Introduction
Perivascular epithelioid cell tumor (PEComa) was
recognized by the World Health Organization in 2002 as ‘mesenchymal
tumors composed of histologically and immunohistochemically
distinctive perivascular epithelioid cells’ (1). PEComa exhibits immunoreactivity for
HMB-45, melan-A, actin, microphthalmia-associated transcription
factor and desmin, and possesses smooth muscle and melanocytic
components (2). According to PubMed
(www.ncbi.nlm.nih.gov/pubmed), ~100
cases of PEComa have been reported in the English literature with
various primary sites. The largest series of 35 cases was reported
by Doyle et al (3) in 2013. A
total of 20 patients out of the 35 were gastrointestinal in origin,
13 patients developed metastasis to various sites, and 5 patients
succumbed to advanced disease (3).
In 10% of PEComas cases, genetic alterations of
tuberous sclerosis complex (TSC), due to losses of 9q34 (TSC1) or
16q13.3 (TSC2), have been reported (4). These genetic alterations activate
mechanistic target of rapamycin (mTOR) in AMP-activated protein
kinase and Ras/mitogen-activated protein kinase pathways, resulting
in high mTOR activity (5). This leads
to a lack of regulation of cell proliferation, migration and
differentiation (6). Since 2007,
several cases of treatment with mTOR inhibitors in advanced PEComa
have been described (7–12).
The current case presents a patient with small bowel
PEComa that metastasized to the brain and lungs. Following
resection of the brain metastasis, the patient was treated with
everolimus, a mTOR inhibitor, resulting in improvement in the
