Introduction
Recent therapies for oral cancer have improved the
curability of oral cancer and the 5-year survival rate of patients.
This is a result of multidisciplinary therapy, in which wide
resection and immediate reconstructive surgery brought about by
advances in reconstructive surgery are combined with chemotherapy
and radiation therapy (1–3). In addition, various therapies, including
intra-arterial chemotherapy, that aim to preserve oral function
through minimal invasion have been investigated to address the
postoperative functional deterioration that accompanies surgical
therapy (4). Additional therapies are
being used with chemotherapy to reduce adverse effects by obtaining
precise antitumor efficacy with minimum doses. These include
multidrug therapy (docetaxel with cisplatin and fluorouracil) in
preference to single-drug therapy (cisplatin), as well as
superselective intra-arterial chemotherapy (5,6). Drug
resistance has also been investigated (7). Therefore, the clinical application of
sensitivity tests for anticancer drugs is highly desirable to
ensure that drugs are used effectively and appropriately.
Prediction of the anticancer drug sensitivity of a particular tumor
when chemotherapy is used not only contributes to improving patient
outcome, but also prevents ineffective drug administration, thus
reducing unnecessary physical and financial burdens (8–11).
In vivo anticancer drug sensitivity tests
that have been developed at present include the nude mouse method
and subrenal capsule assay method, and the clinical application of
these methods has been investigated (12,13). There
is also considerable interest in in vitro methods, which,
compared with in vivo methods, are easier to perform, less
expensive and generate results within a shorter time. In 1995,
Kobayashi et al (14)
developed the collagen gel droplet-embedded culture drug
sensitivity test (CD-DST), which combines the collagen gel droplet
culture method, which is a simple method of three-dimensional (3D)
culture that allows extremely small clinical samples to be tested,
with a serum-free medium step and quantitative evaluation by image
analysis. CD-DST has little effect on non-cancerous cells, allowing
accurate measurements of cancerous cells only (14–16). This
method has been used chiefly on tumors of the digestive system
(17,18). Compared to such cancers of the primary
organs, oral squamous cell carcinoma (OSCC) and other oral cancers
generally have a smaller tumor volume. CD-DST is therefore likely
to be a suitable method for testing the sensitivity of anticancer
drugs on OSCC, as this test can be used with a small volume of
tumor tissue (14–16). However, since anticancer drug contact
concentrations for OSCC have yet to be established by CD-DST;
currently, OSCC is treated using drug contact concentrations
established for the treatment of stomach cancer (19). Therefore, a basic investigation of the
optimal contact concentration of cisplatin (CDDP) and fluorouracil
(5-FU) was conducted using 7 squamous carcinoma cell lines derived
from human oral cancers to establish the anticancer drug
sensitivity for OSCC using CD-DST.
Materials and methods
Materials
The Ethics Committee of The Nippon Dental University
School of Life Dentistry at Niigata (approval no., ECNG-H-119;
Niigata, Japan) approved the present study. In total, 7 squamous
cell carcinoma cell lines derived from human oral cancers were
used, as follows: squamous cell carcinoma of the tongue, SAS,
HSC-3, HSC-4 and OSC-19; squamous cell carcinoma of the gingiva,
Ca9-22; squamous cell carcinoma of the buccal mucosa, HO-1-N-1; and
squamous cell carcinoma of the floor of the mouth, KON. The SAS and
Ca9-22 cell lines were purchased from the Health Science Research
Resources Bank (National Institutes of Biomedical Innovation,
Health and Nutrition, Osaka, Japan), and the HSC-3, HSC-4,
HO-1-N-1, KON and OSC-19 cell lines were purchased from the
Japanese Collection of Research Bioresources (National Institutes
of Biomedical Innovation, Health and Nutrition). The cell lines
were subcultured until use in Dulbecco's modified Eagle's medium
(DMEM/F12; Nihon Pharmaceutical Co., Ltd., Tokyo, Japan)
supplemented with 15% fetal bovine serum (FBS; Life Technologies;
Thermo Fisher Scientific, Inc., Waltham, MA, USA), 0.1% dispensable
amino acid solution (Life Technologies; Thermo Fisher Scientific,
Inc.), 50 U/ml penicillin, 50 µg/ml streptomycin (Life
Technologies; Thermo Fisher Scientific, Inc.), and 0.25 µg/ml
fungizone (Life Technologies; Thermo Fisher Scientific, Inc.). The
subcultures were multilayered under conditions of 37°C, 95% air and
5% CO2 for 72 h. During culture, cells were observed
using an inverted-phase contrast microscope (Olympus, Tokyo,
Japan). The anticancer drugs used in this study were CDDP
(Bristol-Myers Squibb, Tokyo, Japan) and 5-FU (Kyowa Hakko Kirin,
Tokyo, Japan). The 8 experimental animals were female nude BALB/c
nu/nu mice aged 5–7 weeks (CLEA Japan, Inc., Tokyo, Japan).
Investigation of the specificity of
anticancer drug sensitivity by reverse transcription-polymerase
chain reaction (RT-PCR)
Total RNA was extracted from the Ca9-22, HSC-3,
HSC-4, HO-1-N-1, KON, OSC-19 and SAS cell lines seeded onto Petri
dishes using the RNeasy Mini kit® (Qiagen, Hilden,
Germany), according to the manufacturer's protocol. Using 1 µg of
total RNA, cDNA was synthesized using the High Capacity cDNA
Reverse Transcription kit (Life Technologies; Thermo Fisher
Scientific, Inc.). PCR amplification was performed using Platinum
PCR Super Mix (Life Technologies; Thermo Fisher Scientific, Inc.)
with the following gene-specific primers: Multidrug resistance gene
1 (MDR1) (20); multidrug resistance
associated protein gene 1 (MRP1) (20); MRP2 (20); excision repair cross-complementing
factor 1 (ERCC1) (21,22); thymidine synthase (23); dihydropyrimidine dehydrogenase (DPD)
(23); thymidine phosphorylase (TP)
(23); and orotate
phosphoribosyltransferase (OPRT) (23) (Table I).
The PCR cycle consisted of the following conditions: Denaturation
at 94°C for 15 sec; annealing at 55°C for 15 sec; and extension at
72°C for 60 sec. The cycle was repeated 35 times (Table I). The tests were conducted using the
Applied Biosystems® 2720 Thermal Cycler (Applied
Biosystems; Thermo Fisher Scientific, Inc.). The internal control
was glyceraldehyde-3-phosphate dehydrogenase, with PCR
amplification performed under the same conditions. Electrophoresis
was performed on a 2% agarose gel (Nippon Gene, Co., Ltd., Tokyo,
Japan), and the PCR products were visualized by ethidium bromide
staining for identification of the bands.
 | Table I.Sequence of polymerase chain reaction
primers. |
Table I.
