Introduction
Cervical cancer, the second most common malignancy
in women worldwide, is often diagnosed at a local and advanced
stage (1). Radiotherapy is one of the
most important treatments (1).
However, radioresistance (inherent and acquired) often circumvents
the efficacy of radiotherapy (2).
Many patients have tumour recurrence due to radiotherapeutic
resistance (2). However, the
mechanisms of radioresistance are poorly understood.
MicroRNAs (miRNAs or miRs) are a class of non-coding
RNAs, ~20-22-nucleotides long, which negatively regulate the
expression of many cancer-related genes by binding to
3′-untranslated regions (UTRs) (3–5).
Considerable evidence suggests that miRNAs are associated with
multiple processes, including the tumorigenesis, proliferation and
migration of many types of cancer (6–9). For
example, miRNA-610 downregulated vasodilator-stimulated
phosphoprotein to influence the invasion and migration of gastric
cancer cells (10), and miRNA-144
promoted cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of phosphatase and
tensin homolog (11). Growing
evidence suggests that some miRNAs are related to radioresistance,
such as miRNA-31, miRNA-181, miRNA-324-3p and miRNA-214 (12–15).
However, the functions of miRNAs in radioresistance are still
largely unknown. In this study, X-rays induced the expression of
miRNA-320 in the radioresistant cervix cancer subline C33AR.
Furthermore, target prediction suggested that miRNA-320 influences
cervical cancer radiosensitivity by targeting β-catenin. The
activation of β-catenin plays a crucial role in human cancers
through the canonical Wnt/β-catenin signaling pathway, and the
expression of β-catenin in cervical carcinoma causes a malignant
phenotype (16). Our findings
therefore suggest that a decrease in the expression of miRNA-320
promotes radioresistance in the C33AR cervical cancer cell line by
permitting β-catenin expression.
Materials and methods
Cell culture and transfection
The human cervical cell line C33A was obtained from
the Shanghai Life Science Institute Cell Library (Shanghai, China).
C33A and the acquired radioresistant cell line C33AR were cultured
in Minimum Essential Medium (MEM) (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% fetal bovine serum
(HyClone; GE Healthcare Life Sciences), penicillin-streptomycin
liquid (Invitrogen; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) and 0.25 µg/ml amphotericin B (Amresco, Inc., Framingham, MA,
USA) in a humidified atmosphere of 5% CO2 at 37°C. The
miRNA-320 agomir (a novel class of chemically engineered miRNA
mimic) and negative control were purchased from Guangzhou RiboBio
Co., Ltd. (Guangzhou, China). Cells were transfected with 100 nM
miRNA-320 agomir or negative control using Lipofectamine 2000 in
Opti-MEM (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's instructions. A miRNA-320 inhibitor antiagomir
(Guangzhou RiboBio Co., Ltd.) was designed to suppress the
expression of miRNA-320. Small interference RNA (siRNA) was used to
inhibit β-catenin expression. The siRNAs against β-catenin as well
as the non-targeting control siRNAs were obtained from Shanghai
GenePharma Co., Ltd. (Shanghai, China). Cells were transfected with
siRNAs at a concentration of 25 nM using Lipofectamine 2000. The
sequence of the β-catenin siRNA was
5′-GGACACAGCAGCAAUUUGUTT-3′.
Establishing a radioresistant cervical
cancer cell line
Cells were cultured to 80% confluence and then
subjected to irradiation. Irradiation parameters were set as
follows: Quality, 6 MV/X-rays; dose rate, 2 Gy/min; field, 35×35
cm; and 2 Gy each time, using a linear accelerator. Cells were kept
at room temperature for ≤30 min during irradiation. One set of
flasks was not irradiated and treated as wild type. After 24 h of
incubation, the medium in each flask was exchanged for fresh medium
to remove detached cells. Cells resistant to radiation were
cultured in the same medium, which was exchanged for fresh medium
every 2 days thereafter. To generate stable radioresistant clones,
all the clones after 60 Gy radiation were expanded for >6 months
without radiation to confirm the radioresistant phenotype before
studies were undertaken. Two clones from C33A cells were
established. The parental cells of wild-type stable clones were
generated under the same conditions without irradiation.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR) and analysis
Total RNA was extracted from cell lines using TRIzol
reagent (Invitrogen; Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocol. For the detection of miRNAs, RNA was
reverse transcribed using a specific RT primer for U6 and miRNA-320
(Guangzhou RiboBio Co., Ltd.) according to the protocol of the
manufacturer. qPCR was carried out with SYBR Green I Mix (Takara
Biotechnology Co., Ltd., Dalian, China) in a 20-µl reaction volume
(12.5 µl SYBR Green I Mix, 0.5 µl ROX reference dye II, 200 mM
forward and reverse primer, 2 µl complementary DNA template and 8
µl double-distilled H2O) on the iCycler iQ Real-Time PCR
Detection System (Bio-Rad Laboratories, Inc., Hercules, CA, USA)
using the following protocol: 95°C for 20 sec, followed by 40
cycles of 95°C for 10 sec, 60°C for 20 sec and 70°C for 10 sec. The
primers for miR-212/320/132/15a/16 for RT-qPCR were designed and
synthesized by Guangzhou RiboBio Co., Ltd. The sequences for the
remaining primers were as follows: β-catenin forward,
5′-GAAACGGCTTTCAGTTGAGC-3′ and reverse, 5′-CTGGCCATATCCACCAGAGT-3′;
c-MYC forward, 5′-GGGTAGTGGAAAACCAGCAGC-3′ and reverse,
5′-CCTCCTCGTCGCAGTAGAAATA-3′; cyclin D1 forward,
5′-GAGGAACAGAAGTGCGAGGAG-3′ and reverse,
5′-GGATGGAGTTGTCGGTGTAGAT-3′; and GAPDH forward,
5′-TGGAAGGACTCATGACCACA-3′ and reverse, 5′-TTCAGCTCAGGGATGACCTT-3′.
