Introduction
Esophageal cancer is the eighth most common cancer
and the sixth leading cause of cancer mortality, which causes ~30
million mortalities worldwide and 15 million mortalities in China,
almost half of the total mortality, each year (1–3).
Esophageal squamous cell carcinoma (ESCC) is the dominant
histopathological subtype of esophageal cancer (1–3).
Radiotherapy has been used either as a definitive therapy for
esophageal cancer patients with locally advanced disease or as an
adjuvant therapy following radical esophagectomy for esophageal
cancer patients (1–3). However, an hypoxic microenvironment
exists in esophageal carcinomas, which leads to radiation
resistance and poor clinical outcomes, and may be an important
determinant of radioresistance (4,5). Free
oxygen radicals are generated during radiotherapy that induce DNA
damage and kill tumor cells. The lack of oxygen directly activates
the expression of hypoxia-inducible factor 1 (HIF-1), which
consists of an oxygen-sensitive subunit, HIF-1α, and a
constitutively expressed subunit, HIF-1β (5,6). HIF-1 is
a pivotal regulatory factor that enables tumor cells to endure an
hypoxic microenvironment, and promotes tumor growth, angiogenesis,
invasion and metastasis (6).
Additionally, HIF-1 activates the transcription of downstream genes
such as vascular endothelial growth factor (VEGF), and indirectly
reflects the extent of carcinoma oxygenation (6,7).
Overexpression of HIF-1α has been reported to be associated with a
poor prognosis following radiotherapy in patients with esophageal
cancer (8). The suppression of HIF-1α
expression may reversed by the radioresistant phenotype of hypoxic
cancer cells (9,10).
Sunitinib, a highly selective multi-targeted
receptor tyrosine kinase inhibitor, has been reported to have
direct antitumor effects against various cancers, and to enhance
tumor radiosensitivity in breast tumors (11), pancreatic cancer (12) and colon cancer (13). In particular, sunitinib suppressed
cycling hypoxia in tumors and maximized the effects of combination
therapy with anti-angiogenic drugs (14). Furthermore, sunitinib was shown to
downregulate the expression of HIF-1α, and subsequently, that of
VEGF, in human embryonic stem cells (15) and HT-29 colon cancer cells (16).
However, whether sunitinib suppresses HIF-1α in
esophageal cancer cells has not been elucidated yet. In the present
study, it was demonstrated that sunitinib could inhibit HIF-1α and
VEGF expression in ESCC cells, and thus mediate the
radiosensitization of ESCC cells to irradiation (IR)
significantly.
Materials and methods
Reagents and cell lines
Sunitinib (S1042; Selleck Chemicals, Houston, TX,
USA) was dissolved in dimethyl sulfoxide (DMSO; Sigma-Aldrich;
Merck Millipore, Darmstadt, Germany) as a concentrated stock
solution of 10 mg/ml. The human malignant esophageal cancer cell
line ECA109 was obtained from the Shanghai Institute of Cell
Biology (Shanghai, China). Cells were cultured in Dulbecco's
modified Eagle's medium (Gibco; Thermo Fisher Scientific, Inc.,
Waltham, MA, USA) supplemented with 10% fetal bovine serum
(Hyclone; GE Healthcare Life Sciences, Logan, UT, USA) and 1%
penicillin/streptomycin (Invitrogen; Thermo Fisher Scientific,
Inc.). Cells were kept under conditions of 5% CO2 in an
incubator at 37°C.
Hypoxia and IR protocols
Hypoxia was induced by incubating cells in an
hypoxia chamber [a glass chamber maintaining 0.5–1.0% partial
pressure of oxygen (pO2)]. IR was performed at 566
cGy/min using an X-ray medical linear accelerator (Elekta AB,
Stockholm, Sweden). Cells were irradiated at a single dose at room
temperature.
MTT assay
Cell cytotoxicity effect was measured by MTT assay.
ECA109 cells were seeded into 96-well plates at a concentration of
5–6×103 cells/well and allowed to adhere. Next, the
cells were treated with increasing sunitinib doses (0, 1, 2.5, 5,
10, 15, 20 and 25 µM). After 24 or 48 h of exposure to sunitinib,
10 µl of 5 mg/ml MTT reagent was added to each well. After
incubation for 4 h, the supernatants were removed, and 150 µl of
DMSO was added to dissolve the MTT crystals (formazan). The
absorbance of the plates was measured at a wavelength of 490 nm
using a microplate reader (ELx800; BioTek Instruments, Inc.,
Winooski, VT, USA). The half maximal inhibitory concentration
(IC50) values were calculated using the SPSS 17.0
software (SPSS, Inc., Chicago, IL, USA). Each experiment was
performed thrice.