Sequence of polymerase chain reaction
primers.
| Gene | Forward primer,
5′-3′ | Reverse primer,
5′-3′ | Annealing
temperature, °C |
|---|
| MDR1 |
CAGTGTTTGCCATAGTATTTTCAAGGATTG |
CCCTTTAACACTAGAAGCATCAC | 42 |
| MRP1 |
CGGAAACCATCCACGACCCTAATCC |
ACCTCCTCATTCGCATCCACCTTGG | 42 |
| MRP2 |
CTGCCTCTTCAGAATCTTAG |
CCCAAGTTGCAGGCTGGCC | 55 |
| ERCC1 |
GGGAATTTGGCGACGTAATTC |
GCGGAGGCTGAGGAACGA | 55 |
| DPD |
TGTTCGGACAGAGCAAGATG |
CTTCAATCCGGCCATTTCTA | 55 |
| OPRT |
ACGCCGGGGCGCCTGGGAGTTTGA |
TTTCCAGCCAGTGACTTTCAGGAGGAC | 55 |
| TS |
ACCAACCCTGACGACAGAAG |
ATGCGGATTGTACCCTTCAA | 55 |
| TP |
AGGAGACCTCGGTGCTGAC |
TGAGAATGGAGGCTGTGATG | 55 |
| GAPDH |
GTCAAGGCTGAGAACGGGAA |
GCTTCACCACCTTCTTGATG | 55 |
Sensitivity tests and anticancer drug
concentrations
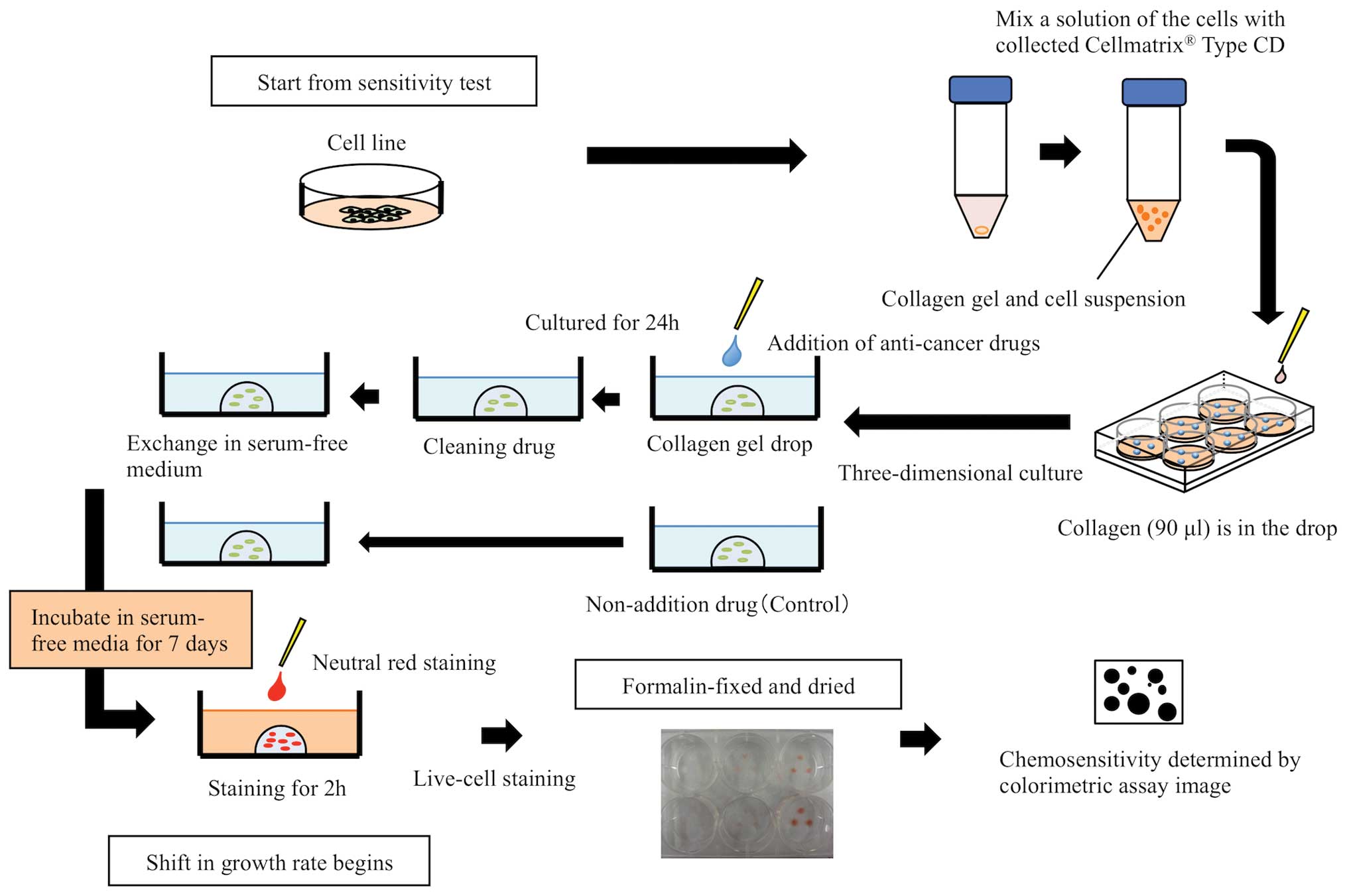
CD-DST was performed according to the method
described by Kobayashi et al (14–16), using
the Primaster® human cancer cell primary culture kit
(Kurabo Industries Ltd., Osaka, Japan). The protocol was performed
three times for each cell line (Fig.
1). Solution A (Cellmatrix® Type CD), solution B
(F-12 medium at 10-fold concentration), and solution C
(reconstitution buffer containing 50 mM NaOH, 260 mM
NaHCO3, and 200 mM HEPES), which were included in the
collagen gel culture kit, were mixed at a ratio of 8:1:1 by volume
to homogeneity to make a collagen solution. Each cell line was
mixed with this collagen solution to 1–5×105 cells/ml,
and the suspension was transferred into a 6-well multiplate
(FALCON®, NY, USA) using a micropipette in three 30-µl
droplets, making a total volume of 90 µl per well (15,16). In
addition, three 30-µl droplets were transferred onto a 40-mm dish
as a reference to determine the growth rate. Subsequent to gelation
in a CO2 incubator at 37°C for 1 h, the droplets in each
well were overlaid with DMEM/F12 containing 10% FBS and cultured
for 24 h. Drug contact was thus performed under conditions
approximating physiological conditions. The maximum concentration
was twice the maximum blood concentration during clinical
administration, and the minimum concentration was the steady state
concentration. CDDP was added at concentrations of 0.15, 0.3, 1.25,
2.5, 5.0 and 10.0 µg/ml. 5-FU was added at concentrations of 0.4,
0.9, 1.8, 3.8, 7.5, 15.0 and 30.0 µg/ml, and the droplets were
returned to the CO2 incubator and left in contact with
the drug for 24 h. Following contact, the culture medium in each
well was removed by suction, and the droplets were washed twice
with phosphate-buffered saline (PBS; Takara Bio Inc., Otsu, Japan)
to remove the anticancer drug. The cells were then growth-cultured
for 7 days in serum-free medium. At this time, to compare the cell
growth rate, the reference plate was stained and fixed. Subsequent
to cultivation for 7 days, neutral red solution (Kurabo Industries
Ltd.) was added to each well, and the cells were incubated at 37°C
for another 2 h. The cells were fixed by replacing the solution
with 10% neutral formalin (Wako Pure Chemical Industries Ltd.,
Osaka, Japan) for 40 min. The culture plates were then washed three
times in PBS and air-dried for 10 h. Drug efficacy was determined
by image analysis (Solution Systems Inc., Chiba, Japan).