∆ quantification cycle (Cq) was calculated by subtracting the Cq of
U6 from the Cq of miRNA-320. ∆∆Cq was then calculated by
subtracting the ∆Cq of the control from the ∆Cq of the treatment
group. The fold-change of miRNA expression was calculated by the
equation 2−∆∆Cq (10).
Flow cytometric analysis of cell cycle
distribution
Cells were harvested and washed with cold PBS three
times, fixed with 70% ethanol at 4°C for 24 h, washed again three
times with cold PBS and then stained with 50 mg/ml propidium iodide
for 0.5 h at 37°C (Beyotime Institute of Biotechnology, Haimen,
China). DNA content was analyzed on the Cytomics FC 500 (Beckman
Coulter, Inc., Brea, CA, USA).
Colony-formation assay
Cells in an exponential growth phase were plated
into a 6-well plate at 100, 200, 400, 800, 1,000 and 2,000 cells
per well, and then irradiated with 0, 2, 4, 6, 8 and 10 Gy. Cells
were then incubated at 37°C in 5% CO2, 95% air for 9–12
days. When most cell clones had reached >50 cells, they were
stained with 0.5% (w/v) crystal violet (Sigma-Aldrich; Merck
Millipore, Darmstadt, Germany). The number of clones in the
radioresistant group was compared with that in the wild-type group.
The survival curves were obtained and analyzed with GraphPad Prism
4 statistical software (GraphPad Software, Inc., La Jolla, CA,
USA). Three independent experiments were performed.
Cell proliferation assay
Cell proliferation was assessed using the WST-1 Cell
Proliferation Reagent (Dojindo Molecular Technologies, Inc.,
Kumamoto, Japan), according to the manufacturer's protocol. The
C33AR cells were plated into a 96-well cell culture plate at 2,000
cells/well and then incubated at 37°C overnight to allow settling.
Then, they were transfected with miRNA-320 agomir and negative
control, and treated with 4 Gy X-ray radiation. Subsequently, 10 µl
of WST-1 reagent was added in each well and incubated for 2 h at
37°C. Absorbance was subsequently determined at a wavelength of 450
nm (for measurements) and 650 nm (as reference) by a microplate
reader (EnSpire; PerkinElmer, Inc., Waltham, MA, USA). Cell
proliferation was calculated by subtracting the absorbance values
of the samples from that of the medium alone (background level).
The relative cell proliferation was normalized to that of the
control group.
β-catenin RNA interference in the
cervical cancer radioresistant cell line C33AR
The β-catenin siRNA oligonucleotides with the
sequence si-1 sense 5′-GCAGUUGUAAACUUGAUUATT-3′; si-2 sense
5′-CCCAAGCUUUAGUAAAUAUTT-3′; and si-3 sense
5′-GGACACAGCAGCAAUUUGUTT-3′, along with the corresponding antisense
oligonucleotides, were synthesized by Shanghai GenePharma Co., Ltd.
A negative control siRNA (Shanghai GenePharma Co., Ltd.) was used
as a control siRNA. siRNA transfection was performed using
Lipofectamine 2000 as indicated in the manufacturer's instructions.
Briefly, subconfluent C33AR cells were plated in 6-well plates in
regular growth medium. The next day, they were transfected with
either 100 nM control siRNA or the β-catenin siRNAs for 6 h,
followed by recovery in serum-containing medium. After 48 h of
siRNAs transfection, the cells were harvested for protein isolation
analysis.
Western blotting
Cells were washed with PBS and lysed in
radioimmunoprecipitation assay buffer containing 50 mM Tris-HCl,
150 mM NaCl, 1% NP-40, 0.1% SDS, 0.5% sodium deoxycholate, 2 mM
sodium fluoride, 2 mM Na3VO4, 1 mM EDTA, 1 mM
EGTA and a protease inhibitor cocktail (Roche Diagnostics, Basel,
Switzerland). Cell lysates were quantified for protein content by
the bicinchoninic acid (BCA) method using a BCA kit (Bio-Rad
Laboratories, Inc.). Then, 40 µg of protein was resolved in 12%
SDS-PAGE and transferred onto a polyvinylidene fluoride membrane
(EMD Millipore, Billerica, MA, USA). Membranes were blocked in 5%
bovine serum albumin (Beyotime Institute of Biotechnology) in TBS
with Tween-20 (TBST) for 2 h, and then probed with primary
antibodies against β-catenin (ab22656; Abcam, Cambridge, MA, USA),
cyclin D1 (ab134175; Abcam), c-MYC (ab32072; Abcam) and GAPDH
(KM1002T; Sungene Biotech Co., Ltd., Tianjin, China) at a 1:1,000
dilution at 4°C overnight, washed extensively with TBST three
times, and incubated with secondary antibodies conjugated with
horseradish peroxidase (Pierce; Thermo Fisher Scientific, Inc.)at
room temperature for 2 h at a 1:5,000 dilution. Immunoreactive
protein was examined using an enhanced chemiluminescence kit
(Beyotime Institute of Biotechnology).
Statistical analysis
The results of the quantitative data in this study
are expressed as the mean ± standard deviation. The Student's
t-test was used to evaluate the significant difference
between two groups of data in all pertinent experiments with the
statistical package SPSS 17.0 (SPSS, Inc., Chicago, IL, USA).
P<0.05 (using a two-tailed paired t-test) was considered
to indicate a statistically significant difference between two
groups of data.
