainly observed in the cytoplasm of tumor cells (Fig. 1L).
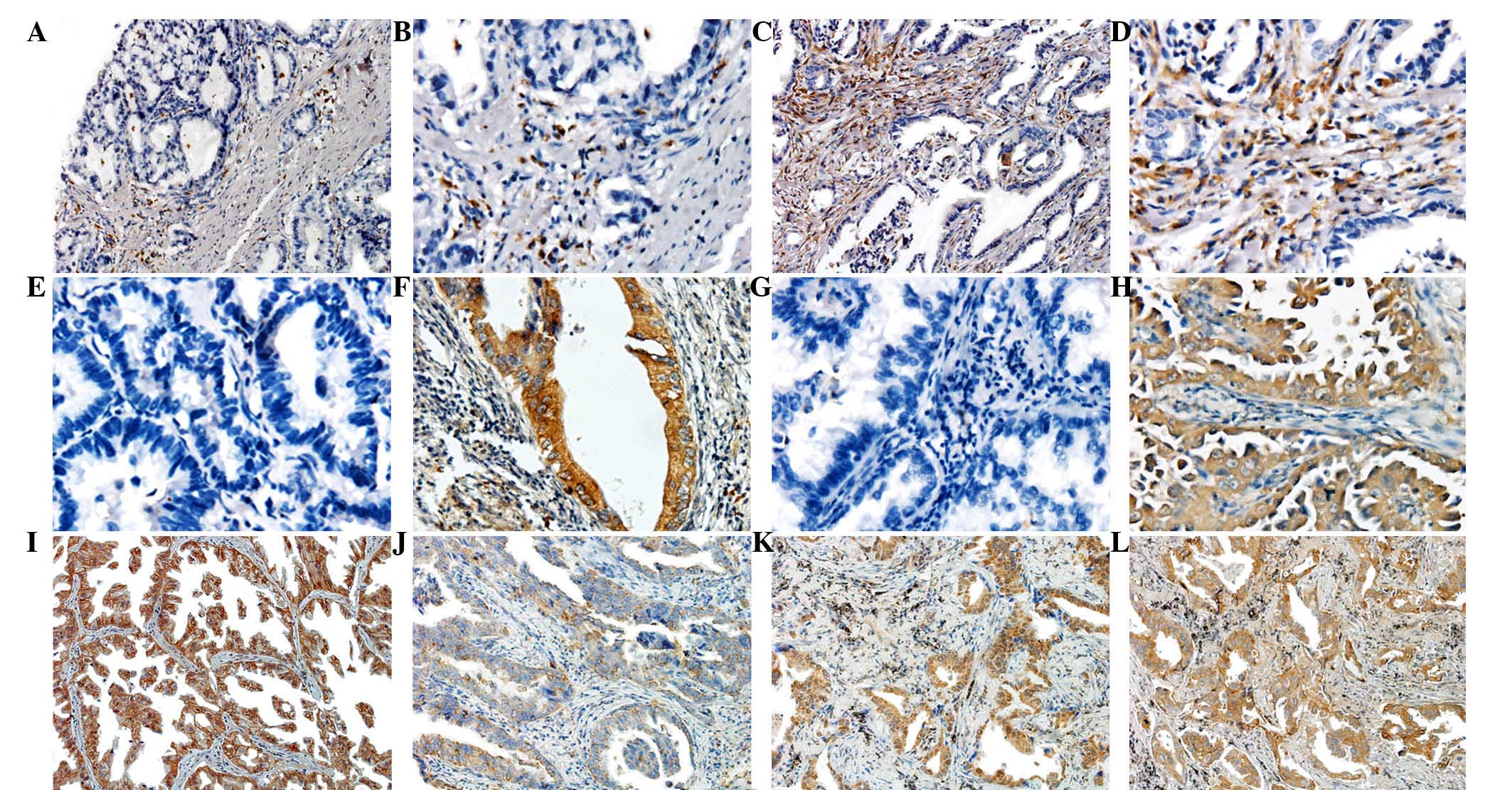 | Figure 1.Typical expression images of
immunochemistry staining in tissue microarrays. (A and B) Low
expression of CD68 in MIA tissues. Original magnification, (A) ×100
and (B) ×200. (C and D) High expression of CD68 in LPA tissues.
Original magnification, (C) ×100 and (D) ×200. (E) Negative
expression of IL-6 in MIA tissues. Original magnification, ×200.
(F) Positive expression of IL-6 in LPA tissues. Original
magnification, ×200. (G) Negative expression of CSF-1 in MIA
tissues. Original magnification, ×200. (H) Positive expression of
CSF-1 in LPA tissues. Original magnification, ×200. (I) High
E-cadherin expression in MIA tissues. Original magnification, ×100.
(J) Low E-cadherin expression in LPA tissues. Original
magnification, ×100. (K) High Snail expression in LPA tissues.
Original magnification, ×100. (L) High MMP-2 expression in LPA
tissues. Original magnification, ×100. CD, cluster of
differentiation; MIA, minimally invasive adenocarcinoma; LPA,
lepidic predominant adenocarcinoma; CSF, colony-stimulating factor;
IL, interleukin; MMP, metalloproteinase. |
Stepwise progression from AIS/MIA to invasive LPA
was assumed. The clinicopathological differences between AIS/MIA
and LPA were investigated. As shown in Table I, significant differences were present
among postoperative (P)-T stage (P<0.001), P-TNM stage
(P=0.030), number of involved nodal stations (P<0.001),
E-cadherin expression (P=0.038) and Snail expression (P=0.010).