Determination of efficacy by image
analysis
Antitumor efficacy was determined according to the
method of Koezuka et al (24)
using the Primage® image analysis device (Kurabo
Industries Ltd.). Imaging of the plate samples obtained from the
sensitivity tests was performed by acquiring grayscale images using
the image analysis device. Images other than those of the cancer
cells that were unnecessary for the analysis were separated and
removed from the sample images using a shade (density gradation
demarcation) or shape (grain shape separation) extraction function.
Cancer cell growth and antitumor efficacy were determined by
measuring the volume of the colony from the cancer cell images. The
treatment group, which was in contact with the anticancer drug, and
the control group, which was not in contact with the drug, were
compared by calculating the ratio of the colony volume of the
treatment group (VT) and the colony volume of the
control group (VC) (VT/VC). This
ratio was the T/C value; a T/C value ≤50% indicated high
sensitivity and a T/C value >50% indicated low sensitivity. The
standard for an evaluable colony was a tumor cell growth rate at
the time of image analysis processing of ≥0.8 times the rate at the
start of the experiment.
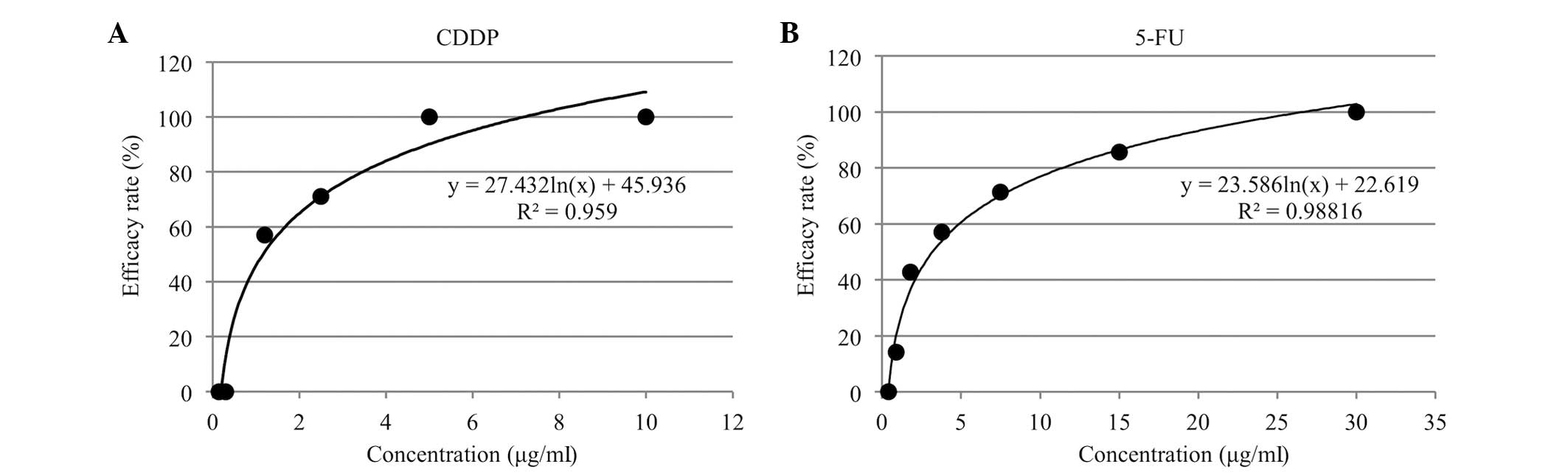
Calculation of optimal contact
concentration
The optimal contact concentrations of CDDP and 5-FU
were established using the method described by Nagai et al
(25). Using the T/C values obtained
from the anticancer drug sensitivity tests for the 7 cell lines, an
efficacy ratio curve was plotted with the cumulative efficacy rate
(%) on the vertical axis against anticancer drug concentration
(µg/ml) on the horizontal axis. From this, the logarithmic
trendline y = a.ln(x) + b was calculated. Using the already-known
clinical response rates of oral cancer to single-drug treatment
with the two anticancer drugs [CDDP, 26.3% (26); 5-FU, 13.0% (27)] on the Y-axis, the optimal contact
concentration could then be read off along the X-axis.
Antitumor efficacy of CDDP in
cancer-bearing nude mice
The nude mouse method was used to investigate
whether the CD-DST results of the calculated optimal contact
concentration was associated with those of the 7 cell lines. Cells
(0.3 ml of a suspension of 1×107 cells/ml of Hanks'
solution) from 6 cell lines (excluding KON) were transferred to
nude mice under the flank skin. When the size of the tumor was
100–300 mm3 [tumor size = 0.5 × (longest diameter ×
shortest diameter2)], the experiment was commenced. The
dose of the anticancer drug was the clinically equivalent dose
(CED) reported by Inaba et al (28,29). This
is the dose at which the peripheral concentration of the anticancer
drug in nude mouse experimental models was the same as the
peripheral concentration in human patients treated with an
effective dose. On the basis of CED, 7.0 mg/kg CDDP was
administered as a single intravenous injection to the tail vein to
reproduce the antitumor efficacy of clinical administration in
humans (28,29). The antitumor efficacy of the
anticancer drug was determined by calculating the T/C value at 21
days post-administration from the relative tumor weight of the drug
treatment group and the control group, according to the method
described by Geran et al (30). A T/C value ≤50% indicated high
sensitivity and a T/C value >50% indicated low sensitivity. The
calculated T/C values were compared to those from the CD-DST model
for each cell line. All procedures were performed to minimize pain
and discomfort according to the Guidelines for the Care and Use of
Laboratory Animals, The Nippon Dental University School of Life
Dentistry at Niigata (approval no., 131).