Results
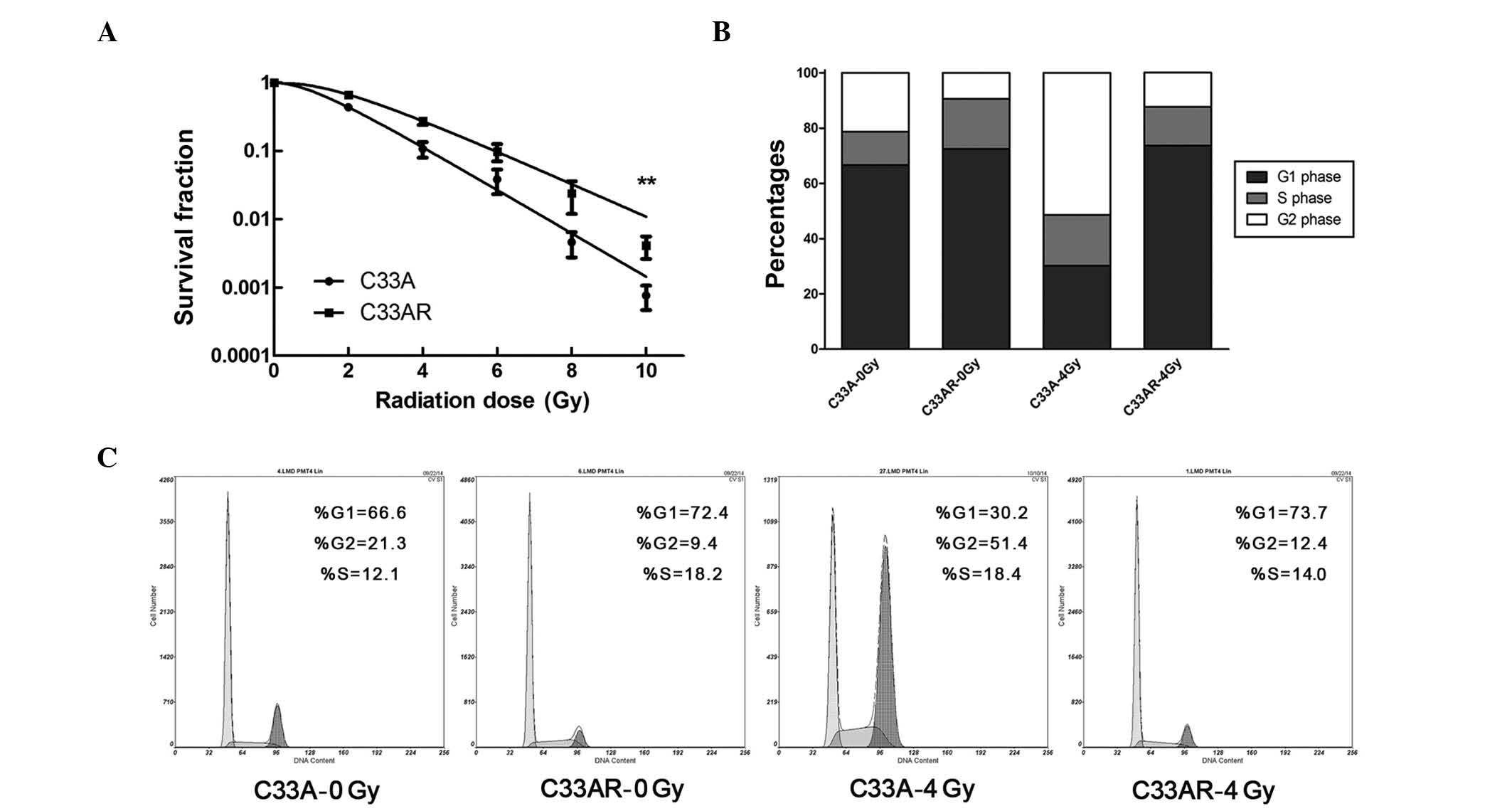
Establishing and validating
radioresistant C33AR cells
To generate a radioresistant cell line, C33A cells
in exponential growth phase were exposed to X-rays at a dose of 2
Gy and a dose rate of 2 Gy/min. An interval of 1 to 3 weeks between
each ionizing radiation (IR) allowed the surviving cells to
regenerate. The whole process of IR and culture lasted for ~1 year,
and the surviving cell line was termed C33AR. To verify phenotypes,
C33AR cells were irradiated and examined by the colony-formation
assay. Compared with C33A, C33AR showed no change in foci formation
when IR was absent, but gained more foci and higher survival
fractions when exposed to IR (Fig.
1A), establishing C33AR as a stable radioresistant cell line.
Next, flow cytometry was used to evaluate alterations in the cell
cycle (Fig. 1B and C). After
irradiation, C33AR cells in the G1 and S phases significantly
increased. Conversely, the proportion of C33AR in the G2/M phase
decreased.