Cell proliferation assay
ECA109 cells were seeded into 96-well plates. The
cells were incubated overnight and then treated with the indicated
concentrations of sunitinib (1 or 2.5 µM) under normoxic or hypoxic
conditions for 24 h, and then subjected to X-rays at 8 Gy. After 24
h, cell proliferation was assessed by MTT assay. The percentage
cell growth inhibition for each group was calculated by adjusting
the control group to 100%. Each experiment was performed
thrice.
Clonogenic assay
ECA109 cells were seeded into 6-well plates. The
cells were incubated overnight and then treated with the indicated
concentrations of sunitinib (1 or 2.5 µM) or DMSO (control) under
normoxic or hypoxic conditions for 24 h, and then subjected to
X-rays at 2, 4, 6 or 8 Gy. Subsequently, the cells were incubated
at 37°C for 10–14 days under normoxic conditions, fixed with
methanol and stained with Giemsa for 30 min. Finally, the plates
were examined under the microscope, and the number of colonies with
≥50 cells was counted. The cell survival curves were fitted
according to a single-hit multi-target model, and the survival
enhancement ratio (SER) was calculated as the ratio of the mean
inactivation dose in control cells divided by the mean inactivation
dose in sunitinib-treated cells. Each experiment was performed
thrice.
Apoptosis assay
Annexin-V/fluorescein isothiocyanate (FITC) and
propidium iodide (PI) dual staining was performed to determine the
percentage of apoptotic cells. The cells were seeded into 6-well
plates and treated with or without sunitinib (1 or 2.5 µM) under
normoxic or hypoxic conditions for 24 h. Subsequently, the cells
were subjected to X-ray IR (8 Gy). The cells were collected 48 h
after IR and analyzed with BD Pharmingen™ FITC Annexin V Apoptosis
Detection kit (BD Biosciences, Franklin Lakes, NJ, USA) by flow
cytometry. Each experiment was performed thrice.
Cell cycle analysis
ECA109 cells were incubated in 6-well plates
(1×106 cells/well) and then divided into the following
groups: Normoxia (Norm), hypoxia (Hypo), sunitinib 1 µM (SU 1 µM)
and sunitinib 2.5 µM (SU 2.5 µM). The groups of SU 1 µM and SU 2.5
µM were pretreated with 1 µM or 2.5 µM sunitinib. After 24 h, all
cells were collected and washed with cold 1X PBS, and then
resuspended in 70% ethanol at 4°C overnight. The cells were
incubated with 6 µl of 1 g/l RNase A, 1 ml of 1 mg/ml PI and 400 µl
of PBS at room temperature for 15 min. The cell cycle distribution
was analyzed using flow cytometry. Each experiment was performed
thrice.
Western blot analysis
Total proteins were extracted from the cells using
SDS Lysis Buffer (Sigma-Aldrich; Merck Millipore) at 24 h after the
last sunitinib treatment under normoxic or hypoxic conditions. The
protein concentrations of the supernatants were determined by
bicinchoninic acid assay. Equal amounts of protein were loaded into
each lane, and proteins were separated by 10% SDS-PAGE and
transferred to polyvinylidene difluoride membranes (EMD Millipore,
Billerica, MA, USA). The membranes were blocked with 5% skim milk,
incubated with primary antibodies against HIF-1α (14179; dilution,
1:250; Cell Signaling Technology, Inc., Danvers, MA, USA), VEGF
(sc-507; dilution, 1:250) and GAPDH (sc-25778; dilution, 1:1,000)
(Santa Cruz Biotechnology, Inc., Dallas, TX, USA) at 4°C overnight,
and then incubated with horseradish peroxidase-conjugated secondary
antibodies (BS13278; dilution, 1:1,000; Bioworld Technology, Inc.,
St. Louis Park, MN, USA) for 1 h at room temperature. The
immunoblotted proteins were visualized with enhanced
chemiluminescence reagents (EMD Millipore), and the signals were
detected using the ChemiDoc™ XRS+ imaging system (Bio-Rad
Laboratories, Inc., Hercules, CA, USA) and analyzed with Quantity
One quantitation software (Bio-Rad Laboratories, Inc.).
Statistical analysis
All data are expressed as means ± standard
deviation. Data were analyzed using SPSS 17.0 software (SPSS,
Inc.). Survival curves were fitted using GraphPad Prism 5.0
(GraphPad Software, Inc., La Jolla, CA, USA). Student's t
test was applied to compare the groups. P<0.05 was considered to
indicate a statistically significant difference.