Statistical analysis
All statistical analyses were performed using the
SPSS version 13.0J statistical software (SPSS, Inc., Chicago, IL,
USA). The Mann-Whitney U test and Pearson's correlation coefficient
were used for comparisons among groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Specificity of anticancer drug
sensitivity of the 7 cell lines
ERCC1 was not expressed in any of the cell lines,
whereas OPRT was expressed in all cell lines. No clear specificity
in the expression of resistance genes or enzymes associated with
metabolism was detected (Fig. 2).
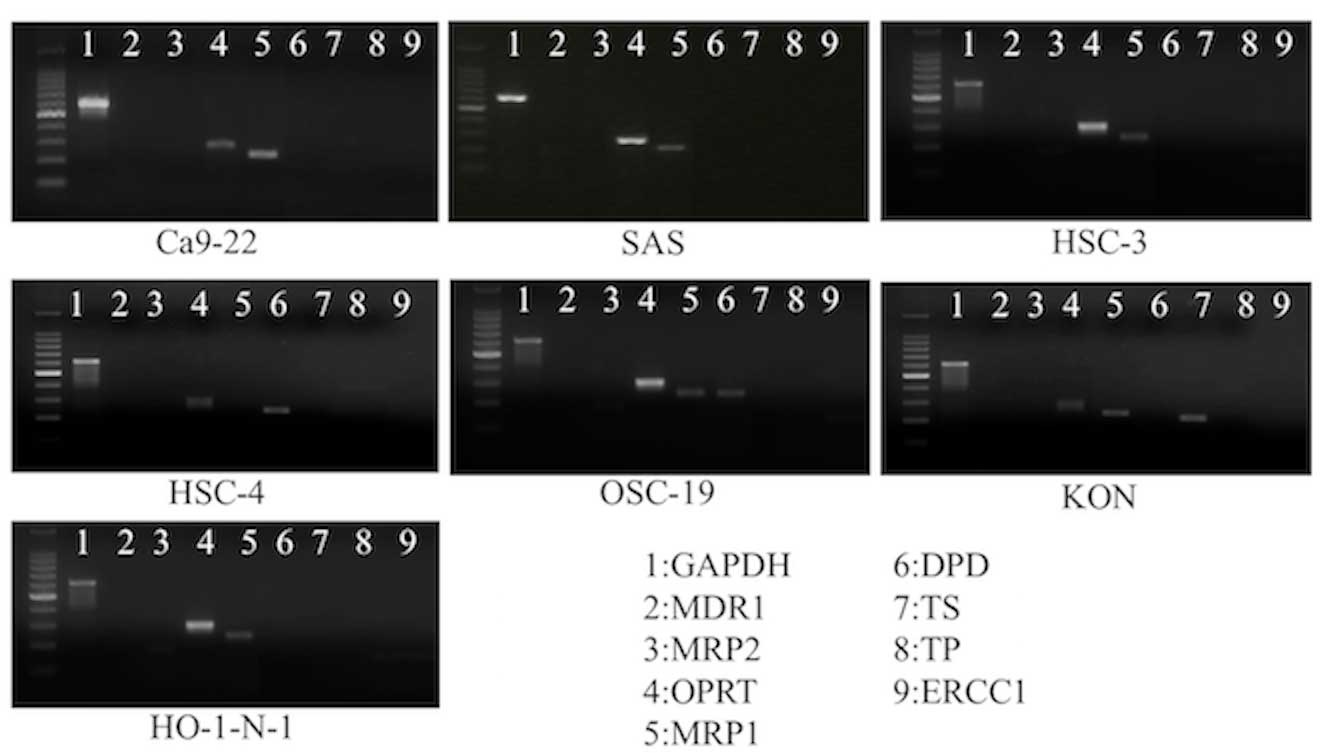 | Figure 2.Results of reverse
transcription-polymerase chain reaction amplification. ERCC1 was
not expressed in any of the cell lines, while expression of OPRT
was found in all cell lines. No clear specificity in the expression
of resistance genes or enzymes associated with metabolism was
observed in the 7 cell lines. GAPDH, glyceraldehyde 3-phosphate
dehydrogenase; MDR1, multidrug resistance gene 1; MRP2, multidrug
resistance associated protein gene 2; OPRT, orotate
phosphoribosyltransferase; TS, thymidine synthase; TP, thymidine
phosphorylase; MRP1, multidrug resistance associated protein gene
1; DPD, dihydropyrimidine dehydrogenase; ERCC1,. excision repair
cross-complementing factor 1. |
Sensitivity of the 7 cell lines to
CDDP and 5-FU at each contact concentration
The results of CD-DST using the 7 cell lines are
shown in Table II. The contact
concentrations at which all cell lines showed low sensitivity were
≤0.3 µg/ml for CDDP and 0.4 µg/ml for 5-FU. Calculation of the
cumulative efficacy rate of the 7 cell lines at each contact
concentration showed that the efficacy rate at 1.25 µg/ml CDDP was
57.1% and at 0.9 µg/ml 5-FU was 14.2%.
 | Table II.Results of the CD-DST method in the
SAS, Ca9-22, HSC-3, HO-1-N-1, HSC-4, KON and OSC-19 cell lines. |
Table II.
Results of the CD-DST method in the
SAS, Ca9-22, HSC-3, HO-1-N-1, HSC-4, KON and OSC-19 cell lines.