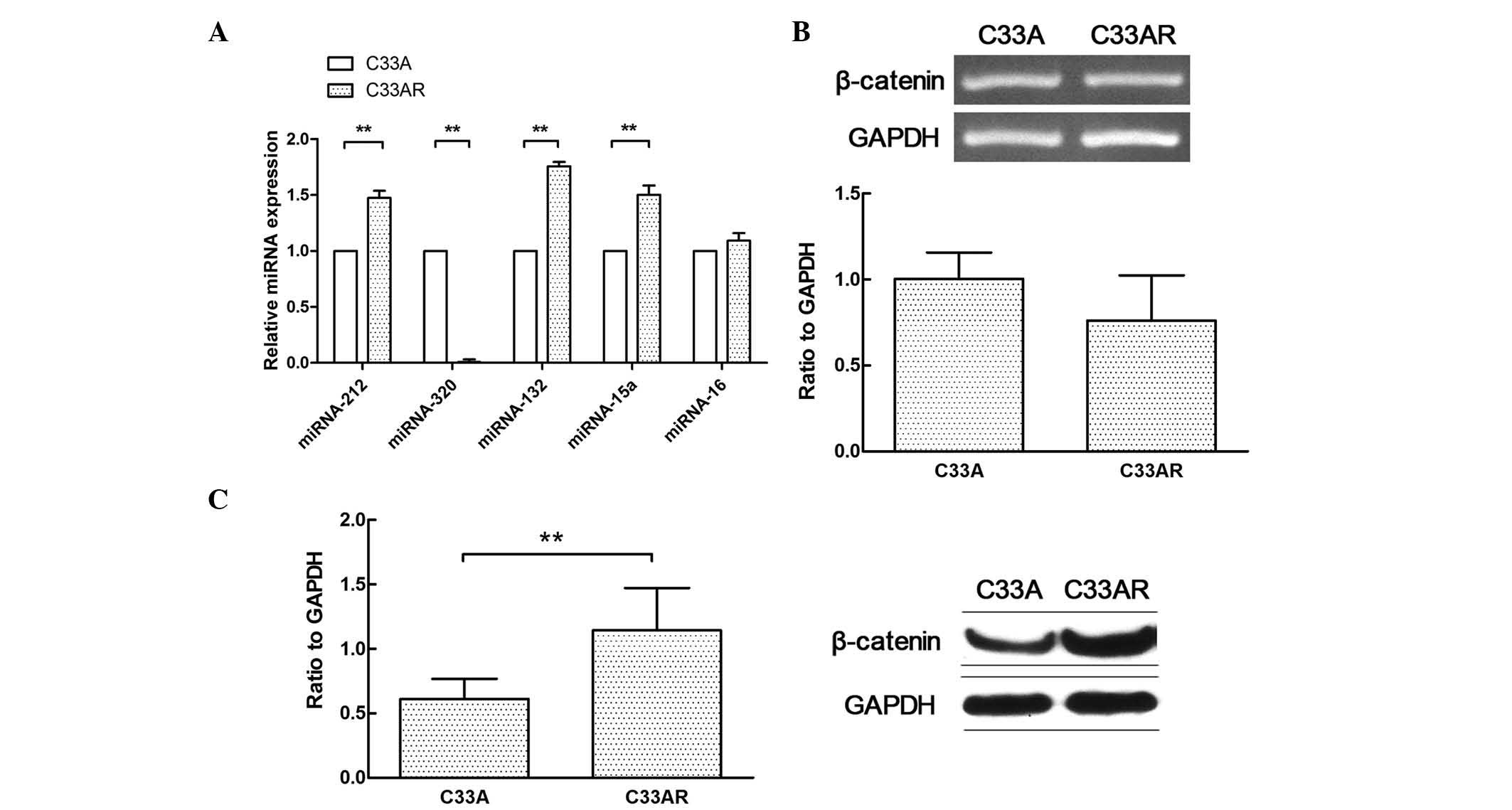
Differential expression of miRNAs and
β-catenin in radioresistant cells
The differential miRNA expression profile between
C33AR cells and their parental C33A cells was determined using
RT-qPCR (Fig. 2A). The expression of
miRNA-320 in C33AR cells was significantly lower than that in C33A
cells. Several differentially expressed miRNAs in this profile were
previously reported to have a role in tumorigenesis, including the
development of radioresistance (17,18). These
results indicated that differentially expressed miRNAs may
contribute to the acquisition of radioresistance in cervical
cancer. The expression of miRNA-320 was significantly changed in
cervical cancer cells after radiation. In addition, β-catenin was
highly expressed in C33AR cells compared with C33A cells at the
protein level but not at the messenger RNA level (Fig. 2B and C).
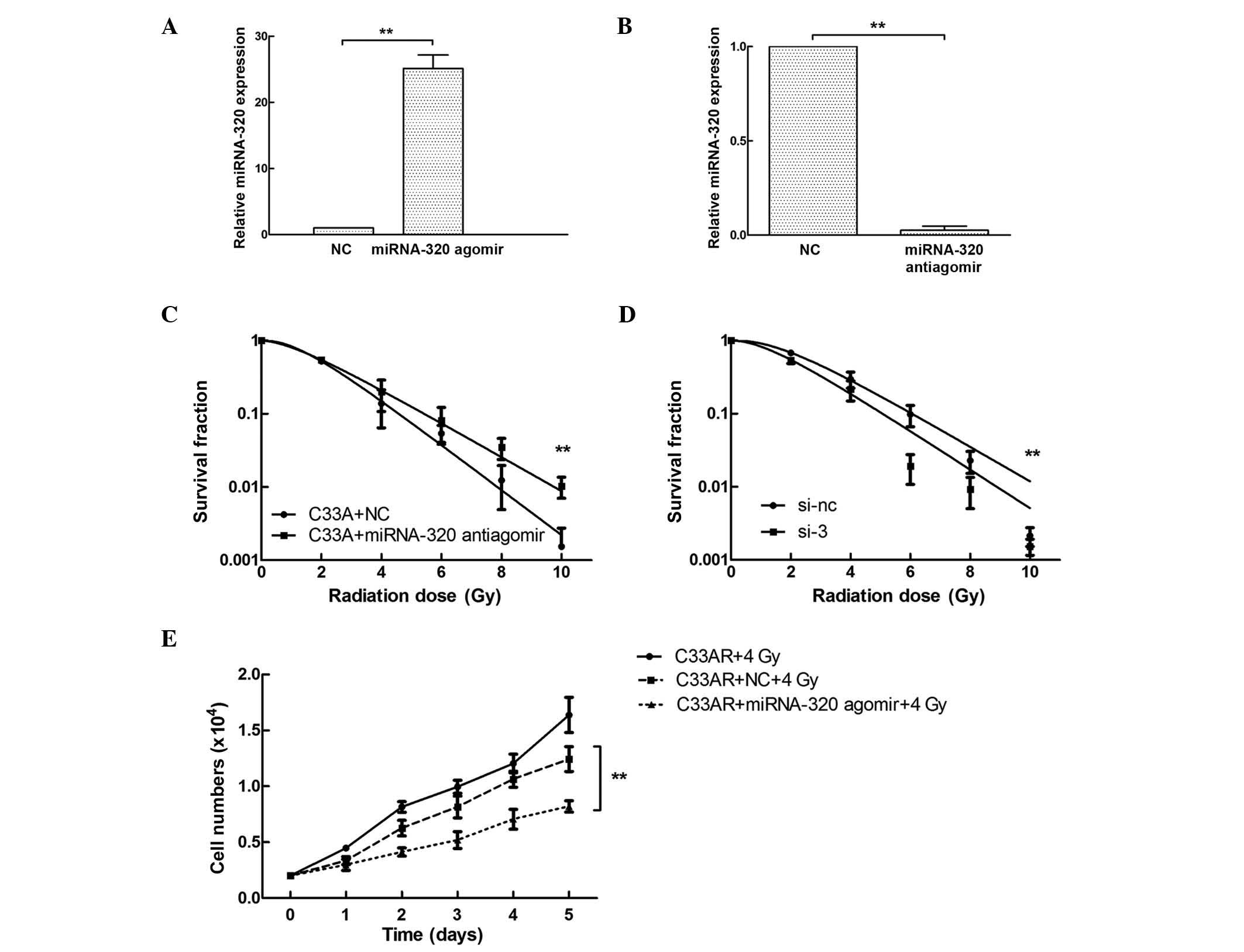
miRNA-320 influences the sensitivity
of C33AR and parental cells to irradiation
Based on the differential expression of miRNA-320
between C33AR and its parental C33A cells, the potential role of
miRNA-320 in cervical cancer radiobiology was examined by
overexpressing or repressing miRNA-320 using synthetic miRNA-320
agomir/antiagomir in C33AR and C33A cells, respectively. The
expression of miRNA-320 was tested by RT-qPCR (Fig. 3A and B). Following miRNA-320
overexpression, the survival rate of C33AR cells decreased compared