Results
Sunitinib inhibits human ESCC cell
proliferation
MTT assay was performed at 24 and 48 h following
sunitinib administration at various concentrations (≤25 µM) to
determine the sensitivity of human ESCC cells to sunitinib as a
single agent. The IC50 value for ECA109 cells at 24 h
was 7.07 µM. Fig. 1A demonstrates
that sunitinib produced a cytotoxic effect in a dose-dependent
manner. The survival rates in 1 or 2.5 µM sunitinib-treated ECA109
cells for 24 h were 89.23 and 78.29%, respectively. These results
indicated a low cytotoxic effect on the growth of ESCC cells. Thus,
these low-cytotoxic concentrations (1 and 2.5 µM) were selected for
the following assays.
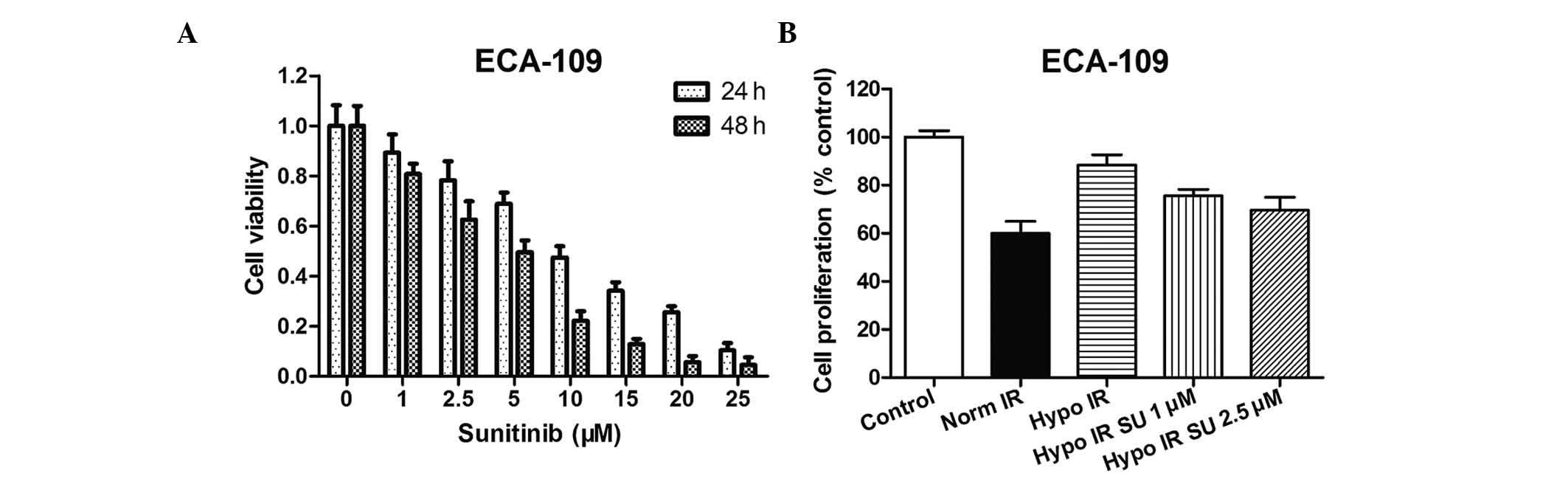 | Figure 1.Effect of sunitinib on cell
proliferation. (A) MTT assay was performed to assess the
cytotoxicity effect of treatment with increasing doses of sunitinib
(0, 1, 2.5, 5, 10, 15, 20 and 25 µM) for 24 or 48 h in ECA109
cells. (B) ECA109 cells were treated with sunitinib (1 or 2.5 µM)
under normoxic or hypoxic conditions for 24 h. Cell proliferation
was measured by MTT assay. The percentage of cell growth inhibition
was calculated by adjusting the control group to 100%. Data were
presented as the men ± standard error of the mean and were
normalized to the control cells. Hypo, hypoxia; Norm, normoxia; SU,
sunitinib; IR, irradiation. |
MTT assay was also performed to assess the effect of
sunitinib on hypoxic ECA109 cells. ECA109 cells under hypoxic
conditions (0.8–1.0% pO2) exhibited a significant
resistance to IR, while sunitinib sensitized hypoxic cancer cells
to IR. The cell growth inhibition with sunitinib treatment was
significantly lower than that without sunitinib treatment (Fig. 1B).
Sunitinib enhances radiation-induced
apoptosis in both normoxic and hypoxic ESCC cells
Annexin-V/PI staining was performed to quantify the
apoptosis of hypoxic ESCC cells exposed to IR after sunitinib
treatment for 48 h (Fig. 2A and B).