Gender, age, smoking status, reception of adjuvant chemotherapy or
radiotherapy, and expression of MMP-2 were not significantly
different between these two groups (P>0.05).
Infiltration of interstitial TAMs and
their association with clinicopathological features
The median numbers of stromal TAMs/hpf were 25.03
(mean ± standard deviation, 24.73±16.87 TAMs/hpf; range, 0.00–71.42
TAMs/hpf). Based on the median numbers of TAMs, 54 patients (18.9%)
were CD68-, and 73 patients (25.6%) were categorized into the
low-TAMs group (≤25 TAMs/hpf), while 158 patients (55.5%) were
categorized into the high-TAMs group (>25 TAMs/hpf). In order to
investigate whether TAMs were associated with tumor progression,
the association of CD68 expression levels and clinicopathological
features was also illustrated in detail. The infiltration degree of
TAMs in tumor stroma was higher in LPA than in AIS/MIA (P=0.012).
In addition, Snail and MMP-2 expression were positively correlated
with the infiltration degree of TAMs (P=0.004 and P=0.009,
respectively). By contrast, E-cadherin exhibited a significantly
negative correlation with the infiltration degree of TAMs
(P=0.038). In the CD68- group, the positive expression rate of
E-cadherin was 35/54 (64.8%). In the low-TAMs group, the positive
expression rate of E-cadherin was 42/73 (57.5%), while in the
high-TAMs group, the positive expression rate of E-cadherin was
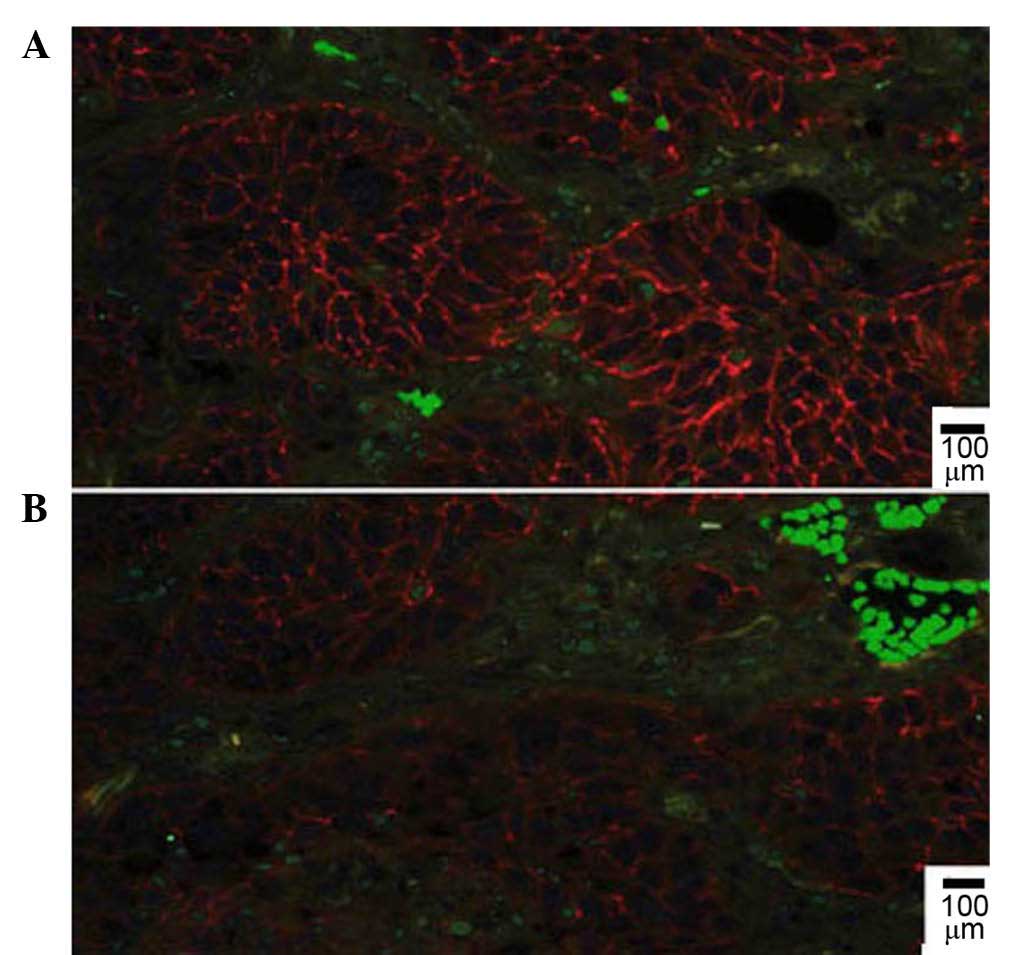
73/158 (46.2%). Immunofluorescent double staining revealed that
E-cadherin localized to the plasma membrane of cells in areas with
low CD68+ TAMs density (Fig. 2A),
while the expression of E-cadherin was compromised and partially
lost in areas with high TAMs density (Fig. 2B).
Prognostic factors of lung
adenocarcinoma
The 5-year OS and DFS rates were 44.0 and 40.0%,
respectively, for the total study population. Univariate analyses
revealed that MMP-2, the EMT markers E-cadherin and Snail, P-T
stage, P-TNM stage, number of involved nodal stations and reception
of adjuvant radiotherapy or chemotherapy were significant
prognostic factors (P<0.05; Table
II). Besides, a significant difference was noticed between
AIS/MIA and LPA for both OS (65.7 vs. 38.6%, P=0.008) and DFS (64.4
vs. 37.3%, P=0.006). The survival data also revealed that the
5-year OS and DFS rates for the CD68+ group were worse than those
for the CD68- group, but better than those for the CD68++ group
(45.4 vs. 53.6 and 35.3%, P=0.014; and 40.3 vs. 51.1 and 33.4%,
P=0.017, respectively).
 | Table II.Univariate survival analysis
according to clinicopathological factors. |
Table II.