with that of C33AR cells transfected with negative control vector
10 days after IR stimulation (Fig.
3C). These results revealed that overexpression of miRNA-320
significantly increases the sensitivity of C33AR cells to
irradiation. The expression of miRNA-320 in C33A cells was
inhibited by being transfected with antiagomir, and caused a
decrease in radiosensitivity (Fig.
3D). In the proliferation assay, C33AR cells were transfected
with miRNA-320 agomir and negative control, and exposed to IR with
4 Gy x-rays, and their cell growth was monitored by counting the
cell numbers (Fig. 3E). Increased
expression of miRNA-320 promoted cell death in the experimental
group with 4 Gy IR exposure in the long-term cell culture compared
with the negative control.
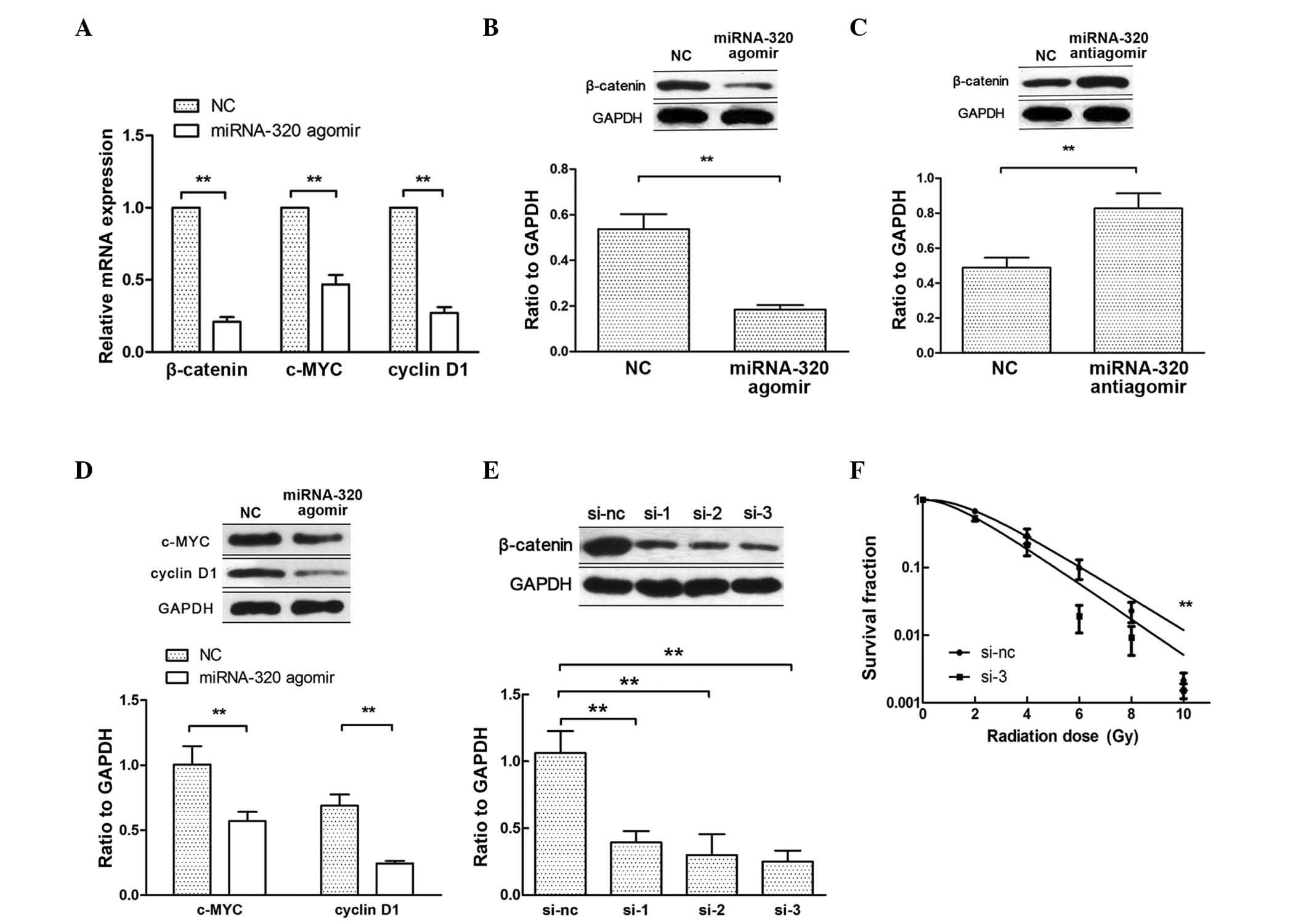
Decrease in miRNA-320 induces the
radioresistance of cervical cancer cells by targeting β-catenin
expression
Hsieh et al have reported that miRNA-320
inhibits endogenous β-catenin expression and nuclear localization
in prostate cancer (19). When
β-catenin translocates to the nucleus, it activates the
transcription of Wnt/β-catenin target genes, such as c-MYC and
cyclin D1 (20). Therefore, to
further explore the mechanism by which miRNA-320 influences cell
radiosensitivity, the expression of β-catenin, c-MYC and cyclin D1
was examined by RT-qPCR and western blotting after transfecting
C33AR cells with miRNA-320 agomir (Fig.