patient's quality of life and a long period of stable disease.
Case report
A 35-year old woman with morbid obesity, but without
any comorbidity, was admitted to The Emek Medical Center (Afula,
Israel) in October 2010 due to acute abdominal pain. Abdominal
computed tomography (CT) demonstrated small bowel obstruction. An
emergency, explorative laparotomy was performed with resection of
the intestinal mass. Pathology conducted on the resected mass
provided a diagnosis of PEComa. A subsequent chest CT revealed the
presence of multiple lung nodules, which were diagnosed as
metastases of PEComa by pathology on samples taken using
fine-needle biopsy.
The patient was lost to follow-up until April 2011
when she was admitted The Emek Medical Center due to severe
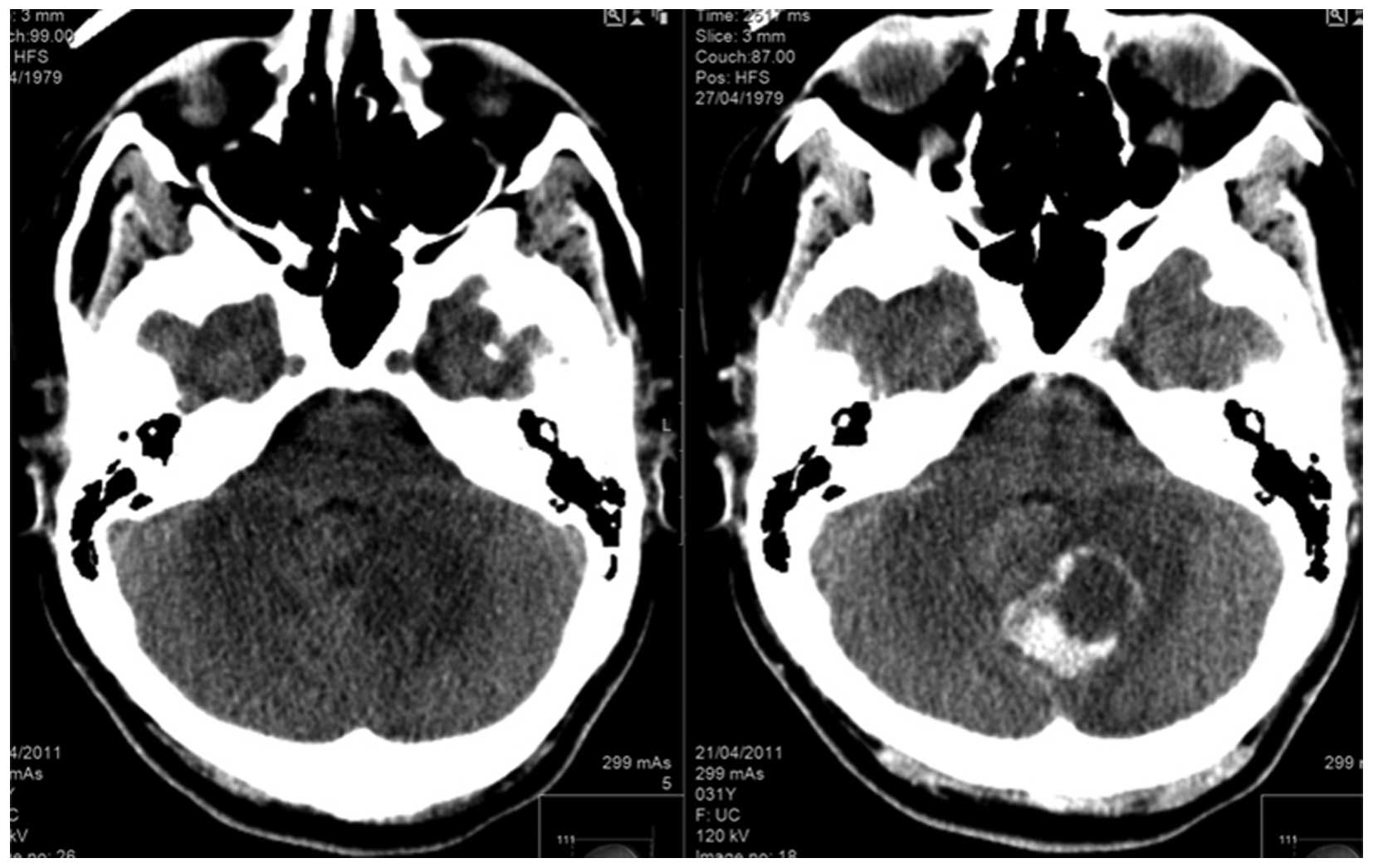
headaches and blurred vision. Brain magnetic resonance imaging
(MR750w 3.0T; GE Healthcare, Milwaukee, WI, USA) demonstrated the
presence of a posterior brain tumor (Fig.
1). Posterior craniotomy and resection of the tumor was
performed. The pathological report diagnosed the tumor as
metastatic PEComa identical to the small bowel origin (Fig. 2).
The patient had an appointment with an oncologist
(Rambam Health Care Campus, Haifa, Israel) for the first time in
January 2012. The patient's Eastern Cooperative Oncology Group
(ECOG) performance status (13) was
2, and she suffered from dyspnea, abdominal pain and weakness. In
addition, the patient used a wheelchair and nasal oxygen.
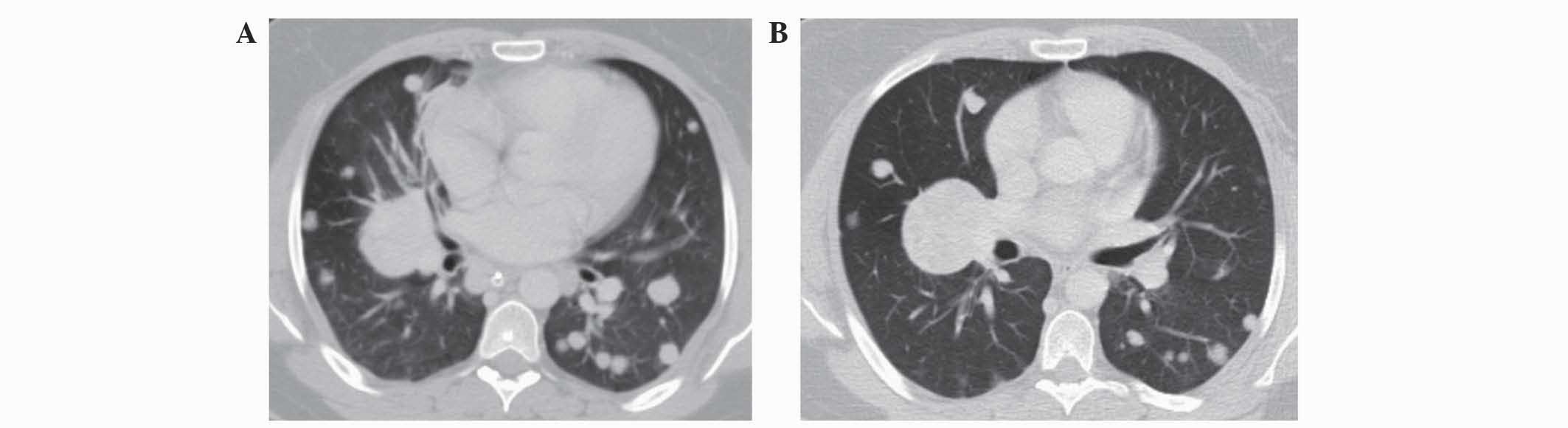
A CT scan for a systemic evaluation demonstrated
multiple bilateral lung metastases (Fig.