Univariate survival analysis
according to clinicopathological factors.
| Variables | Cases, n (%) | 5YOSR, % | P-value | 5YDFSR, % | P-value |
|---|
| Gender |
|
| 0.170 |
| 0.194 |
|
Male | 134 (47.0) | 39.1 |
| 36.7 |
|
|
Female | 151 (53.0) | 45.3 |
| 42.6 |
|
| Age |
|
| 0.943 |
| 0.865 |
| >60
years | 128 (44.9) | 43.4 |
| 41.2 |
|
| ≤60
years | 157 (55.1) | 41.4 |
| 38.7 |
|
| Smoking status |
|
| 0.068 |
| 0.087 |
| Never
smoked | 151 (53.0) | 46.0 |
| 43.4 |
|
|
Smoker | 134 (47.0) | 38.9 |
| 36.0 |
|
| Adenocarcinoma
subtype |
|
| 0.008 |
| 0.006 |
|
AIS/MIA | 37 (22.6) | 65.7 |
| 64.4 |
|
|
LPA | 127 (77.4) | 38.6 |
| 37.3 |
|
| P-T stage |
|
| <0.001 |
| <0.001 |
| T1 | 123 (43.2) | 59.8 |
| 54.8 |
|
| T2 | 96 (33.7) | 38.6 |
| 36.4 |
|
| T3 | 66 (23.1) | 17.4 |
| 14.3 |
|
| P-TNM stage |
|
| <0.001 |
| <0.001 |
| I | 126 (44.2) | 62.6 |
| 58.1 |
|
| II | 59 (20.7) | 38.3 |
| 33.1 |
|
|
IIIA | 100 (35.1) | 19.5 |
| 16.9 |
|
| Number of involved
nodal stations |
|
| <0.001 |
| <0.001 |
| ≤1 | 193 (67.7) | 53.3 |
| 49.1 |
|
|
>1 | 92 (32.3) | 19.2 |
| 18.0 |
|
| Adjuvant
chemotherapy |
|
| 0.025 |
| 0.004 |
|
Yes | 176 (61.8) | 39.3 |
| 32.3 |
|
| No | 109 (38.2) | 51.6 |
| 50.2 |
|
| Adjuvant
radiotherapy |
|
| 0.032 |
| 0.009 |
|
Yes | 43 (15.1) | 24.6 |
| 15.5 |
|
| No | 242 (84.9) | 45.6 |
| 43.9 |
|
| CD68
expression |
|
| 0.014 |
| 0.017 |
| − | 54 (18.9) | 53.6 |
| 51.1 |
|
| + | 73 (25.6) | 45.4 |
| 40.3 |
|
| ++ | 158 (55.5) | 35.3 |
| 33.4 |
|
| CD68/CSF-1/IL-6
expression |
|
| <0.001 |
| <0.001 |
|
CD68- | 54 (18.9) | 45.4 |
| 40.3 |
|
|
CD68+CSF-1-IL-6- | 62 (21.8) | 71.0 |
| 68.1 |
|
|
CD68+CSF-1+IL-6- | 39 (13.7) | 47.6 |
| 46.9 |
|
|
CD68+CSF-1-IL-6+ | 33 (11.6) | 44.7 |
| 41.1 |
|
|
CD68+CSF-1+IL-6+ | 97 (34.0) | 19.9 |
| 14.1 |
|
| MMP-2
expression |
|
| 0.010 |
| 0.007 |
| − | 111 (38.9) | 52.5 |
| 50.8 |
|
| + | 174 (61.1) | 36.3 |
| 33.1 |
|
| E-cadherin
expression |
|
| 0.038 |
| 0.047 |
| − | 135 (47.4) | 35.7 |
| 33.2 |
|
| + | 150 (52.6) | 50.5 |
| 47.7 |
|
| Snail
expression |
|
| 0.004 |
| 0.002 |
| − | 85 (29.8) | 56.3 |
| 51.4 |
|
| + | 200 (70.2) | 36.6 |
| 33.9 |