The results revealed that the apoptosis rate was significantly
lower in the hypoxic group than in the normoxic group (P<0.05).
Following treatment with sunitinib at 1 or 2.5 µM, the apoptosis
rate was higher compared with that of hypoxia alone
(P<0.05).
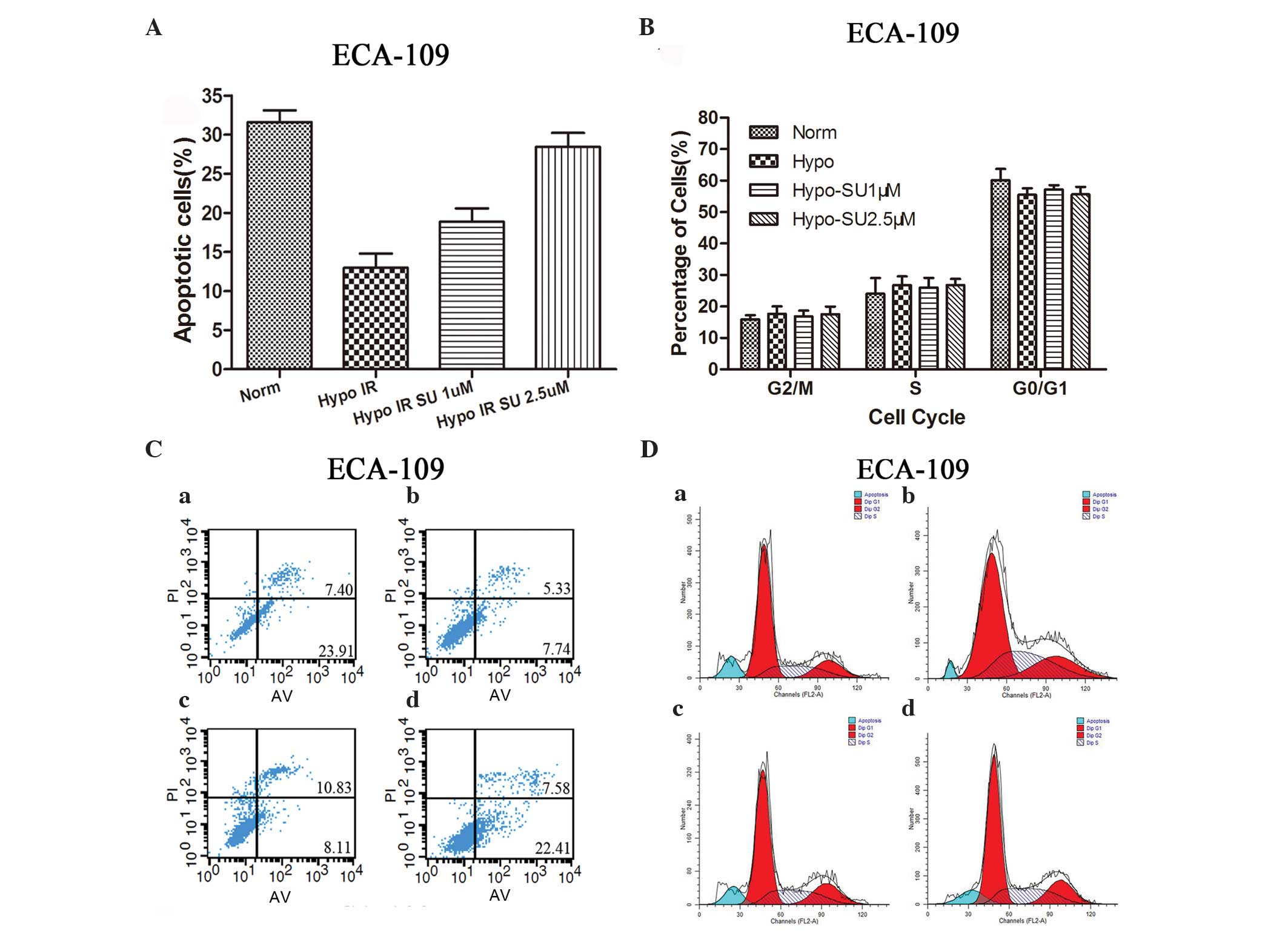 | Figure 2.Effect of sunitinib on cell apoptosis
and cell cycle distribution. (A and C) ECA109 cells were treated
with sunitinib (1 or 2.5 µM) under normoxic or hypoxic conditions
for 24 h and then subjected to X-ray IR (8 Gy). Following 48 h, the
percentage of apoptotic cells was evaluated using flow cytometry.
