Introduction
Eosinophilia is known to cause complications in
allergic disease and parasitic infections, and may occasionally
cause complications in malignant tumors. Previous studies have
indicated that eosinophilia-associated complications may arise not
only in hematopoietic malignancies, but also in lung cancer
(1), gastric cancer (2) and renal cell carcinoma (3); however, the mechanism has not been
elucidated. Approximately 0.5–1.0% of cases of eosinophilia are
associated with malignancies (2–4).
Eosinophilia in solid malignancies is rarely reported, and there
are a limited number of cases describing eosinophilia in rectal
cancer. A number of previously reported cases have involved
advanced cancers accompanied by metastases, and the majority have
exhibited a poor prognosis. Peripheral hypereosinophilia is
considered to be a poor prognostic sign, which is frequently
associated with extensive metastatic disease (4–8) The
present study reports the case of a patient with
eosinophilia-associated advanced rectal cancer, which was
successfully stabilized using chemotherapy. The possible mechanism
underlying eosinophilia-associated complications in the present
case and the importance of early chemotherapeutic intervention in
treating such cancers are also reviewed.
Case report
In February 2013, a 65-year-old man presented to the
Kirishima Medical Center (Kirishima, Japan) with the primary
complaint of melena, and was diagnosed with rectal cancer. The
patient had tested positive for fecal occult blood during a routine
medical examination 1 year earlier, but had left the condition
uncontrolled. The patient's prior medical history included an
unspecified joint surgery to the left shoulder 6 years earlier.
During a visit to the Department of Cardiovascular and
Gastroenterological Surgery, Kagoshima University Medical and
Dental Hospital (Kagoshima, Japan) in March 2013, the patient
presented with severe anemia caused by tumor hemorrhage and was
immediately hospitalized with a diagnosis of hemorrhagic rectal
cancer. Upon hospital admission, the baseline characteristics were
as follows: Body temperature, 36.6°C (normal range, 36.6–37.0°C);
blood pressure, 105/60 mmHg (normal range, <120/80 mmHg); and
pulse rate, 83 beats/min (normal range, 50–80 beats/min). Physical
examination revealed swelling of the left upper extremity. In
addition, the abdomen was flat, soft and non-tender, and no tumor
was palpable. Digital rectal examination identified a mass
accompanied by bleeding proximal to the left anterior wall, located
~7 cm from the anal verge. Laboratory blood test results upon
admission were as follows: White blood cells, 15,630/µl (normal
range, 4,500–8,500/µl); eosinophils, 39% (normal range, 1–4%);
hemoglobin, 6.1 g/dl (normal range, 14.0–18.0 g/dl); platelets,
11.1×104/µl (normal range, 13.0–32.0×104/µl);
prothrombin time-international normalized ratio, 2.17 (normal
range, 0.85–1.15); and activated partial thromboplastin time, 57.7
sec (normal range, 25.1–36.5 sec). These results indicated
coagulopathy. Furthermore, the lactate dehydrogenase level was
mildly increased to 288 IU/l (normal range, 119–229 IU/l).
Hepatorenal function tests revealed normal results as follows:
Aspartate aminotransferase, 16 IU/l (normal range, 13–33 IU/l);
alanine aminotransferase, 13 IU/l (normal range, 6–30 IU/l); urea
nitrogen, 19.4 mg/dl (normal range, 8–22 mg/dl); and creatinine,
0.82 mg/dl (normal range, 0.6–1.1 mg/dl).
Elevated levels of tumor markers were observed as
follows: Carcinoembryonic antigen, 9.1 ng/ml (normal range,
<5.68 ng/ml) and carbohydrate antigen 19–9, 72.6 U/ml (normal
range, <37.0 U/ml). A stool examination for parasites and ova
yielded negative results. Lower gastrointestinal endoscopy revealed
a full-circumference tumor accompanied by bleeding in the
mid-rectum, which obstructed the passage of the endoscope probe.