4A, B and D). Upon transfection with miRNA-320 antiagomir in
C33A cells, the expression of β-catenin increased at the protein
level (Fig. 4C). To confirm that
miRNA-320 modulates the radiobiological behavior of cervical cancer
cells by repressing β-catenin expression, rescue experiments were
performed. First, β-catenin expression was inhibited using siRNAs
(Fig. 4E) and then, the knockdown of
β-catenin increased C33AR radiosensitivity (Fig. 4F). The inhibition of β-catenin rescued
miRNA-320-mediated cell radioresistance. These results indicated
that a decrease in miRNA-320 inhibits cervical cancer cell
radiosensitivity in vitro by negatively regulating β-catenin
expression and Wnt/β-catenin signaling activity.
Discussion
Radiotherapy is the main treatment for cervical
cancer, especially at an early stage of the disease. However, many
patients are not sufficiently radiosensitive and relapse soon after
radiotherapy (1). The tumor radiation
response is thus a major factor for the effect (and closely related
to) tumor radiosensitivity. Numerous biological processes
participate in the regulation of tumor radiation response,
including DNA damage response and repair, cell cycle checkpoint,
apoptosis control, and metabolism (21).
Although some achievements have been made in
previous studies (22,23), the exact molecular mechanisms
underlying radioresistance remain to be elucidated. In the past
years, there has been increasing evidence regarding miRNAs as
important regulators of radiotherapeutic resistance and other
biological effects (24,25). In this study, a radioresistant
cervical cancer cell line was identified using the clonogenic
assay. Biological analyses revealed an increased percentage of
G1-phase cells in the C33AR cell line compared with the parental
cell line, suggesting its radioresistance, since it is generally
accepted that cells are usually most sensitive to radiation in the
late G2/M phase and most resistant to radiation in the mid-to-late
S and early G1 phases (26).
The RT-qPCR results indicated that miRNA-320 was
significantly decreased in the C33AR cell line. The in vitro
functional analysis in our study demonstrated that a decrease in
miRNA-320 confers radioresistance to the C33AR cell line, and that
miRNA-320 overexpression induces increased radiosensitivity in
C33AR cells. This alteration suggests that the differential
expression of some miRNAs may be important for surviving the
cytotoxic effects of radiation, thus supporting previous results
demonstrated in other cancer types (27,28).
miRNA-320 is located on chromosome 8p21.3, a region
frequently reported to undergo a loss of heterozygosity during the
progression of prostate cancer (29).
Altered miRNA-320 expression has also been linked to a defect in
post-transcriptional processing and chromosomal deletions (29). The attenuation of miRNA-320 expression
has also been demonstrated to be important for the initiation
(30) and progression (31) of a number of cellular processes. The
downregulation of miRNA-320 and the following radioresistant effect
in C33AR cells support a critical role for this miRNA in modulating
the cellular response to radiation. This is supported not only by
the downregulation of miRNA-320 in our model but also in the larynx
squamous carcinoma acquired radioresistant cell line Hep2R
(32) in a previous study. However,
the overexpression of miRNA-320 in the C33A cell line did not
enhance radiosensitivity (data not shown). This may be explained by
a plateau effect, given the relatively high basal expression of
miRNA-320 in these cells, or it may suggest that the modulatory
effect of miRNA-320 on the radiosensitivity of C33AR cells may
depend on other molecular genetic changes that occurred during the
generation of this radioresistant subline.
Radiation can induce different alterations in
various oncogenes or cancer suppressor genes, including P53,
Bcl-2-associated X protein, P21 and DNA-dependent protein kinase
(33–35). Therefore, bioinformatic algorithms,
including PicTar (pictar.bio.nyu.edu/), MicroRNA.org
(www.microrna.org/microrna/home.do) and Targetscan
(www.targetscan.org/), were used to
determine that β-catenin, a well-known oncogene (36), is a potential target with the highest
predictive value for miRNA-320. In addition, a previous study
confirmed it as a target of miRNA-320 (19). The study, performed in prostate cancer
cells, demonstrated that miRNA-320 influences stem cell-like
characteristics by directly downregulating the Wnt/β-catenin
signaling pathway (19). In the
present study, miRNA-320 was identified as a critical contributor
to radioresistance by directly targeting β-catenin in cervical
cancer. Previous studies have demonstrated that the activation of
the Wnt signaling pathway is a key radioprotective mechanism in
irradiated cancer cells (37–40). Woodward et al reported that Wnt
and β-catenin signaling may contribute to the radioresistance of
breast cancer stem cells (41), and
Watson et al reported that cells with silenced β-catenin are
more sensitive to radiation compared with the parental cell line
(42). The present report is the
first to demonstrate an association between miRNA-320
downregulation and radioresistance through a negative regulation of
β-catenin. Although the colony-formation assay confirmed that the
differential expression of miRNA-320 and the inverse expression of
β-catenin are related to radioresistance, it remains unclear how
the miRNA-320/β-catenin signaling pathway participates in
establishing radioresistance in cervical cancer. Further work is
required to elucidate these factors and to assess their importance
in the radiation response of cervical cancer.