(B and D) ECA109 cells were divided into four groups: (a) Norm, (b)
Hypo, (c) SU 1 µM and (d) SU 2.5 µM μM. Cells were subjected to
X-ray IR (6 Gy) and analyzed using flow cytometry 24 h later. Data
were presented as the mean ± standard error of the mean. Hypo,
hypoxia; Norm, normoxia; SU, sunitinib; IR, irradiation; PI,
propidium iodide; AV, Annexin V/FITA; Dip, Diploid; FL2-A,
FL2-area. |
Sunitinib radiosensitizes ESCC cells,
but does not alter their cell cycle distribution
The percentage of cells in each phase of the cell
cycle in the different groups are summarized in Fig. 2C and D. Compared with the normoxia
group, no significant accumulation of ECA109 cells in the G0/G1 or
G2/M phases was noted in the hypoxia alone group or in the hypoxia
group treated with sunitinib at 1 or 2.5 µM.
Sunitinib significantly enhances ESCC
cell radiosensitivity
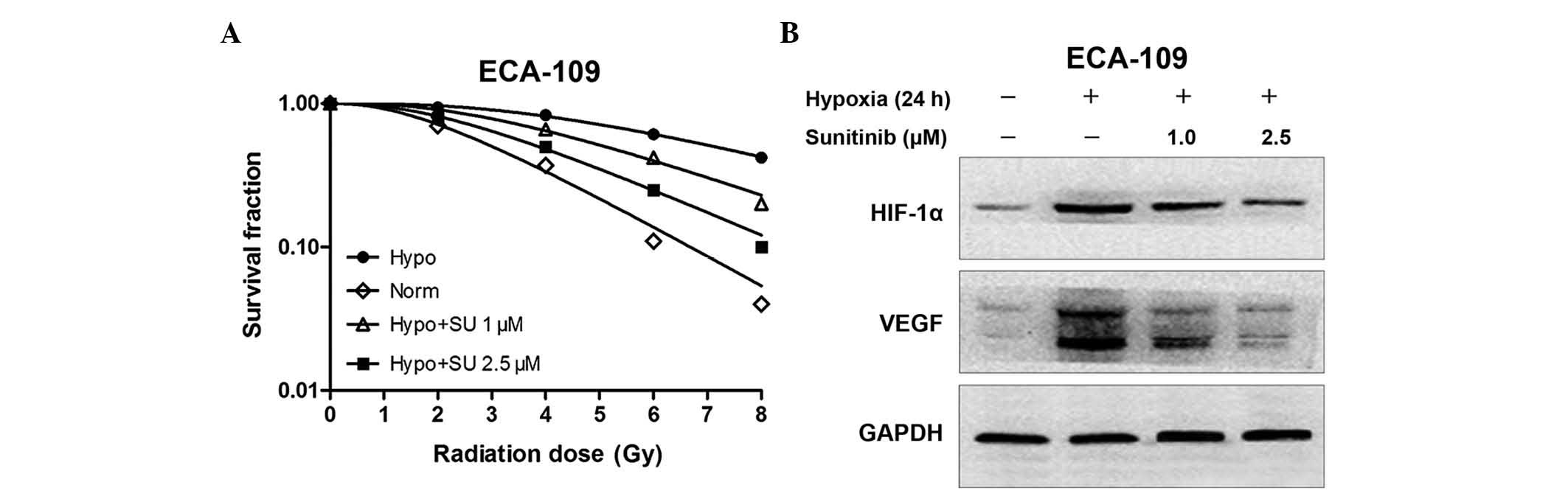
Clonogenic survival assays were performed to assess
the potential radiosensitization activity of sunitinib. Cells under
hypoxic conditions (0.8–1.0% pO2) exhibited a
significant increase in their ability to form colonies following
IR, indicating their resistance to IR. However, sunitinib
sensitized hypoxic cancer cells to IR significantly. The
dose-survival curves are shown in Fig.
3A. The surviving fraction (SF) data were fitted into the
following single-hit multi-target model formula:
SF=1-(1-e−D/D0)n, where D0 is the
mean lethal dose and Dq is the quasi-threshold dose. The
results revealed that the SFs at 2 Gy (SF2)s were 0.48 and 0.67 for
ECA109 cells under normoxic and hypoxic conditions, respectively.
Upon treatment with sunitinib at 1 or 2.5 µM, the SF2 of hypoxic
cells increased to 0.61 or 0.58 in ECA109 cells, respectively. The
survival enhancement ratio (SER) was calculated according to the
clonogenic results: SER = D0 (test group)/D0
(hypo group). The SERs of hypoxic cells were 1.31 or 1.59 in ECA109
cells treated with sunitinib at 1 or 2.5 µM, respectively, compared
with the hypoxic condition alone (Table
I). These data demonstrated that treating hypoxic ESCC cells
with sunitinib resulted in a significant radiosensitization
effect.
 | Table I.Radiosensitization activity of
sunitinib in hypoxic ECA109 cells. |
Table I.