|
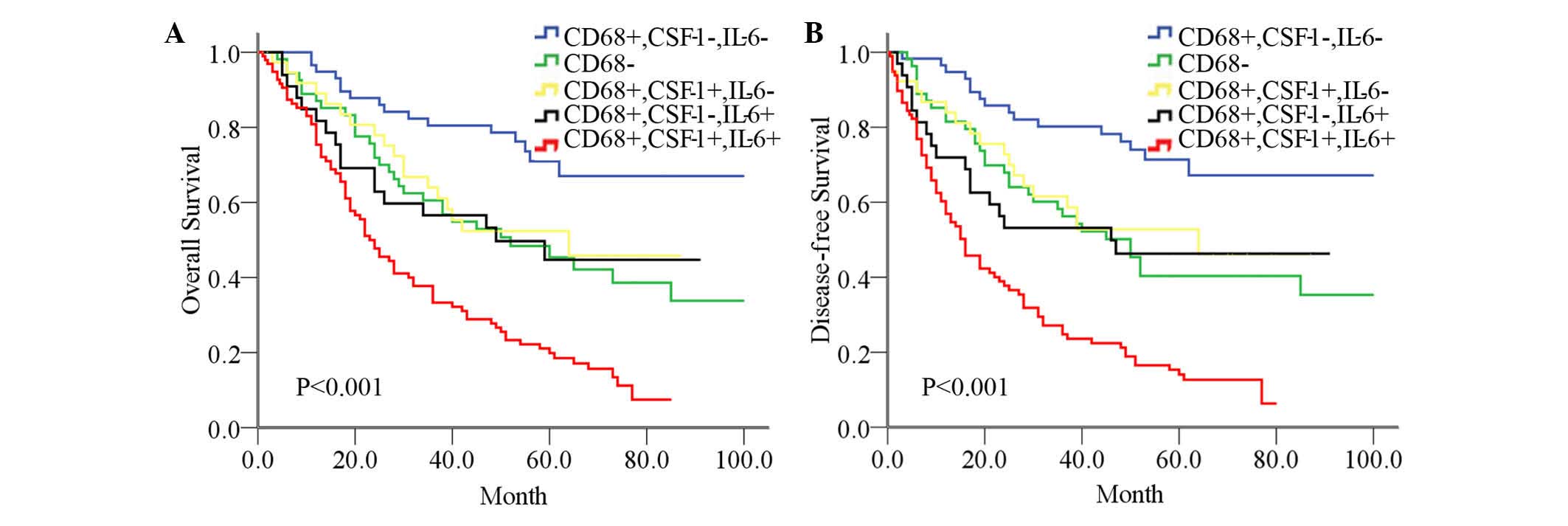
To elucidate the phenotype and biological function
of TAMs, the levels of tumor-derived CSF-1 and IL-6 were analyzed,
since these molecules are associated with TAMs functional
plasticity (10,11). CSF-1 and IL-6 could also be used as
prognostic factors for survival (P<0.001, data not shown).
Additional analysis demonstrated that the 5-year OS (19.9%) and DFS
(14.1%) rates in the CD68+CSF-1+IL-6+ group were the worst, while
the prognosis of the CD68+CSF-1-IL-6- group was the best (71.0 and
68.1%, respectively). The 5-year survival rates for the CD68- group
and the CD68+CSF-1+IL-6- group or the CD68+CSF-1-IL-6+ group were
intermediate between those of the group with the worst and the
group with the best rates (P<0.001; Fig. 3). Multivariate analysis determined
that the combination of CD68/CSF-1/IL-6 [OS hazard ratio
(HR)=3.360, DFS HR=4.179) remained significant and was an
independent prognostic factor for survival (P<0.001; Table III).
 | Table III.Multivariate analysis of factors
associated with overall survival and disease-free survival. |
Table III.
Multivariate analysis of factors
associated with overall survival and disease-free survival.
|
| Overall
survival | Disease-free
survival |
|---|
|
|
|
|
|---|
| Variables | Hazard ratio (95%
CI) | P-value | Hazard ratio (95%
CI) | P-value |
|---|
| Adenocarcinoma
subtype (LPA vs. AIS/MIA) | 1.297
(0.951–1.768) | 0.100 | 1.376
(1.009–1.876) | 0.044 |
| Number of involved
nodal stations (>1 vs. ≤1) | 1.664
(0.993–2.788) | 0.053 | 1.973
(1.176–3.310) | 0.010 |
| P-TNM stage (II and
IIIA vs. I) | 2.021
(1.119–3.649) | 0.020 | 1.862
(1.028–3.371) | 0.040 |
| P-T stage (T2 and
T3 vs. T1) | 1.399
(0.838–2.336) | 0.199 | 1.461
(0.893–2.390) | 0.131 |
| Adjuvant
chemotherapy (yes vs. no) | 0.625
(0.351–1.112) | 0.110 | 0.865
(0.493–1.517) | 0.613 |
| Adjuvant
radiotherapy (yes vs. no) | 1.430
(0.864–2.366) | 0.164 | 1.759
(1.062–2.915) | 0.028 |
| E-cadherin (+ vs.
-) | 0.511
(0.323–0.810) | 0.004 | 0.587
(0.375–0.919) | 0.020 |
| Snail (+ vs.
-) | 0.995
(0.546–1.815) | 0.987 | 1.143
(0.627–2.083) | 0.662 |
| MMP-2 (+ vs.