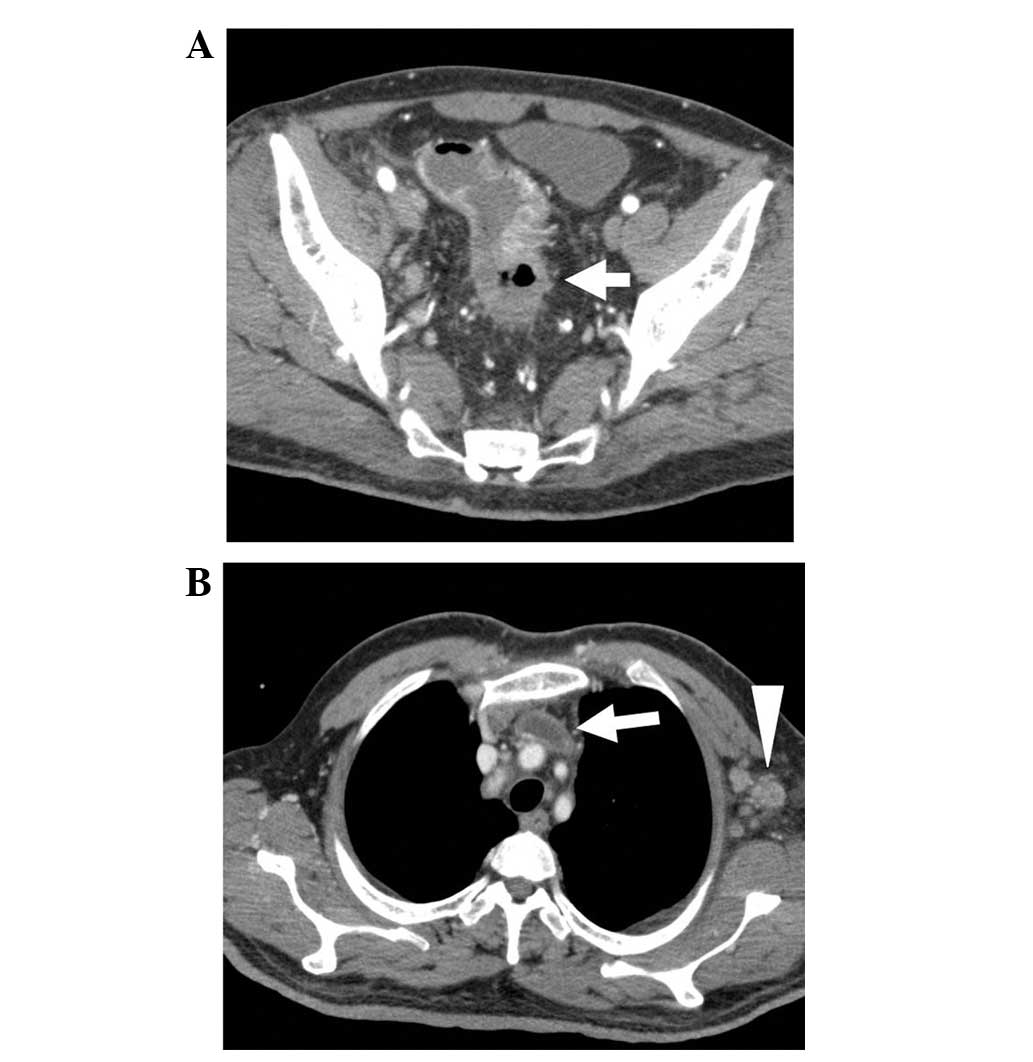
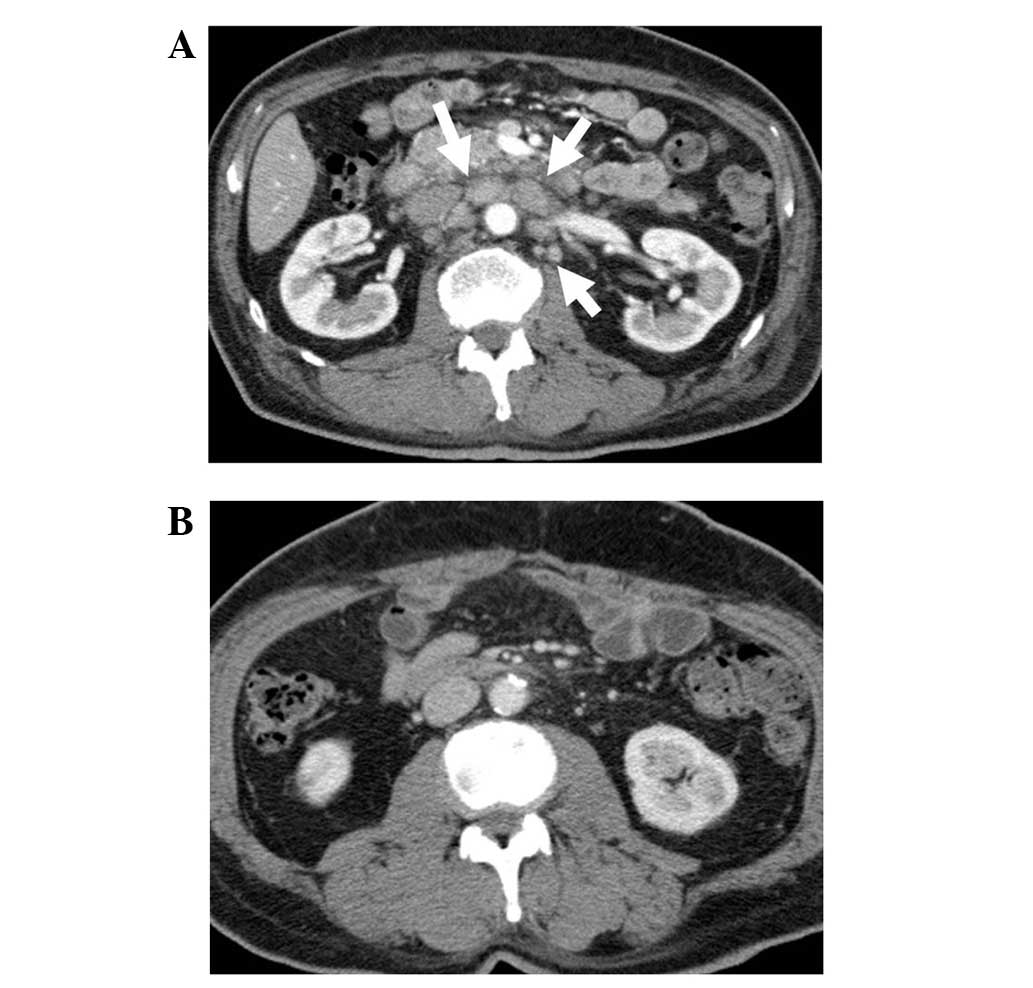
Chest/abdominal computed tomography (CT) revealed irregular wall
thickening extending from the sigmoid colon to the upper rectum,
and extraserosal invasion of the rectal tumor was suspected
(Fig. 1A). Enlarged para-aortic,
supraclavicular and left axillary lymph nodes were observed, in
addition to swelling of the regional lymph nodes. A thrombus was
also observed in the subclavian/brachiocephalic vein, extending
from the left internal jugular vein (Fig.