Radiosensitization activity of
sunitinib in hypoxic ECA109 cells.
| ECA109 cells |
D0a |
Dqa | SF2b | SER |
|---|
| Hypo | 4.15 | 5.22 | 0.67 | – |
| Norm | 2.07 | 1.99 | 0.48 | 2.0 |
| Hypo SU 1 µM | 3.18 | 3.60 | 0.61 | 1.31 |
| Hypo SU 2.5 µM | 2.61 | 2.59 | 0.58 | 1.59 |
Sunitinib radiosensitizes ESCC cells
by inhibiting the expression of HIF-1α and VEGF
Western blot analysis was performed to confirm the
effect of sunitinib on the VEGF and HIF-1α expression induced by
hypoxia. ECA109 cells were treated with 1 or 2.5 µM sunitinib for
24 h. It was observed that hypoxia increased the expression of VEGF
and HIF-1α. However, sunitinib could inhibit the expression of VEGF
and HIF-1α, particularly at high doses (Fig. 3B).
Discussion
In spite of the excellent progress in IR techniques
and treatment strategies, achievements in advanced esophageal
cancers are still unsatisfactory, with a 5-year survival rate of
20–30% and a locoregional control rate of only 45% (1,17). Thus,
novel radiosensitizing agents to overcome the resistance to
conventional radiotherapeutic interventions are urgently required.
The present study demonstrated for the first time that sunitinib
could significantly promote the radiosensitivity of ESCC ECA109
cells, and that this promotion was associated with the inhibition
HIF-1α and VEGF expression induced by the hypoxic microenvironment.
The present study confirmed that sunitinib could apparently inhibit
human ESCC cell viability and proliferation. It was also noticed
that sunitinib radiosensitized esophageal cancer cells by
inhibiting the clonogenic growth of hypoxic ECA109 cells following
IR. Compared with that of the hypoxia and IR groups, the apoptosis
rate of the group treated with sunitinib increased in a
dose-dependent manner. The radiosensitivity of sunitinib in hypoxic
ESCC cells was associated with the inhibition of hypoxia-induced
HIF-1α and VEGF expression. Compared with the normoxia group, there
were no changes in cell cycle distribution in the groups subjected
to sunitinib. Thus, the radiosensitizing effect of sunitinib may be
independent of the cell cycle distribution. These results will
expand our understanding of the effect of sunitinib activity, and
suggest that sunitinib may be a potential radiosensitizing agent
for the treatment of esophageal cancer.
Radiotherapy is a crucial treatment modality for
esophageal carcinoma (2). However,
due to radioresistance, the radiotherapy effects are often
unsuccessful (2). Recent studies have
demonstrated that an hypoxic microenvironment is one of the crucial
factors in radioresistance to radiation therapy (RT) in solid
tumors, resulting from the unbalance between increased oxygen
consumption by the extensive growth of tumor cells and decreased
oxygen delivery by disordered tumor blood vessels (18). The tumor vasculature was also observed
to be an effective target for the cytotoxic effects of RT (19). The inherent resistance of the tumor
blood vessels to the cytotoxic effects of RT required to be
overcome (19). HIF-1α is a
well-recognized hallmark of hypoxic microenvironments and an
important regulator of the hypoxic response, participating in the
regulation of aerobic glycolysis to enable the growth of cancer
cells (14,20). Previous evidence has suggested that
radiation prevented the accumulation of HIF-1α, which protected the
tumor vasculature from radiation damage by inducing VEGF expression
(6). In addition, tumor cells under
hypoxic conditions exhibited cancer cell phenotypes with enhanced
pro-survival pathways, acquiring increased malignant potential and
resistance to radiotherapy (21,22).
Furthermore, preclinical studies consistently demonstrated an
increase in radiosensitization upon suppression of HIF-1α and VEGF
(23,24).