-) | 1.414
(0.856–2.333) | 0.176 | 1.362
(0.815–2.274) | 0.238 |
| CD68+CSF-1+IL-6+
vs. othersa | 3.360
(2.093–5.396) | <0.001 | 4.179
(2.628–6.646) | <0.001 |
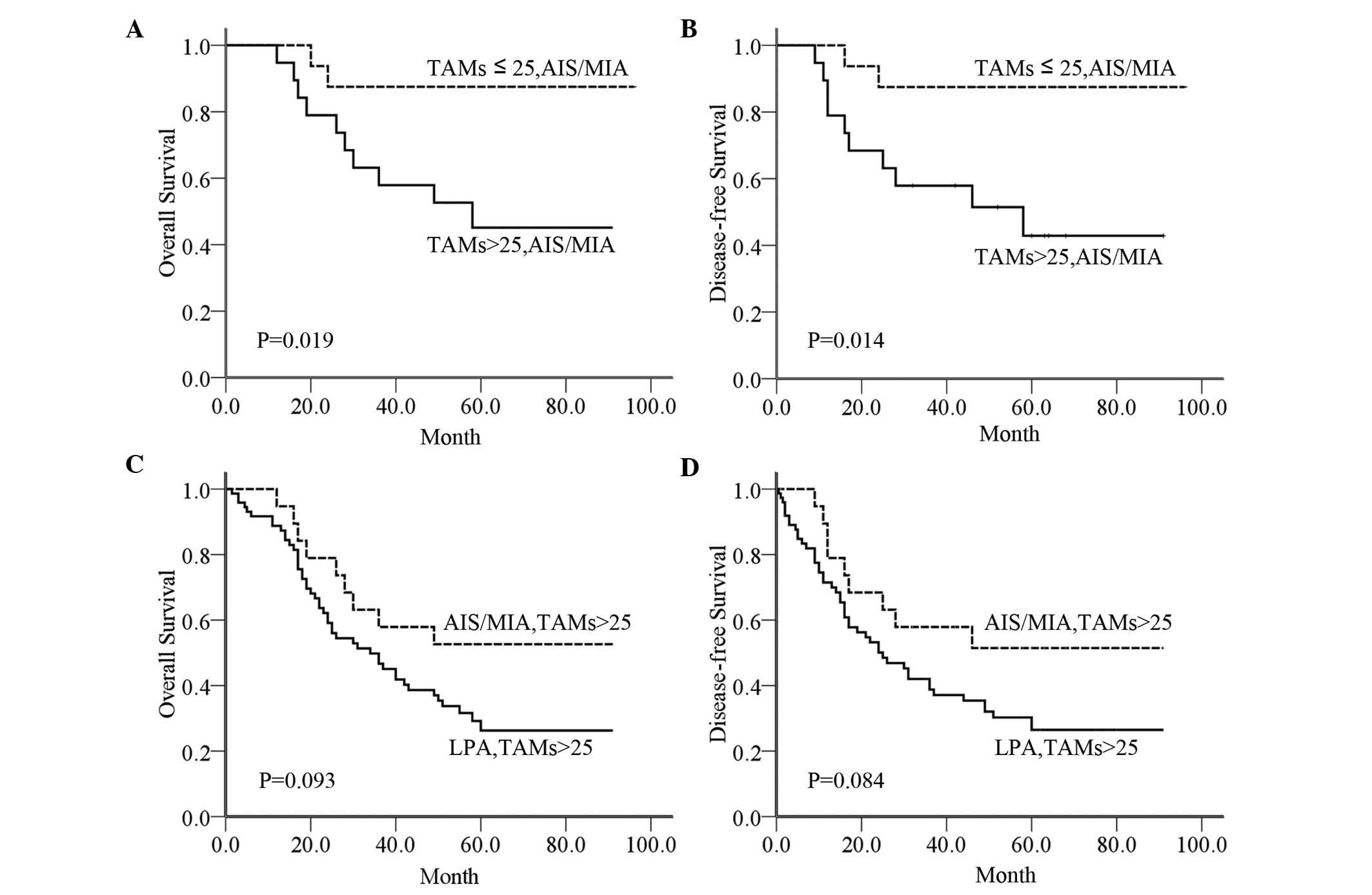
TAMs may serve an active role in
facilitating AIS/MIA progression to LPA
In order to further validate that TAMs regulated the
progression of lung adenocarcinoma, a stratified analysis was
performed according to adenocarcinoma subtype and CD68 expression
in TAMs (Table IV). Our results
indicated that the 5-year OS and DFS rates in AIS/MIA patients with
a number of TAMs ≤25 were better than those in AIS/MIA patients
with TAMs >25 (P=0.019 and P=0.014, respectively; Fig. 4A and B). By contrast, in patients with
TAMs >25, the 5-year OS and DFS rates were not significantly
different between AIS/MIA and LPA (P=0.093 and P=0.084,
respectively; Fig. 4C and D). In
AIS/MIA patients, there was a similar statistically significant
difference between the CD68+CSF-1+IL-6+ group and other groups
(CD68-, CD68+CSF-1+IL-6-, CD68+CSF-1-IL-6+ and CD68+CSF-1-IL-6-
groups) (P=0.008 and P=0.012, respectively; Fig. 5A and B). However, no significant
difference in patients with positive expression for all three
markers (CD68, CSF-1 and IL-6) was observed between AIS/MIA and LPA
(P=0.580 and P=0.301, respectively; Fig.
5C and D).