3A), without evidence of brain or abdominal recurrence. In May
2012, the patient was administered with a mTOR inhibitor as a
first-line treatment for metastatic disease (10 mg oral everolimus
once daily). Following two months of treatment, improvement in the
patient's lung metastases (Fig. 3B),
quality of life and symptoms was observed. All symptoms of dyspnea,
abdominal pain and weakness were improved, and the patient was able
to stop using the wheelchair. Radiological evaluations revealed
stable disease under continued treatment with everolimus that
lasted for 18 months. During this period, the patient was
asymptomatic and had an ECOG performance status of 0. The patient
was followed up every two months, and follow-up appointments
consisted of history and physical evaluation, cell blood count,
blood biochemistry and CT.
In November 2013, the patient complained of right
pelvic pain radiating down the right leg, with ipsilateral low
extremity lymphedema. A CT scan revealed the presence of novel
masses above the right knee and in the pelvic soft tissue, and the
already diagnosed lung lesions. Biopsy from the leg mass
demonstrated that the pathology was that of metastatic PEComa.
The patient was started on second-line therapy with
doxorubicin (75 mg/m2 every 21 days for 4 cycles). A
response was observed two months later in the soft tissue masses,
with reduction of the leg lymphedema, but the disease progressed in
the lungs with novel metastases observed five months subsequent to
treatment. The patient succumbed to the disease in January 2015.
Written informed consent was obtained from the patient's family for
the publication of this study.
Discussion
Primary PEComa of the small intestine is rare and
has been reported in only a few cases (7,14,15). The current patient was initially
diagnosed with small bowel PEComa with metastatic disease to the
lungs, and possibly to the brain, although the brain metastases
were only identified four months later when the patient became
symptomatic. Brain metastases from PEComa are extremely rare and
have only been reported in two additional cases (14,16).
Until the era of mTOR inhibitors, the only option
for the treatment of metastatic disease was chemotherapy. However,
no formal recommendations were provided for first-line
chemotherapy, and treatment strategies differed between reported
cases. Based on the genetic alterations of the tuberous sclerosis
complex that was identified in certain patients with PEComas
(6), resulting in high mTOR activity,
several cases reported treatment of this type of disease with mTOR
inhibitors. These cases and the current case are summarized in
Table I. Italiano et al
(8) reported a radiological complete
response following neoadjuvant treatment with temsirolimus as a
first-line treatment in a patient with lung metastasis, and a
partial response in another patient with cardiac metastasis as a
second-line treatment following combination chemotherapy. Wagner
et al (9) reported three cases
treated with sirolimus either as a first-line treatment leading to
stable disease (two cases), or as a second-line treatment with a
partial response (one case). Neoadjuvant treatment with
temsirolimus for primary uterine PEComa in a young female patient
(10) and with sirolimus for a
patient with primary liver PEComa (17) resulted in partial response and no
recurrence following surgery. Dickson et al (7) also reported complete response or
prolonged partial response in 4 out of 5 cases with abdominal
PEComas. These patients were treated with rapamycin, sirolimus and
everolimus.
 | Table I.Reported cases of advanced
perivascular epithelioid cell tumors treated with mechanistic
target of rapamycin inhibitors. |
Table I.