In the present study, sunitinib, an oral
multi-tyrosine kinase inhibitor, was used, since this agent has
demonstrated beneficial effects in clinical phase II studies with
patients with advanced esophageal or gastroesophageal junction
cancer (25). Sunitinib exhibited
broad and potent antitumor activity in breast tumors, pancreatic
cancer and colon cancer (11–13). Additionally, sunitinib had been shown
to transiently improve tumor oxygenation, normalize tumor
vasculature, suppress tumor cycling hypoxia and enhance the tumor
response to radiotherapy (14,26). In
particular, sunitinib was revealed to inhibit cellular signaling
via HIF-1α and subsequent VEGF in human embryonic stem cell
(15), HT-29 colon cancer cells
(16) and melanoma xenografts
(27). Therefore, the present study
first investigated the potential of sunitinib as a potent HIF-1α
inhibitor in ESCC cells, and observed that HIF-1α and VEGF
expression were suppressed by sunitinib. These data suggest that
sunitinib could sensitize hypoxic ESCC cells to radiotherapy by
inhibiting HIF-1α and VEGF expression. As the current study was
conducted in vitro, future studies should be performed to
determine the radiosensitization effect of sunitinib in
vivo. Second, the mechanisms by which sunitinib suppressed the
expression of HIF-1α and VEGF were difficult to define, and it is
uncertain whether the observed suppression was direct or
indirect.
In conclusion, sunitinib increased the
radiosensitivity of ESCC cells and led to the suppression of HIF-1α
in the present in vitro study. These results provide support
that sunitinib may be a novel radiosensitizer and a promising agent
in adjuvant therapy to enhance the effects of radiotherapy for
ESCC. However, future studies are required to investigate the
molecular mechanisms and confirm these effects prior to its
clinical use.
Acknowledgements
The present study was supported by a project funded
by the Priority Academic Program Development of Jiangsu Higher
Education Institution (Nanjing, China; grant no. JX10231801),
grants from the Key Academic Discipline of Jiangsu Province
‘Medical Aspects of Specific Environments’ (Nanjing, China), the
National Natural Science Foundation of Jiangsu (Nanjing, China;
grant no. BK20151174), the Scientific Research of Changzhou
(Changzhou, China; grant nos. CJ20159038 and CE20155046) and the
Changzhou High Level Medical Talents Training Project (Changzhou,
China; grant no. 2016C2LJ026).
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Shridhar R, Almhanna K, Meredith KL,
Biagioli MC, Chuong MD, Cruz A and Hoffe SE: Radiation therapy and
esophageal cancer. Cancer Control. 20:97–110. 2013.PubMed/NCBI
|
|
3
|
Li S, Jiang S, Jiang W, Zhou Y, Shen XY,
Luo T, Kong LP and Wang HQ: Anticancer effects of crocetin in human
esophageal squamous cell carcinoma KYSE-150 cells. Oncol Lett.
9:1254–1260. 2015.PubMed/NCBI
|
|
4
|
Williams KJ, Telfer BA, Xenaki D, Sheridan
MR, Desbaillets I, Peters HJ, Honess D, Harris AL, Dachs GU, van
der Kogel A and Stratford IJ: Enhanced response to radiotherapy in
tumours deficient in the function of hypoxia-inducible factor-1.
Radiother Oncol. 75:89–98. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hsiao HT, Xing L, Deng X, Sun X, Ling CC
and Li GC: Hypoxia-targeted triple suicide gene therapy
radiosensitizes human colorectal cancer cells. Oncol Rep.
32:723–729. 2014.PubMed/NCBI
|
|
6
|
Semenza GL: Hypoxia-inducible factors:
Mediators of cancer progression and targets for cancer therapy.
Trends Pharmacol Sci. 33:207–214. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Harada H, Inoue M, Itasaka S, Hirota K,
Morinibu A, Shinomiya K, Zeng L, Ou G, Zhu Y, Yoshimura M, et al:
Cancer cells that survive radiation therapy acquire HIF-1 activity
and translocate towards tumour blood vessels. Nat Commun.
3:7832012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Sohda M, Ishikawa H, Masuda N, Kato H,
Miyazaki T, Nakajima M, Fukuchi M, Manda R, Fukai Y, Sakurai H and
Kuwano H: Pretreatment evaluation of combined HIF-1alpha, p53 and
p21 expression is a useful and sensitive indicator of response to
radiation and chemotherapy in esophageal cancer. Int J Cancer.