As traditional radiotherapy may result in the
potential radioresistance of cervical cancer, the classical
schedule requires a radiosensitive drug regimen to enhance its
curative effect. The roles of miRNAs in the regulation of tumor
radiosensitivity suggest that miRNAs will be a promising target for
clinical diagnosis and treatment. In addition, the potential
improvement of radiotherapeutic effects through activating or
inhibiting the expression of certain miRNAs and downstream target
genes is extremely promising. A thorough understanding of tumor
radiosensitivity and the regulatory mechanisms of miRNAs will bring
new hope to more cancer patients.
Acknowledgements
We thank the support provided by the National
Natural Science Foundation of China (Beijing, China; grant nos.
81071908 and 81272996) and the Fundamental Research Funds for the
Central Universities (Hubei, China; grant no. 2042014).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Waggoner SE: Cervical cancer. Lancet.
361:2217–2225. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bernstein E, Caudy AA, Hammond SM and
Hannon GJ: Role for a bidentate ribonuclease in the initiation step
of RNA interference. Nature. 409:363–366. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Sevignani C, Calin GA, Siracusa LD and
Croce CM: Mammalian microRNAs: A small world for fine-tuning gene
expression. Mamm Genome. 17:189–202. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zhang B, Wang Q and Pan X: MicroRNAs and
their regulatory roles in animals and plants. J Cell Physiol.
210:279–289. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Mendell JT and Olson EN: MicroRNAs in
stress signaling and human disease. Cell. 148:1172–1187. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Nikitina EG, Urazova LN and Stegny VN:
MicroRNAs and human cancer. Exp Oncol. 34:2–8. 2012.PubMed/NCBI
|
|
8
|
Xie H, Zhao Y, Caramuta S, Larsson C and
Lui WO: miR-205 expression promotes cell proliferation and
migration of human cervical cancer cells. PLoS One. 7:e469902012.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Aigner A: MicroRNAs (miRNAs) in cancer
invasion and metastasis: Therapeutic approaches based on
metastasis-related miRNAs. J Mol Med (Berl). 89:445–457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang J, Zhang J, Wu J, Luo D, Su K, Shi W,
Liu J, Tian Y and Wei L: MicroRNA-610 inhibits the migration and
invasion of gastric cancer cells by suppressing the expression of
vasodilator-stimulated phosphoprotein. Eur J Cancer. 48:1904–1913.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zhang LY, Ho-Fun Lee V, Wong AM, Kwong DL,
Zhu YH, Dong SS, Kong KL, Chen J, Tsao SW, Guan XY and Fu L:
MicroRNA-144 promotes cell proliferation, migration and invasion in
nasopharyngeal carcinoma through repression of PTEN.
Carcinogenesis. 34:454–463. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Lynam-Lennon N, Reynolds JV, Marignol L,
Sheils OM, Pidgeon GP and Maher SG: MicroRNA-31 modulates tumour
sensitivity to radiation in oesophageal adenocarcinoma. J Mol Med
(Berl). 90:1449–1458. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen G, Zhu W, Shi D, Lv L, Zhang C, Liu P
and Hu W: MicroRNA-181a sensitizes human malignant glioma U87MG
cells to radiation by targeting Bcl-2. Oncol Rep. 23:997–1003.
2010.PubMed/NCBI
|
|
14
|
Li G, Liu Y, Su Z, Ren S, Zhu G, Tian Y
and Qiu Y: MicroRNA-324-3p regulates nasopharyngeal carcinoma
radioresistance by directly targeting WNT2B. Eur J Cancer.
49:2596–2607. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Salim H, Akbar NS, Zong D, Vaculova AH,
Lewensohn R, Moshfegh A, Viktorsson K and Zhivotovsky B: miRNA-214
modulates radiotherapy response of non-small cell lung cancer cells
through regulation of p38MAPK, apoptosis and senescence. Br J
Cancer. 107:1361–1373. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Shinohara A, Yokoyama Y, Wan X, Takahashi
Y, Mori Y, Takami T, Shimokawa K and Tamaya T: Cytoplasmic/nuclear
expression without mutation of exon 3 of the beta-catenin gene is
frequent in the development of the neoplasm of the uterine cervix.