| A, CDDP |
|---|
|
|---|
|
| Cell line | Cumulative
efficacy |
|---|
|
|
|
|
|---|
| Cells, µg/ml | SAS | Ca9-22 | HSC-3 | HO-1-N-1 | HSC-4 | KON | OSC-19 | Cell lines, n | Rate, % |
|---|
|
0.15 | 92.1 | 112.9 | 107.1 | 97.9 | 101.4 | 90.3 | 108.9 | 0/7 |
0.0 |
|
0.30 | 91.4 |
87.5 |
98.3 | 90.5 |
86.1 | 88.9 |
90.7 | 0/7 |
0.0 |
|
1.25 | 38.1 |
35.5 |
88.2 | 72.9 |
43.1 | 45.1 |
79.7 | 4/7 |
57.1 |
|
2.50 |
9.6 |
8.2 |
65.6 | 28.2 |
21.9 | 22.9 |
67.7 | 5/7 |
71.4 |
|
5.00 |
3.8 |
6.9 |
35.3 |
4.9 |
9.2 |
8.3 |
44.4 | 7/7 | 100.0 |
| 10.00 |
2.2 |
3.4 |
8.5 |
1.4 |
2.3 |
6.2 |
36.0 | 7/7 | 100.0 |
|
| B, 5-FU |
|
|
| Cell line | Cumulative
efficacy |
|---|
|
|
|
|
|---|
| Cells, µg/ml | SAS | Ca9-22 | HSC-3 | HO-1-N-1 | HSC-4 | KON | OSC-19 | Cell lines, n | Rate, % |
|
|
0.4 | 110.0 | 50.1 | 86.8 | 87.0 | 77.5 | 75.6 | 81.6 | 0/7 |
0.0 |
|
0.9 |
88.2 | 23.8 | 55.1 | 86.3 | 63.3 | 50.4 | 76.6 | 1/7 |
14.2 |
|
1.8 |
34.3 | 18.5 | 53.8 | 73.7 | 62.6 | 40.5 | 76.0 | 3/7 |
42.8 |
|
3.8 |
13.1 |
4.5 | 52.2 | 59.6 | 28.9 | 20.1 | 71.7 | 4/7 |
57.1 |
|
7.5 |
6.9 |
4.3 | 51.4 | 48.9 | 29.9 | 12.2 | 60.4 | 5/7 |
71.4 |
| 15.0 |
3.7 |
3.4 | 43.0 | 21.6 | 20.4 |
6.2 | 59.2 | 6/7 |
85.7 |
| 30.0 |
3.3 |
3.3 | 30.3 |
4.6 | 10.6 |
6.9 | 44.8 | 7/7 | 100.0 |
Calculation of the optimal contact
concentrations of CDDP and 5-FU
The in vitro cumulative efficacy rate curves
of the cumulative efficacy rate (%) against the contact
concentration (µg/ml) for CDDP and 5-FU are shown in Fig. 3A and B. The optimal contact
concentrations, determined by reading off the concentrations for
the known clinical response rates of the single-drug treatment on
the logarithmic trendline, were 0.5 µg/ml for CDDP and 0.7 µg/ml
for 5-FU.
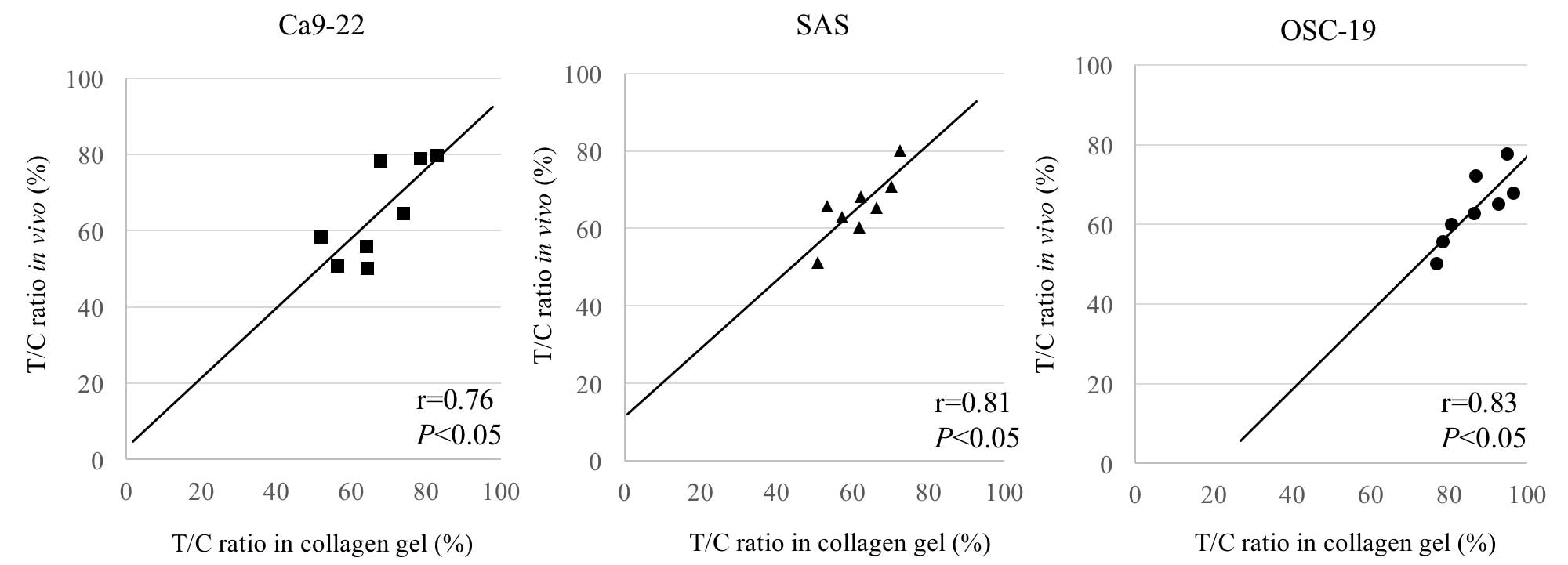
Comparison of CD-DST and nude mouse
results for CDDP
Of the 6 cell lines used (excluding KON),
engraftment was confirmed in the SAS, Ca9-22 and OSC-19 cell lines.
The results of treating nude tumor-bearing mice with CDDP at the
CED were compared with the CD-DST results for the same cell lines
of CDDP administered at the optimal contact concentration (0.5
µg/ml). The T/C values from CD-DST were as follows: Ca9-22, 67.3%;
SAS, 61.7%; and OSC-19, 86.6%. The T/C values from the nude mouse
CED method were as follows: Ca9-22, 64.6%; SAS, 65.6%; and OSC-19,
64.0% (P<0.05). The T/C values obtained from the two different
methods were therefore almost equal (Fig.
4).
Discussion
In previous years, there have been studies of
anticancer drug sensitivity tests used for the selection of
appropriate anticancer drugs for individual tumors (18,31,32). The
nude mouse grafting method for testing drug sensitivity was
reported in 1969 (12), and since
then, SRC methods have been developed (13). However, these drug sensitivity tests
were not well suited for clinical use, due to their low success
rate and high costs, and the time required for the results to be
obtained. In vitro tests, such as human tumor colony assay
and succinate dehydrogenase inhibition, were subsequently
announced, although there were challenges with the success rate of
the primary culture and drug contact concentration (33,34). The
CD-DST method, which was developed in 1995, is an in vitro
method of testing anticancer drug sensitivity that combines the 3D
culture of isolated cells embedded in collagen gel droplets and
image colorimetry (14). The
particular features of this method are that it allows testing using
smaller quantities of cells compared with conventional assays,
eliminates the effects of fibroblasts that may adulterate samples
at the time of harvesting, and allows the evaluation of
physiological drug concentrations (14,15).
Therefore, CD-DST is a useful method for predicting efficacy prior
to the administration of an anticancer drug. Measurement success
rates of ≥80% have been obtained for cancers, including colorectal
cancer (18), lung cancer (31) and breast cancer (32), and a high clinical efficacy prediction
rate of 91% has been obtained (35).