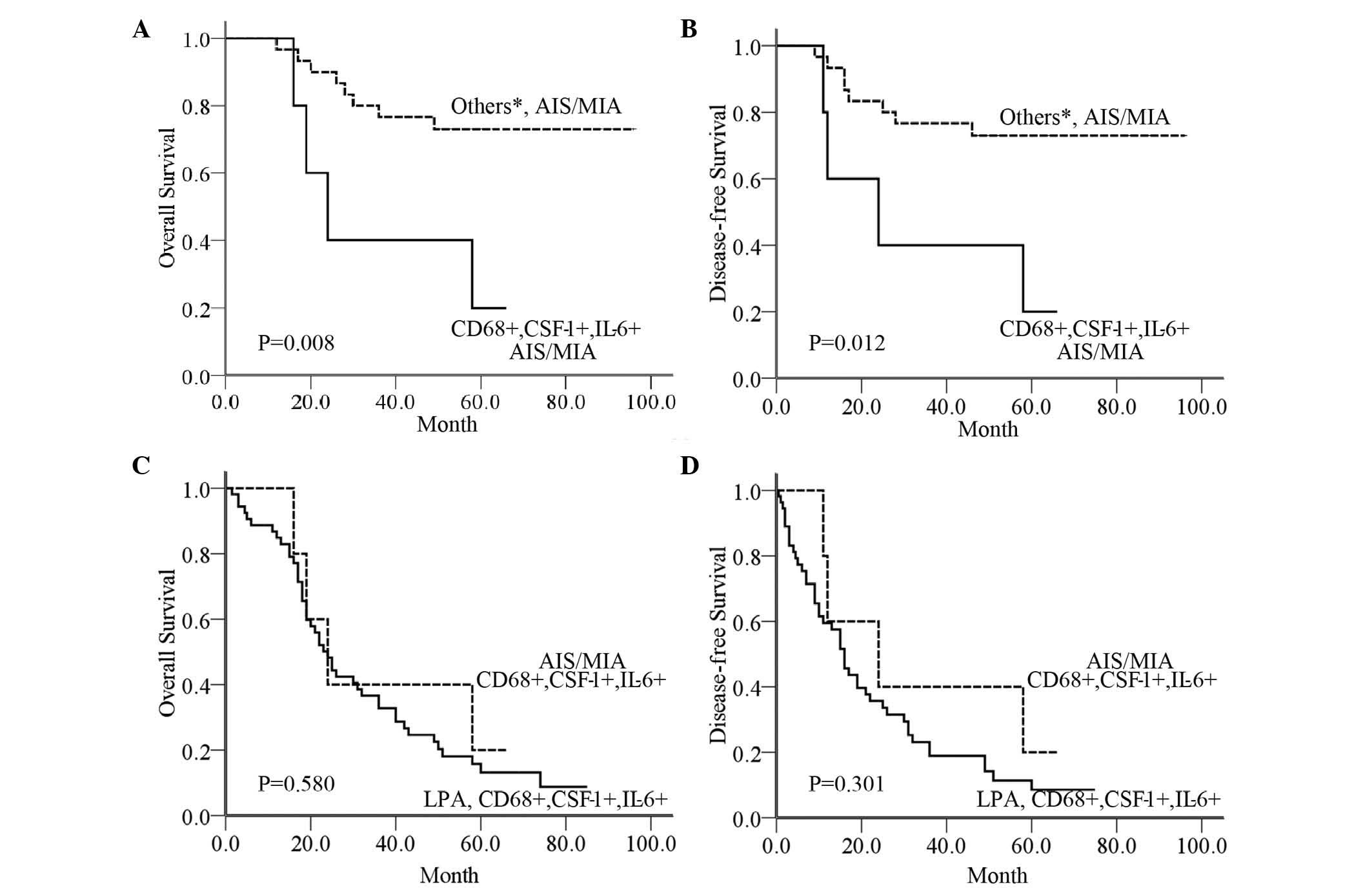 | Figure 5.(A) OS in patients with AIS/MIA in the
CD68+CSF-1+IL-6+ group compared with other groups, including the
CD68-, CD68+CSF-1+IL-6-, CD68+CSF-1-IL-6+ and CD68+CSF-1-IL-6-
groups. (B) DFS in patients with AIS/MIA in the CD68+ CSF-1+ IL-6+
group compared with other groups (C) Comparison of OS in AIS/MIA
patients with CD68+CSF-1+IL-6+ and in LPA patients with
CD68+CSF-1+IL-6+. (D) Comparison of DFS in AIS/MIA patients with
CD68+CSF-1+IL-6+ and in LPA patients with CD68+CSF-1+IL-6+. *Others
include the CD68+CSF-1+IL-6- group, the CD68+CSF-1-IL-6+ group, the
CD68+CSF-1-IL-6- group and the CD68- group. AIS, adenocarcinoma
in situ; MIA, minimally invasive adenocarcinoma; CD, cluster
of differentiation; CSF, colony-stimulating factor; IL,
interleukin; OS, overall survival; DFS, disease-free survival. |
 | Table IV.Stratified analysis according to
adenocarcinoma subtype and number of CD68+ TAMs. |
Table IV.
Stratified analysis according to
adenocarcinoma subtype and number of CD68+ TAMs.
| Variables | Cases, % | 5YOSR, % | P-value | 5YDFSR, % | P-value |
|---|
| AIS/MIA, number of
TAMs |
|
| 0.019 |
| 0.014 |
|
AIS/MIA, TAMs >25 | 14 | 45.1 |
| 42.9 |
|
|
AIS/MIA, TAMs ≤25 | 23 | 87.5 |
| 87.5 |
|
| TAMs >25,
adenocarcinoma subtype |
|
| 0.093 |
| 0.084 |
| TAMs
>25, AIS/MIA | 14 | 45.1 |
| 42.9 |
|
| TAMs
>25, LPA | 81 | 26.1 |
| 25.9 |
|
| AIS/MIA,
CD68/CSF-1/IL-6 |
|
| 0.008 |
| 0.012 |
|
AIS/MIA, CD68+CSF-1+IL-6+ | 6 | 20.0 |
| 20.0 |
|
|
AIS/MIA, othersa | 31 | 73.0 |
| 73.0 |
|
| CD68+CSF-1+IL-6+,
adenocarcinoma subtype |
|
| 0.580 |
| 0.301 |
|
CD68+CSF-1+IL-6+, AIS/MIA | 6 | 20.0 |
| 20.0 |
|
|
CD68+CSF-1+IL-6+, LPA | 55 | 13.1 |
|
8.5 |
|
Discussion
Accumulating evidence has shown that TAMs in the
tumor microenvironment can significantly enhance the malignant
phenotypes of tumors by promoting tumor growth and invasiveness
(10,11). Experimental models have demonstrated
that the lack of macrophage recruitment to the tumor site results
in decreased tumorigenic ability (12,13), and
clinical evidence has shown a strongly correlation between
increased TAMs density in tumor stroma and poor prognosis in
different types of solid tumors (14–16). Along
with these previous results, our study demonstrated that high
infiltration of TAMs was negatively associated with human lung
adenocarcinoma prognosis (P<0.05), and that the infiltration
degree of TAMs in the tumor stroma was higher in LPA than in
AIS/MIA (P<0.05). These results study indicated that highly
infiltrating TAMs may promote tumor invasion and progression.