Reported cases of advanced
perivascular epithelioid cell tumors treated with mechanistic
target of rapamycin inhibitors.
| Author, year | Age | Gender | Primary origin | Metastatic site | mTOR inhibitor | Time to tumor
progression | OS time, months | (Ref.) |
|---|
| Dickson et al,
2013 | 24 | F | Retroperitoneal | Local mass | Sirolimus | NR | 22+ | (7) |
| Dickson et al,
2013 | 40 | F | Retroperitoneal | Pelvic-lymph
nodes | Sirolimus | NR | 16+ | (7) |
| Dickson et al,
2013 | 57 | M | Small bowel | Abdominal spread | Sirolimus | NR | 14+ | (7) |
| Dickson et al,
2013 | 65 | M | Adrenal | Lungs, soft
tissue | Sirolimus | 2 months | 36 | (7) |
| Dickson et al,
2013 | 37 | F | Liver | NA | Everolimus (neo) | NR | 6+ | (7) |
| Italiano et
al, 2010 | 69 | F | Uterine | Lung | Temsirolimus
(neo) | NR | 9+ | (8) |
| Italiano et
al, 2010 | 55 | F | Uterine | Liver, thoracic
lesion | Temsirolimus | 7 months | 7+ | (8) |
| Wagner et al,
2010 | 70 | M | Kidney | Local recurrence | Sirolimus | 12 months | 12+ | (9) |
| Wagner et al,
2010 | 65 | M | Retroperitoneal | Multifocal local
recurrences | Sirolimus | 16 months | 12+ | (9) |
| Wagner et al,
2010 | 61 | F | Cervix | Bilateral lungs | Sirolimus | 1 months | 3 | (9) |
| Bunch and Sunde,
2014 | 19 | F | Uterine | Local mass | Temsirolimus
(neo) | NR | 15+ | (10) |
| Benson et al,
2014 | NA | NA | Gynecological | NA | Sirolimus | 3 months | NA | (11) |
| Benson et al,
2014 | NA | NA | Gynecological | NA | Sirolimus | 45 months | NA | (11) |
| Benson et al,
2014 | NA | NA | Gastrointestinal | NA | Sirolimus | 21+ months | NA | (11) |
| Benson et al,
2014 | NA | NA | Retroperitoneal | NA | Sirolimus | ~2 days | NA | (11) |
| Benson et al,
2014 | NA | NA | Retroperitoneal | NA | Sirolimus | 2 months | NA | (11) |
| Benson et al,
2014 | NA | NA | Bone-skull | NA | Sirolimus | 7 months | NA | (11) |
| Benson et al,
2014 | NA | NA | Renal | NA | Sirolimus | 10+ months | NA | (11) |
| Benson et
al, 2014 | NA | NA |
Gastrointestinal | NA | Sirolimus | ~2 days | NA | (11) |
| Benson et
al, 2014 | NA | NA | Renal | NA | Sirolimus | 3+ months | NA | (11) |
| Benson et
al, 2014 | NA | NA | Renal | NA | Temsirolimus | 5 months | NA | (11) |
| Gennatas et
al, 2012 | 63 | F |
Retroperitoneal | Lungs, abdomen | Everolimus | 37 months |
37+ | (12) |
| Bergamo et
al, 2014 | 31 | F | Liver | Local mass | Sirolimus
(neo) | NR | 9+ | (17) |
| Present case,
2015 | 35 | F | Small bowel | Brain, lungs | Everolimus | 18 months | 30 | – |
The largest case series was reported by Benson et
al (11) with 10 cases treated at
the Royal Marsden Hospital (London, UK). In that study, a partial
response was observed in 5 out of 7 assessed patients and
symptomatic responses were documented in 7 out of 10 patients. The
1-year survival rate for this series of patients was calculated at
78.8% with a median overall survival time of 2.4 years (95%
confidence interval, 0.3–4.5 years) and a median follow-up time of
1.9 years.
Treatment with everolimus was reported in two cases
only: As a neoadjuvant treatment in 1 patient with primary liver
PEComa, which lead to a good partial response allowing the patient
to undergo surgery (7); and in 1
patient with primary retroperitoneal PEComa that had metastasized
to the lungs, which lead to a complete response in the lung lesions
and a partial response in the abdominal mass (12). In the current case, treatment with
everolimus lead to stable disease in the patient for 18 months,
with symptomatic relief and improvement in the quality of life of
the patient for a disease that had rapidly progressed prior to this
treatment.