110:838–844. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Moon SY, Chang HW, Roh JL, Kim GC, Choi
SH, Lee SW, Cho KJ, Nam SY and Kim SY: Using YC-1 to overcome the
radioresistance of hypoxic cancer cells. Oral Oncol. 45:915–919.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Staab A, Fleischer M, Loeffler J, Said HM,
Katzer A, Plathow C, Einsele H, Flentje M and Vordermark D: Small
interfering RNA targeting HIF-1α reduces hypoxia-dependent
transcription and radiosensitizes hypoxic HT 1080 human
fibrosarcoma cells in vitro. Strahlenther Onkol. 187:252–259. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
El Kaffas A, Al-Mahrouki A, Tran WT, Giles
A and Czarnota GJ: Sunitinib effects on the radiation response of
endothelial and breast tumor cells. Microvasc Res. 92:1–9. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Cuneo KC, Geng L, Fu A, Orton D, Hallahan
DE and Chakravarthy AB: SU11248 (sunitinib) sensitizes pancreatic
cancer to the cytotoxic effects of ionizing radiation. Int J Radiat
Oncol Biol Phys. 71:873–879. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Sun J, Sun Q, Brown MF, Dudgeon C,
Chandler J, Xu X, Shu Y, Zhang L and Yu J: The multi-targeted
kinase inhibitor sunitinib induces apoptosis in colon cancer cells
via PUMA. PloS One. 7:e431582012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Matsumoto S, Batra S, Saito K, Yasui H,
Choudhuri R, Gadisetti C, Subramanian S, Devasahayam N, Munasinghe
JP, Mitchell JB and Krishna MC: Antiangiogenic agent sunitinib
transiently increases tumor oxygenation and suppresses cycling
hypoxia. Cancer Res. 71:6350–6359. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Chen G, Xu X, Zhang L, Fu Y, Wang M, Gu H
and Xie X: Blocking autocrine VEGF signaling by sunitinib, an
anti-cancer drug, promotes embryonic stem cell self-renewal and
somatic cell reprogramming. Cell Res. 24:1121–1136. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shin HW, Cho CH, Kim TY and Park JW:
Sunitinib deregulates tumor adaptation to hypoxia by inhibiting
HIF-1alpha synthesis in HT-29 colon cancer cells. Biochem Biophys
Res Commun. 398:205–211. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Minsky BD, Pajak TF, Ginsberg RJ, Pisansky
TM, Martenson J, Komaki R, Okawara G, Rosenthal SA and Kelsen DP:
INT 0123 (Radiation Therapy Oncology Group 94–05) phase III trial
of combined-modality therapy for esophageal cancer: High-dose
versus standard-dose radiation therapy. J Clin Oncol. 20:1167–1174.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Yoshimura M, Itasaka S, Harada H and
Hiraoka M: Microenvironment and radiation therapy. Biomed Res Int.
2013:6853082013. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kim DW, Huamani J, Fu A and Hallahan DE:
Molecular strategies targeting the host component of cancer to
enhance tumor response to radiation therapy. Int J Radiat Oncol
Biol Phys. 64:38–46. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Sadri N and Zhang PJ: Hypoxia-inducible
factors: Mediators of cancer progression; prognostic and
therapeutic targets in soft tissue sarcomas. Cancers (Basel).
5:320–333. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Martinive P, Defresne F, Bouzin C, Saliez
J, Lair F, Grégoire V, Michiels C, Dessy C and Feron O:
Preconditioning of the tumor vasculature and tumor cells by
intermittent hypoxia: Implications for anticancer therapies. Cancer
Res. 66:11736–11744. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Dewhirst MW, Cao Y and Moeller B: Cycling
hypoxia and free radicals regulate angiogenesis and radiotherapy
response. Nat Rev Cancer. 8:425–437. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang C, Yang X, Zhang Q, Yang B, Xu L,
Qin Q, Zhu H, Liu J, Cai J, Tao G, et al: Berberine radiosensitizes
human nasopharyngeal carcinoma by suppressing hypoxia-inducible
factor-1alpha expression. Acta Otolaryngol. 134:185–192. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Meijer TW, Kaanders JH, Span PN and
Bussink J: Targeting hypoxia, HIF-1, and tumor glucose metabolism
to improve radiotherapy efficacy. Clin Cancer Res. 18:5585–5594.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Schmitt JM, Sommers SR, Fisher W, Ansari
R, Robin E, Koneru K, McClean J, Liu Z, Tong Y and Hanna N:
Sunitinib plus paclitaxel in patients with advanced esophageal
cancer: A phase II study from the Hoosier Oncology Group. J Thorac
Oncol. 7:760–763. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Chen FH, Chiang CS, Wang CC, Fu SY, Tsai
CS, Jung SM, Wen CJ, Lee CC and Hong JH: Vasculatures in tumors
growing from preirradiated tissues: Formed by vasculogenesis and
resistant to radiation and antiangiogenic therapy. Int J Radiat
Oncol Biol Phys. 80:1512–1521. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Gaustad JV, Pozdniakova V, Hompland T,
Simonsen TG and Rofstad EK: Magnetic resonance imaging identifies
early effects of sunitinib treatment in human melanoma xenografts.
J Exp Clin Cancer Res. 32:932013. View Article : Google Scholar : PubMed/NCBI
|