Furthermore, in the tumor microenvironment,
neoplastic cells can shape the differentiation and functional
orientation of TAMs, which, in turn, exert several pro-tumoral
functions, including secretion of growth factors and matrix
proteases, promotion of angiogenesis, and suppression of adaptive
immunity (17–19). Recent studies have also shown that
TAMs are recruited into tumor regions by a range of bioactive
chemokines in the tumor microenvironment, including CSF-1, IL-6,
IL-10, which are mainly produced by the tumor cells themselves, and
are educated toward a tumor-promoting phenotype (20–22). The
study these cytokines is currently ongoing at our group, and the
interaction between cancer cells and TAMs is under way. The
detailed signaling pathway and underlying regulatory mechanisms
still require to be further in-depth investigated. In the present
study, univariate and multivariate analyses for survival
demonstrated that the combination of CD68, CSF-1 and IL-6 was the
most significant and independent prognostic factor (P<0.001).
The 5-year survival rates in the group with positive expression of
CD68, CSF-1 and IL-6 were the worst. The prognosis of the
CD68+CSF-1-IL-6- group was the best, and better than that of the
CD68- group. These findings have been confirmed by previous
studies. For instance, Duluc et al (23) noticed that tumor-associated leukemia
inhibitory factor and IL-6, which are present at high
concentrations in ovarian cancer ascites, redirect monocyte
differentiation into tumor-promoting TAMs by increasing CSF-1
consumption. In addition, Dijkgraaf et al (24) considered that prostaglandin E2 and
IL-6 were associated with chemoresistance and tumor-induced
differentiation of tumor-promoting macrophages. Based on these
results, it was assumed that the combination of CSF-1 and IL-6 had
the ability to strongly induce the formation of tumor-promoting
TAMs.
In order to further validate that TAMs regulated the
progression of lung adenocarcinoma, a stratified analysis was
performed according to AIS/MIA type and CD68 expression in TAMs. In
our study, AIS/MIA patients had a significantly better prognosis
than LPA patients, whereas LPA had significantly worse prognosis
(P<0.05). The 5-year survival rates in AIS/MIA patients with
TAMs ≤25 were better than those in AIS/MIA patients with TAMs
>25 (P<0.05). In AIS/MIA patients, there was a similar
statistically significant difference between the CD68+CSF-1+IL-6+
group and the other groups (CD68-, CD68+CSF-1+IL-6-,
CD68+CSF-1-IL-6+ and CD68+CSF-1-IL-6- groups) (P<0.05). By
contrast, in patients with TAMs >25 and in patients with
positive expression of CD68, CSF-1 and IL-6, the survival rates
were not significantly different between AIS/MIA and LPA
(P>0.05). Accordingly, it was likely that TAMs may play an
active role in facilitating AIS/MIA progression to LPA.
A characterized progression that epithelial-derived
tumor cells undergo is termed EMT, which involves loss of polarity
and adhesion, increased mobility and invasiveness, and acquisition
of an invasive mesenchymal phenotype (6,25,26). The loss of E-cadherin has been shown
to be associated with increased tumor invasiveness, metastasis and
poor prognosis (25,27). Snail and Slug have been established as
repressors of E-cadherin, one of the key molecules in the EMT
process, both in early development and in cancer progression
(27). MMP-2 is regarded as a crucial
enzyme for tumor progression, invasion and metastasis due to its
capability to degrade basement membrane components (28). Therefore, we hypothesized that TAMs
may facilitate adenocarcinoma progression by inducing EMT and
degrading the extracellular matrix, thereby contributing to tumor
heterogeneity and grade. The results of the present study indicated
that Snail and MMP-2 expression were positively correlated with the
infiltration degree of TAMs, whereas E-cadherin expression
exhibited a modest negative correlation with CD68+ densities
(P<0.05). It was certified that various cytokines secreted by
activated TAMs can induce the EMT phenotype in cancer cells
(29,30). As EMT is associated with both drug
resistance and patient relapse, it is possible to speculate that
therapeutic targeting of TAMs could improve disease outcome.
Considering the importance of TAMs in promoting tumor progression,
treatment only targeting the malignant cells would not be
efficient. The results presented herein also provide important new
insight into the significance of cancer-stroma cell interactions in
influencing the outcome of cancer therapy, which should be helpful
for the rational design of an anticancer strategy.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81201649 and 30801377) and Tianjin Natural Science Foundation of
China (Tianjin, China; grant no. 11JCYBJC13300).
References
|
1
|
Devesa SS, Bray F, Vizcaino AP and Parkin
DM: International lung cancer trends by histologic type: Male:
Female differences diminishing and adenocarcinoma rates rising. Int
J Cancer. 117:294–299. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Soh J, Toyooka S, Ichihara S, Asano H,
Kobayashi N, Suehisa H, Otani H, Yamamoto H, Ichimura K, Kiura K,
et al: Sequential molecular changes during multistage pathogenesis
of small peripheral adenocarcinomas of the lung. J Thorac Oncol.