The CD-DST method is therefore expected to have future application
as an anticancer drug sensitivity test in fundamental research and
clinical practice involving OSCC. However, at present, optimal
anticancer drug contact concentrations have not been established
using the CD-DST method for OSCC; instead, tests are performed
using the contact concentrations for stomach cancer
(adenocarcinoma) (36). Sensitivity
has been clearly shown to differ according to the organ or tissue
type; therefore, there is a possibility of erroneous evaluation of
the antitumor efficacy of anticancer drugs for OSCC (18,31,32).
Establishing the optimal contact concentrations of anticancer drugs
for OSCC is therefore warranted.
In the present study, cell lines were used instead
of clinical specimens to establish optimal contact concentrations.
First, the specificity of each cell line was examined using RT-PCR.
No clear specificity in the expression of resistance genes or
enzymes associated with metabolism was observed in the 7 cell
lines. No ERCC1 expression was detected in the 7 cell lines. ERCC1
is a protein involved in nucleotide excision repair of DNA, and it
affects CDDP resistance (37,38). It also enhances DNA repair capacity by
acting as a rate-limiting enzyme that removes CDDP-DNA compounds in
the nucleotide cleavage and modification pathway (39). The group that did not express ERCC1
may be expected to exhibit a greater effect of CDDP than the group
that exhibited ERCC1; therefore, ERCC1 has increasingly broad
implications as a prognostic factor in CDDP-based chemotherapy
(40). By contrast, OPRT was
expressed in all cell lines. OPRT is considered to be involved in
DNA synthesis inhibition as well as RNA dysfunction by converting
5-FU to 5-fluorouridinemonophosphate (41). Although it was previously reported
that the sensitivity of 5-FU could be predicted from OPRT enzyme
activity (42), it has also been
reported that there is no correlation between OPRT expression and
antitumor efficacy (43,44). Watanabe et al (45) reported that a metabolic pathway
mediated by OPRT is central to drug efficacy expression in OSCC,
and that a high expression of OPRT is essential for drug
sensitivity. The present study showed that the 7 cell lines
possessed no clear indication of drug resistance and no specificity
in their drug sensitivity, thus prompting their use in the present
study.
First, the sensitivities of the 7 OSCC cell lines
toward CDDP and 5-FU were investigated at different contact
concentrations using the CD-DST method. The optimal contact
concentration of CDDP was 0.3 µg/ml, which is higher than that for
stomach cancer (0.2 µg/ml), and all cell lines showed low
sensitivity. It therefore appears that the optimal contact
concentration of CDDP for OSCC treatment is increased compared with
the optimal contact concentration for stomach cancer. With 5-FU,
all cell lines showed low sensitivity at 0.4 µg/ml, which is lower
than the optimal contact concentration for stomach cancer (1.0
µg/ml). At 0.9 µg/ml, 1 of the 7 cell lines (Ca9-22 cells) showed
high sensitivity, and the cumulative efficacy rate was 14.2%.
Taking into consideration that the clinical response rate obtained
by single-drug treatment with 5-FU was 13.0%, it could be deduced
that the optimal contact concentration of 5-FU is likely be lower
for OSCC than for stomach cancer.
The optimal contact concentrations of CDDP and 5-FU
for OSCC were calculated from the CD-DST of the 7 cell lines,
according to the method described by Nagai et al (25) and were compared with the contact
concentrations currently used clinically for stomach cancer (0.2
µg/ml) (24). CDDP had an optimal
contact concentration value of 0.5 µg/ml, which was increased
compared with that of stomach cancer (0.2 µg/ml), whereas 5-FU had
an optimal contact concentration value of 0.7 µg/ml, which was
decreased compared with that of stomach cancer (1.0 µg/ml). Using
CD-DST, Nagai et al (25)
found the optimal contact concentration of CDDP to be ~1.0 µg/ml
for uterine body and cervical cancers and 2.0 µg/ml for ovarian
cancer, which are all increased compared with the contact
concentrations of stomach cancer (0.2 µg/ml). Thus, the sensitivity
to an anticancer drug varies according to the organ or tissue type.
In addition, the clinical response rate obtained by single-drug
treatment with CDDP has been reported to be 57.8% for ovarian
cancer (8), 39.4% for cervical cancer
(9), and 19.1% for stomach cancer
(10). There is therefore a trend for
the contact concentration to decrease at lower clinical response
rates. The clinical response rate of CDDP for OSCC is 26.3%
(26); therefore, on the basis of the
aforementioned trend, the optimal contact concentration of 0.5
µg/ml appears valid.
An in vivo verification test was performed to
examine the clinical efficacy of CDDP, in which nude mice were used
as models of clinical efficacy in humans. The cell lines were
transplanted into nude mice, and the tumor-bearing nude mice were
administered a single dose of CDDP by injection into the tail vein
with the CED of 7.0 mg kg established by Inaba et al
(29) in order to reproduce the
antitumor efficacy of anticancer drugs in humans. This method was
used to predict the clinical antitumor efficacy. The in
vitro antitumor efficacy is generally determined by the
concentration of the drug in culture and the time for which it
acts. However, it is extremely challenging to reproduce changes in
blood concentration at the clinical dose in mice. Using the CED
in vivo facilitated the determination of the antitumor
efficacy similar to when the drug is administered to humans
(26,27). The present in vivo verification
test was performed with the CED of CDDP, and the clinical effects
appear to have been reproduced. The T/C value from CD-DST, with an
optimal contact concentration of 0.5 µg/ml, and the T/C values from
the CED nude mouse method were almost in agreement for the SAS,
Ca9-22 and OSC-19 cell lines (P<0.05), indicating that CD-DST is
likely to be predictive of clinical efficacy.
CD-DST combines the collagen gel droplet culture
method, a simple 3D culture method that allows the analysis of
extremely small clinical samples, with a serum-free medium step and
quantitative evaluation by image analysis. Furthermore, CD-DST has
little effect on non-cancerous cells, allowing accurate
measurements of cancerous cells only. Thus, CD-DST is an ideal
anticancer drug sensitivity test that overcomes the challenges of
other sensitivity tests, and CD-DST is widely used in clinical
practice for lung, breast and colorectal cancer (18,31,32). In
addition to OSCC, chemotherapy based on anticancer drug sensitivity
tests offers patients increased freedom of choice. CD-DST is likely
to be important in the selection of treatment, in terms of function
preservation and quality of life, and also as a treatment option in
unresectable, intractable or recurrent cases. However, the optimal
contact concentrations determined in the present study are a basic
stage for performing tests using clinical samples. Additional
sensitivity tests performed on clinical samples are required, using
the calculated optimal contact concentrations to evaluate
reproducibility on the basis of clinical data.