Gynecol Oncol. 82:450–455. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Mei Z, Su T, Ye J, Yang C, Zhang S and Xie
C: The miR-15 family enhances the radiosensitivity of breast cancer
cells by targeting G2 checkpoints. Radiat Res. 183:196–207. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Jiang X, Chen X, Chen L, Ma Y, Zhou L, Qi
Q, Liu Y, Zhang S, Luo J and Zhou X: Upregulation of the
miR-212/132 cluster suppresses proliferation of human lung cancer
cells. Oncol Rep. 33:705–712. 2015.PubMed/NCBI
|
|
19
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Valenta T, Hausmann G and Basler K: The
many faces and functions of β-catenin. Embo J. 31:2714–2736. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhao L, Lu X and Cao Y: MicroRNA and
signal transduction pathways in tumor radiation response. Cell
Signal. 25:1625–1634. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
López J, Poitevin A, Mendoza-Martínez V,
Pérez-Plasencia C and García-Carrancá A: Cancer-initiating cells
derived from established cervical cell lines exhibit stem-cell
markers and increased radioresistance. BMC Cancer. 12:482012.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ke G, Liang L, Yang JM, Huang X, Han D,
Huang S, Zhao Y, Zha R, He X and Wu X: MiR-181a confers resistance
of cervical cancer to radiation therapy through targeting the
pro-apoptotic PRKCD gene. Oncogene. 32:3019–3027. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Xu XM, Wang XB, Chen MM, Liu T, Li YX, Jia
WH, Liu M, Li X and Tang H: MicroRNA-19a and −19b regulate cervical
carcinoma cell proliferation and invasion by targeting CUL5. Cancer
Lett. 322:148–158. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Tang T, Wong HK, Gu W, Yu MY, To KF, Wang
CC, Wong YF, Cheung TH, Chung TK and Choy KW: MicroRNA-182 plays an
onco-miRNA role in cervical cancer. Gynecol Oncol. 129:199–208.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Pawlik TM and Keyomarsi K: Role of cell
cycle in mediating sensitivity to radiotherapy. Int J Radiat Oncol
Biol Phys. 59:928–942. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Shiiba M, Shinozuka K, Saito K, Fushimi K,
Kasamatsu A, Ogawara K, Uzawa K, Ito H, Takiguchi Y and Tanzawa H:
MicroRNA-125b regulates proliferation and radioresistance of oral
squamous cell carcinoma. Br J Cancer. 108:1817–1821. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
John-Aryankalayil M, Palayoor ST, Makinde
AY, Cerna D, Simone CB II, Falduto MT, Magnuson SR and Coleman CN:
Fractionated radiation alters oncomir and tumor suppressor miRNAs
in human prostate cancer cells. Radiat Res. 178:105–117. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kagan J, Stein J, Babaian RJ, Joe YS,
Pisters LL, Glassman AB, von Eschenbach AC and Troncoso P:
Homozygous deletions at 8p22 and 8p21 in prostate cancer implicate
these regions as the sites for candidate tumor suppressor genes.
Oncogene. 11:2121–2126. 1995.PubMed/NCBI
|
|
30
|
Kim BM and Choi MY: Non-canonical
microRNAs miR-320 and miR-702 promote proliferation in
Dgcr8-deficient embryonic stem cells. Biochem Biophys Res Commun.
426:183–189. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Ren XP, Wu J, Wang X, Sartor MA, Qian J,
Jones K, Nicolaou P, Pritchard TJ and Fan GC: MicroRNA-320 is
involved in the regulation of cardiac ischemia/reperfusion injury
by targeting heat-shock protein 20. Circulation. 119:2357–2366.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Zhou FX, Xiong J, Luo ZG, Dai J, Yu HJ,
Liao ZK, Lei H, Xie CH and Zhou YF: cDNA expression analysis of a
human radiosensitive-radioresistant cell line model identifies
telomere function as a hallmark of radioresistance. Radiat Res.
174:550–557. 2010. View
Article : Google Scholar : PubMed/NCBI
|
|
33
|
Zhang M, Atkinson RL and Rosen JM:
Selective targeting of radiation-resistant tumor-initiating cells.
Proc Natl Acad Sci USA. 107:3522–3527. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Theys J, Yahyanejad S, Habets R, Span P,
Dubois L, Paesmans K, Kattenbeld B, Cleutjens J, Groot AJ,
Schuurbiers OC, et al: High NOTCH activity induces radiation
resistance in non small cell lung cancer. Radiother Oncol.
108:440–445. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Huerta S, Gao X, Dineen S, Kapur P, Saha D
and Meyer J: Role of p53, Bax, p21 and DNA-PKcs in radiation
sensitivity of HCT-116 cells and xenografts. Surgery. 154:143–151.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kim KH, Seol HJ, Kim EH, Rheey J, Jin JH,
Lee Y, Joo KM, Lee J and Nam DH: Wnt/β-catenin signaling is a key
downstream mediator of MET signaling in glioblastoma stem cells.
Neuro Oncol. 15:161–171. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Orford K, Orford CC and Byers SW:
Exogenous expression of beta-catenin regulates contact inhibition,
anchorage-independent growth, anoikis, and radiation-induced cell
cycle arrest. J Cell Biol. 146:855–868. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Nakashima M, Meirmanov S, Matsufuji R,
Hayashida M, Fukuda E, Naito S, Matsuu M, Shichijo K, Kondo H, Ito
M, et al: Altered expression of beta-catenin during
radiation-induced colonic carcinogenesis. Pathol Res Pract.
198:717–724. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Rodningen OK, Overgaard J, Alsner J,
Hastie T and Børresen-Dale AL: Microarray analysis of the
transcriptional response to single or multiple doses of ionizing
radiation in human subcutaneous fibroblasts. Radiother Oncol.
77:231–240. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Gassler N, Herr I, Keith M, Autschbach F,
Schmitz-Winnenthal H, Ulrich A, Otto HF, Kartenbeck J and Z'graggen
K: Wnt-signaling and apoptosis after neoadjuvant short-term
radiotherapy for rectal cancer. Int J Oncol. 25:1543–1549.
2004.PubMed/NCBI
|
|
41
|
Woodward WA, Chen MS, Behbod F, Alfaro MP,
Buchholz TA and Rosen JM: WNT/beta-catenin mediates radiation
resistance of mouse mammary progenitor cells. Proc Natl Acad Sci
USA. 104:618–623. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Watson RL, Spalding AC, Zielske SP, Morgan
M, Kim AC, Bommer GT, Eldar-Finkelman H, Giordano T, Fearon ER,
Hammer GD, et al: GSK3beta and beta-catenin modulate radiation
cytotoxicity in pancreatic cancer. Neoplasia. 12:357–365. 2010.
View Article : Google Scholar : PubMed/NCBI
|