In conclusion, treatment with mTOR inhibitors as a
first-line treatment option in advanced PEComa patients appears
reasonable, according to the increasing amount of data reported by
cases that present this rare malignancy.
References
|
1
|
Folpe AL: Neoplasms with perivascular
epithelioid cell differentiation (PEComas)World Health Organization
Classification of Tumors: Pathology and Genetics of Tumors of Soft
Tissue and Bone. Fletcher CDM. Unni KK and Mertens F: IARC Press;
Lyon: pp. 221–223. 2002
|
|
2
|
Martignoni G, Pea M, Reghellin D, Zamboni
G and Bonetti F: PEComas: The past, the present and the future.
Virchows Arch. 452:119–132. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Doyle LA, Hornick JL and Fletcher CD:
PEComa of the gastrointestinal tract: Clinicopathologic study of 35
cases with evaluation of prognostic parameters. Am J Surg Path.
37:1769–1782. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hornick JL and Fletcher CD: PEComa: What
do we know so far? Histopathology. 48:75–82. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Napolioni V, Moavero R and Curatolo P:
Recent advances in neurobiology of Tuberous Sclerosis Complex.
Brain Dev. 31:104–113. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kenerson H, Folpe AL, Takayama TK and
Yeung RS: Activation of the mTOR pathway in sporadic
angiomyolipomas and other perivascular epithelioid cell neoplasms.
Hum Pathol. 38:1361–1371. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Dickson MA, Schwartz GK, Antonescu CR,
Kwiatkowski DJ and Malinowska IA: Extrarenal perivascular
epithelioid cell tumors (PEComas) respond to mTOR inhibition:
Clinical and molecular correlates. Int J Cancer. 132:1711–1717.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Italiano A, Delcambre C, Hostein I, Cazeau
AL, Marty M, Avril A, Coindre JM and Bui B: Treatment with the mTOR
inhibitor temserolimus in patients with malignant PEComa. Ann
Oncol. 21:1135–1137. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Wagner AJ, Malinowska-Kolodziej I, Morgan
JA, Qin W, Fletcher CD, Vena N, Ligon AH, Antonescu CR, Ramaiya NH,
Demetri GD, et al: Clinical activity of mTOR inhibitor with
sirolimus in malignant perivascular epithelioid cell tumors:
Targeting the pathogenic activation of mTORC1 in tumors. J Clin
Oncol. 28:835–840. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Bunch K and Sunde J: Fertility sparing
treatment of a malignant uterine perivascular epithelioid cell
tumor: A case report. Gynecol Oncol Case Rep. 8:14–16. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Benson C, Vitfell-Rasmussen J, Maruzzo M,
Fisher C, Tunariu N, Mitchell S, Al-Muderis O, Thway K, Larkin J
and Judson I: A retrospective study of patients with malignant
PEComa receiving treatment with sirolimus or temsirolimus: The
royal marsden hospital experience. Anticancer Res. 34:3663–3668.
2014.PubMed/NCBI
|
|
12
|
Gennatas C, Michalaki V, Kairi PV,
Kondi-Paphiti A and Voros D: Successful treatment with the mTOR
inhibitor everolimus in a patient with perivascular epithelioid
cell tumor. World J Surg Oncol. 10:1812012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Oken MM, Creech RH, Tormey DC, Horton J,
Davis TE, McFadden ET and Carbone PP: Toxicity and Response
Criteria of the Eastern Cooperative Oncology Group. Am J Clin
Oncol. 5:649–655. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Agaimy A and Wünsch PH: Perivascular
epithelioid cell sarcoma (malignant PEComa) of the ileum. Pathol
Res Pract. 202:37–41. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yanai H, Matsuura H, Sonobe H, Shiozaki S
and Kawabata K: Perivascular epithelioid cell tumor of the jejunum.
Pathol Res Pract. 199:47–50. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Parfitt JR, Keith JL, Megyesi JF and Ang
LC: Metastatic PEComa to the brain. Acta Neuropathol. 112:349–351.
2006. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Bergamo F, Maruzzo M, Basso U, Montesco
MC, Zagonel V, Gringeri E and Cillo U: Neoadjuvant sirolimus for a
large hepatic perivascular epithelioid cell tumor (PEComa). World J
Surg Oncol. 12:462014. View Article : Google Scholar : PubMed/NCBI
|