References
|
1
|
Adelstein DJ, Li Y, Adams GL, Wagner H Jr,
Kish JA, Ensley JF, Schuller DE and Forastiere AA: An intergroup
phase III comparison of standard radiation therapy and two
schedules of concurrent chemoradiotherapy in patients with
unresectable squamous cell head and neck cancer. J Clin Oncol.
21:92–98. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wendt TG, Grabenbauer GG, Rödel CM, Thiel
HJ, Aydin H, Rohloff R, Wustrow TP, Iro H, Popella C and Schalhorn
A: Simultaneous radiochemotherapy versus radiotherapy alone in
advanced head and neck cancer: a randomized multicenter study. J
Clin Oncol. 16:1318–1324. 1998.PubMed/NCBI
|
|
3
|
Adelstein DJ, Lavertu P, Saxton JP, Secic
M, Wood BG, Wanamaker JR, Eliachar I, Strome M and Larto MA: Mature
results of a phase III randomized trial comparing concurrent
chemoradiotherapy with radiation therapy alone in patients with
stage III and IV squamous cell carcinoma of the head and neck.
Cancer. 88:876–883. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Fuwa N, Ito Y, Matsumoto A, Kamata M,
Kodaira T, Furutani K, Sasaoka M, Kimura Y and Morita K: A
combination therapy of continuous superselective intraarterial
carboplatin infusion and radiation therapy for locally advanced
head and neck carcinoma. Phase I study. Cancer. 89:2099–2105. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Posner MR, Hershock DM, Blajman CR,
Mickiewicz E, Winquist E, Gorbounova V, Tjulandin S, Shin DM,
Cullen K, Ervin TJ, Murphy BA, et al: TAX 324 Study Group:
Cisplatin and fluorouracil alone or with docetaxel in head and neck
cancer. N Engl J Med. 357:1705–1715. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Forastiere AA, Goepfert H, Maor M, Pajak
TF, Weber R, Morrison W, Glisson B, Trotti A, Ridge JA, Chao C, et
al: Concurrent chemotherapy and radiotherapy for organ preservation
in advanced laryngeal cancer. N Engl J Med. 349:2091–2098. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Suzuki M, Ishikawa H, Tanaka A and Mataga
I: Heterogeneity of anticancer drug sensitivity in squamous cell
carcinoma of the tongue. Human Cell. 24:21–29. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kato T: Phase II study of
CIS-diamminedichloroplatinum (II) by collaborative study. Gan To
Kagaku Ryoho. 9:694–701. 1982.(In Japanese). PubMed/NCBI
|
|
9
|
Noda K, Takeuchi S, Kurihara S, Sugawa T,
Kato T, Ikeda M, Kanazawa K, Tsutsui F, Yamamoto K, Umezu J, et al:
Phase II study of cisplatin in cervical and endometrial carcinomas.
Gan To Kagaku Ryoho. 14:1129–1135. 1987.(In Japanese). PubMed/NCBI
|
|
10
|
Ishibiki K, Kumai K, Kodaira S, Abe O,
Yamamoto K, Oouchi T, Fukaya Y, Kimura K, Takamatsu K, Ootsuka E,
et al: Phase II study with cisplatin in advanced stomach and colon
carcinoma. Cooperative study group of cisplatin for stomach and
colon carcinoma. Gan To Kagaku Ryoho. 16:3185–3193. 1989.(In
Japanese). PubMed/NCBI
|
|
11
|
Kondo T, Kubota T, Tanimura H, Yamaue H,
Akiyama S, Maehara Y, Tanigawa N, Kitajima M and Takagi H:
Cumulative results of chemosensitivity tests for antitumor agents
in Japan. Japan Research Society for Appropriate Cancer
Chemotherapy. Anticancer Res. 20:2389–2392. 2000.PubMed/NCBI
|
|
12
|
Rygaard J and Povlsen CO:
Heterotransplantation of a human malignant tumor to ‘Nude’ mice.
Acta Pathol Microbiol Scand. 77:758–760. 1969. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bogden AE, Cobb WR, Lepage DJ, Haskell PM,
Gulkin TA, Ward A, Kelton DE and Esber HJ: Chemotherapy
responsiveness of human tumors as first transplant generation
xenografts in the normal mouse: Six-day subrenal capsule assay.
Cancer. 48:10–20. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Kobayashi H, Tanisaka K, Kondo N, Mito Y,
Koezuka M, Yokouchi H, Higashiyama M, Kodama K, Doi O, Yamada M, et
al: Development of new in vitro chemosensitivity test using
collagen gel droplet embedded culture and its clinical usefulness.
Gan To Kagaku Ryoho. 22:1933–1939. 1995.(In Japanese). PubMed/NCBI
|
|
15
|
Kobayashi H, Higashiyama M, Minamigawa K,
Tanisaka K, Takano T, Yokouchi H, Kodama K and Hata T: Examination
of in vitro chemosensitivity test using collagen gel droplet
culture method with colorimetric endpoint quantification. Jpn J
Cancer Res. 92:203–210. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Kobayashi H: Development of a new in vitro
chemosensitivity test using collagen gel droplet culture and image
analysis for clinical usefulness. Recent Results Cancer Res.
161:48–61. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hanatani Y, Kobayashi H, Kodaira S, Takami
H, Asagoe T and Kaneshiro E: An in vitro chemosensitivity test for
gastric cancer using collagen gel droplet embedded culture. Oncol
Rep. 7:1027–1033. 2000.PubMed/NCBI
|
|
18
|
Araki Y, lsomoto H, Matsumoto A, Kaibara
A, Yasunaga M, Hayashi K, Yatsugi H and Yamauchi K: An in vitro
chemosensitivity test for colorectal cancer using collagen-gel
droplet embedded cultures. Kurume Med J. 46:163–166. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shintani S, Hino S, Nakashiro K and
Hamakawa H: Clinical trial of chemotherapy identified according to
chemosensitivity assay for oral cancer patients with unresectable
recurrent lesions. Gan To Kagaku Ryoho. 33:357–360. 2006.(In
Japanese). PubMed/NCBI
|
|
20
|
Matsumoto Y, Tamiya T and Nagano S:
Resistance to topoisomerase II inhibitors in human glioma cell
lines overexpressing multidrug resistant associated protein (MRP)
2. J Med Invest. 52:41–48. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Selvakumaram M, Pisarcik DA, Bao R, Yeung
AT and Hamilton TC: Enhanced cisplatin cytotoxicity by disturbing
the nucleotide excision repair pathway in ovarian cancer cell
lines. Cancer Res. 63:1311–1316. 2003.PubMed/NCBI
|
|
22
|
Bartolucci R, Wei J, Senchez JJ,
Perez-Roca L, Chaib I, Puma F, Farabi R, Mendez P, Roila F, Okamoto
T, et al: XPG mRNA expression levels modulate prognosis in resected
non-small-cell lung cancer in conjunction with BRCA1 and ERCC1
expression. Clin Lung Cancer. 10:47–52. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Park JS, Yoon S Young, Kim JM, Yeom YI,
Kim YS and Kim NS: Identification of novel genes associated with
the response to 5-FU treatment in gastric cancer cell lines using a
cDNA microarray. Cancer Lett. 214:19–33. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Koezuka M, Kondo N, Kobayashi H, Hara S,
Yasutomi M, Nishida S, Hashimoto S and Asano H: Drug sensitivity
test for human cancer-cells using collagen gel embedded culture and
image analysis. Int J Oncol. 2:953–959. 1993.PubMed/NCBI
|
|
25
|
Nagai N, Minamikawa K, Mukai K, Hirata E,
Komatsu M and Kobayashi H: Predicting the chemosensitivity of
ovarian and uterine cancers with the collagen gel droplet culture
drug-sensitivity test. Anticancer Drugs. 16:525–531. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Inuyama Y and Takeda C: A cooperative
phase II study of cisplatin in patients with head and neck cancer.