3:340–347. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Mantovani A, Allavena P, Sica A and
Balkwill F: Cancer-related inflammation. Nature. 454:436–444. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Solinas G, Germano G, Mantovani A and
Allavena P: Tumor-associated macrophages (TAM) as major players of
the cancer-related inflammation. J Leukoc Biol. 86:1065–1073. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mantovani A, Schioppa T, Porta C, Allavena
P and Sica A: Role of tumor-associated macrophages in tumor
progression and invasion. Cancer Metastasis Rev. 25:315–322. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Thiery JP, Acloque H, Huang RY and Nieto
MA: Epithelial-mesenchymal transitions in development and disease.
Cell. 139:871–890. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Detterbeck FC, Boffa DJ and Tanoue LT: The
new lung cancer staging system. Chest. 136:260–271. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Richardsen E, Uglehus RD, Due J, Busch C
and Busund LT: The prognostic impact of CSF-1, CSF-1 receptor, CD68
and CD3 in prostatic carcinoma. Histopathology. 53:30–38. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Gwak JM, Jang MH, Kim DI, Seo AN and Park
SY: Prognostic value of tumor-associated macrophages according to
histologic locations and hormone receptor status in breast cancer.
PLoS One. 10:e01257282015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Quail DF and Joyce JA: Microenvironmental
regulation of tumor progression and metastasis. Nat Med.
19:1423–1437. 2013. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Hu M and Polyak K: Microenvironmental
regulation of cancer development. Curr Opin Genet Dev. 18:27–34.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang
PY, Xu HX, Kong LQ, Wang L, Wu WZ and Tang ZY: Depletion of
tumor-associated macrophages enhances the effect of sorafenib in
metastatic liver cancer models by antimetastatic and antiangiogenic
effects. Clin Cancer Res. 16:3420–3430. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zabuawala T, Taffany DA, Sharma SM,
Merchant A, Adair B, Srinivasan R, Rosol TJ, Fernandez S, Huang K,
Leone G and Ostrowski MC: An ets2-driven transcriptional program in
tumor-associated macrophages promotes tumor metastasis. Cancer Res.
70:1323–1333. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Subimerb C, Pinlaor S, Khuntikeo N,
Leelayuwat C, Morris A, McGrath MS and Wongkham S: Tissue invasive
macrophage density is correlated with prognosis in
cholangiocarcinoma. Mol Med Rep. 3:597–605. 2010.PubMed/NCBI
|
|
15
|
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH,
Wang XZ, Zhao YW and Wei YQ: Prognostic significance of
tumor-associated macrophages in solid tumor: A meta-analysis of the
literature. PloS One. 7:e509462012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Steidl C, Lee T, Shah SP, Farinha P, Han
G, Nayar T, Delaney A, Jones SJ, Iqbal J, Weisenburger DD, et al:
Tumor-associated macrophages and survival in classic Hodgkin's
lymphoma. N Engl J Med. 362:875–885. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Qian BZ and Pollard JW: Macrophage
diversity enhances tumor progression and metastasis. Cell.
141:39–51. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Sica A, Larghi P, Mancino A, Rubino L,
Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P and Mantovani
A: Macrophage polarization in tumour progression. Semin Cancer
Biol. 18:349–355. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sica A and Bronte V: Altered macrophage
differentiation and immune dysfunction in tumor development. J Clin
Invest. 117:1155–1166. 2007. View
Article : Google Scholar : PubMed/NCBI
|
|
20
|
Lippitz BE: Cytokine patterns in patients
with cancer: A systematic review. Lancet Oncol. 14:e218–e228. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Bonecchi R, Locati M and Mantovani A:
Chemokines and cancer: A fatal attraction. Cancer cell. 19:434–435.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Sica A, Allavena P and Mantovani A: Cancer
related inflammation: The macrophage connection. Cancer Lett.
267:204–215. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Duluc D, Delneste Y, Tan F, Moles MP,
Grimaud L, Lenoir J, Preisser L, Anegon I, Catala L, Ifrah N, et
al: Tumor-associated leukemia inhibitory factor and IL-6 skew
monocyte differentiation into tumor-associated macrophage-like
cells. Blood. 110:4319–4330. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dijkgraaf EM, Heusinkveld M, Tummers B,
Vogelpoel LT, Goedemans R, Jha V, Nortier JW, Welters MJ, Kroep JR
and van der Burg SH: Chemotherapy alters monocyte differentiation
to favor generation of cancer-supporting M2 macrophages in the
tumor micro-environment. Cancer Res. 73:2480–2492. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Hugo H, Ackland ML, Blick T, Lawrence MG,
Clements JA, Williams ED and Thompson EW: Epithelial-mesenchymal
and mesenchymal-epithelial transitions in carcinoma progression. J
Cell Physiol. 213:374–383. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Yilmaz M and Christofori G: EMT, the
cytoskeleton, and cancer cell invasion. Cancer Metastasis Rev.
28:15–33. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yang J and Weinberg RA:
Epithelial-mesenchymal transition: At the crossroads of development
and tumor metastasis. Dev Cell. 14:818–829. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
González-Arriaga P, Pascual T,
García-Alvarez A, Fernández-Somoano A, López-Cima MF and Tardón A:
Genetic polymorphisms in MMP-2, 9 and 3 genes modify lung cancer
risk and survival. BMC Cancer. 12:1212012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Bonde AK, Tischler V, Kumar S, Soltermann
A and Schwendener RA: Intratumoral macrophages contribute to
epithelial-mesenchymal transition in solid tumors. BMC Cancer.
12:352012. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Gao D, Vahdat LT, Wong S, Chang JC and
Mittal V: Microenvironmental regulation of epithelial-mesenchymal
transitions in cancer. Cancer Res. 72:4883–4889. 2012. View Article : Google Scholar : PubMed/NCBI
|