Gan To Kagaku Ryoho. 13:232–238. 1986.(In Japanese). PubMed/NCBI
|
|
27
|
Jacobs C, Lyman G, Velez-García E, Sridhar
KS, Knight W, Hochster H, Goodnough LT, Mortimer JE, Einhorn LH,
Schacter L, et al: A phase III randomized study comparing cisplatin
and fluorouracil as single agents and in combination for advanced
squamous cell carcinoma of the head and neck. J Clin Oncol.
10:257–263. 1992.PubMed/NCBI
|
|
28
|
Inaba M, Kobayashi T, Tashiro T and
Sakurai Y: Pharmacokinetic approach to rational therapeutic dose
for human tumor-bearing nude mice. Jpn J Cancer Res. 79:509–516.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Inaba M: Pharmacokinetic approach to the
improvement of clinical predictability in the preclinical test for
antitumor agents. Gan To Kagaku Ryoho. 18:1449–1456. 1991.(In
Japanese). PubMed/NCBI
|
|
30
|
Geran RI, Greenberg NH, Macdonald MM, et
al: Protocols against animal tumors and other biological systems.
Cancer Chemother Rep. 3:51–57. 1972.
|
|
31
|
Kawamura M, Inoue Y, Oyama T and Kobayashi
K: Chemosensitivity test for uresectable non-small cell lung
cancer. Nihon Geka Gakkai Zasshi. 103:229–232. 2002.(In Japanese).
PubMed/NCBI
|
|
32
|
Takamura Y, Kobayashi H, Taguchi T,
Motomura K, Inaji H and Noguchi S: Prediction of chemotherapeutic
response by collagen gel droplet embedded culture-drug sensitivity
test in human breast cancers. lnt J Cancer. 98:450–455. 2002.
|
|
33
|
Kondo T, lmamura T and lchibashi H: In
vitro test for sensitivity of tumor to carcinostatic agents. Gan.
57:113–121. 1966.PubMed/NCBI
|
|
34
|
Salomon SE, Hamburger AW, Soehnlen B,
Durie BG, Alberts DS and Moon TE: Quantitation of differential
sensitivity of human-tumor stem cell to anticancer drugs. N Engl J
Med. 298:1321–1327. 1978. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Kobayashi H: CD-DST (Collagen gel droplet
embedded culture drug sensitivity test). Oncology Chemotherapy.
16:231–235. 2000.
|
|
36
|
Shintani S, Hino S, Nakashiro K and
Hamakawa H: Clinical trial of chemotherapy identified according to
chemosensitivity assay for oral cancer patients with unresectable
recurrent lesions. Gan To Kagaku Ryoho. 33:357–360. 2006.PubMed/NCBI
|
|
37
|
Rosell R, Taron M, Barnadas A, Scagliotti
G, Sarries C and Roig B: Nucleotide excision repair pathways
involved in Cisplatin resistance in non-small-cell lung cancer.
Cancer Control. 10:297–305. 2003.PubMed/NCBI
|
|
38
|
Reardon JT, Vaisman A, Chaney SG and
Sancar A: Efficient nucleotide excision repair of cisplatin,
oxaliplatin, and
Bis-aceto-ammine-dichloro-cyclohexylamine-platinum(IV) (JM216)
platinum intrastrand DNA diadducts. Cancer Res. 59:3968–3971.
1999.PubMed/NCBI
|
|
39
|
Takenaka T, Yoshino I, Kouso H, Ohba T,
Yohena T, Osoegawa A, Shoji F and Maehara Y: Combined evaluation of
Rad51 and ERCC1 expressions for sensitivity to platinum agents in
non-small cell lung cancer. Int J Cancer. 121:895–900. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Simon GR, Sharma S, Cantor A, Smith P and
Bepler G: ERCC1 expression is a predictor of survival in resected
patients with non-small cell lung cancer. Chest. 127:978–983. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Fukushima M, Nomura H, Murakami Y,
Shirasaka T and Aiba K: Estimation of pathways of 5-fluorouracil
anabolism in human cancer cells in vitro in vivo. Gan To Kagaku
Ryoho. 23:721–731. 1996.(In Japanese). PubMed/NCBI
|
|
42
|
Sakamoto K, Sugimoto Y, Miyadera K, Oka T
and Fukushima M: Preparation of anti-OPRT antibody for
immunochemical detection. Gan To Kagaku Ryoho. 32:653–658. 2005.(In
Japanese). PubMed/NCBI
|
|
43
|
Ishida H, Shirakawa K, Ohsawa T, Sobajima
J, Hayashi Y, Nakada H, Yokoyama M and Hashimoto D: Expression of
mRNA levels of thymidilate synthase, dihydropyrimidine
dehydrogenase, and orotate phosphoribosyltransferase of colorectal
cancer-relationships among mRNA levels in association with response
to 5-FU based treatment. Gan To Kagaku Ryoho. 32:1929–1934.
2005.(In Japanese). PubMed/NCBI
|
|
44
|
Yamada T, Tanaka N, Yokoi K, Ishikawa N,
Seya T, Kanazawa Y, Shirakawa T, Ohkawa K, Koizumi M, Ohaki Y, et
al: Correlation between clinical pathologic factors and enzymatic
activity of orotate phosphoribosyl transferase (OPRT),
dihydropyrimidine dehydrogenase (DPD) and thymidylate synthase (TS)
in colorectal cancer. Gan To Kagaku Ryoho. 33:789–793. 2006.(In
Japanese). PubMed/NCBI
|
|
45
|
Watanabe M, Satomi T, Matuda K, et al:
Relationship between expression of enzymes activating
fluoropyrimidine anti-tumor agents and response to the agents in
oral squamous cell carcinoma. Japanese Journal of Head and Neck
Cancer. 34:498–502. 2008.
|