1B). No liver or lung metastasis was observed, and no
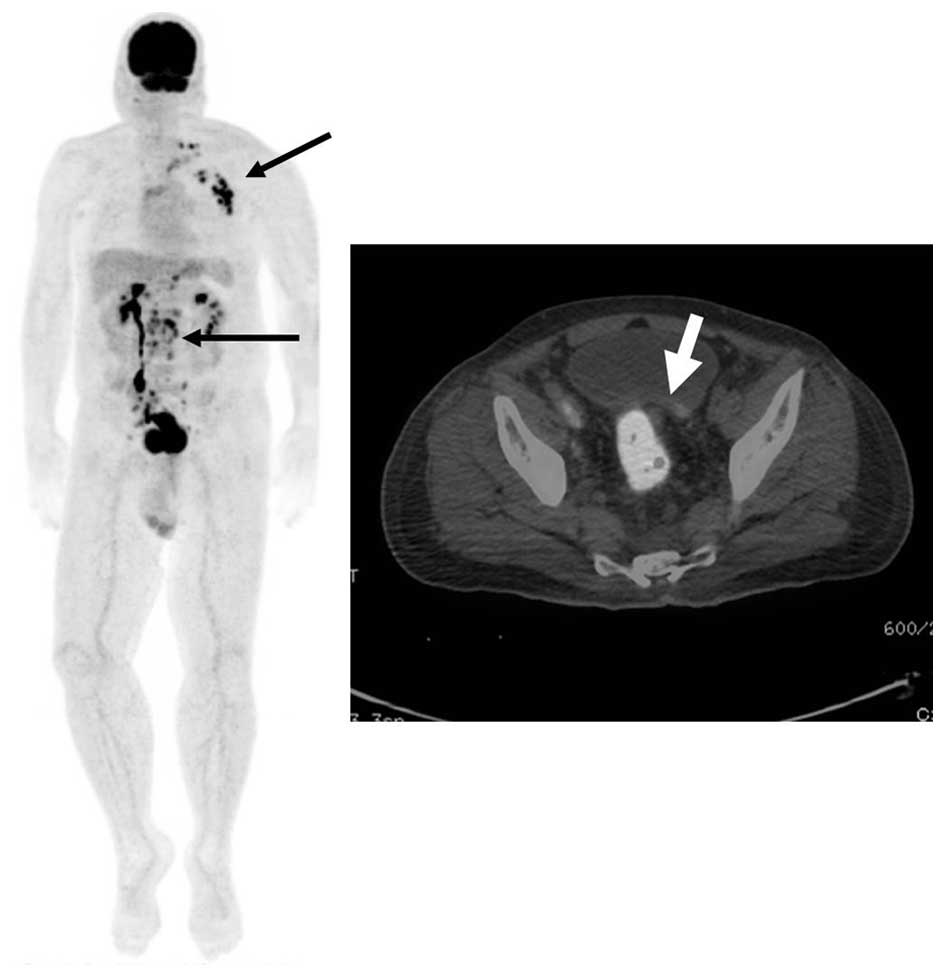
peritoneal dissemination was identified. Positron emission
tomography/CT revealed fluorodeoxyglucose accumulation in the
rectum and lymph nodes, in the region where swelling was indicated
on the CT scans. The maximum standardized uptake values were 15.8
and 9–14.1 in the rectum and lymph nodes, respectively (Fig. 2). Based on the aforementioned
observations, malignant lymphoma was considered as a differential
diagnosis; however, the lactate dehydrogenase level was mildly
elevated in relation to the tumor size, and as no other organ was
involved, the patient was diagnosed with T4aN2bM1a, stage IVA
rectal cancer, according to the International Union Against Cancer
(9). Concentrated red blood cells
(1,680 ml) and fresh frozen plasma (960ml) were administered;
however, the melena persisted and the anemia progressed. Hematuria
was also identified. Subsequently, the patient's condition
deteriorated due disseminated intravascular coagulation (DIC).
Therefore, a primary tumor resection was performed immediately to
control the bleeding. A laparotomy surgical procedure was performed
with an incision in the lower abdominal midline. No peritoneal
dissemination was observed. The tumor of the rectosigmoid colon had
directly infiltrated the bladder via the upper rectum. As a
non-curative resection was performed, concomitant resection of the
bladder was not achieved. Therefore, the infiltrated portion of the
bladder was detached and Hartmann's procedure was performed.
Hemostasis was difficult to achieve inside the pelvic cavity,
however, it was obtained using compression, hemostatic agents
(absorbable hemostat and fibrin adhesive; Surgicel NU-KNIT;
Ethicon, Somerville, NJ, USA) and a blood transfusion (red blood
cells, 1,920 ml; fresh frozen plasma, 800 ml).
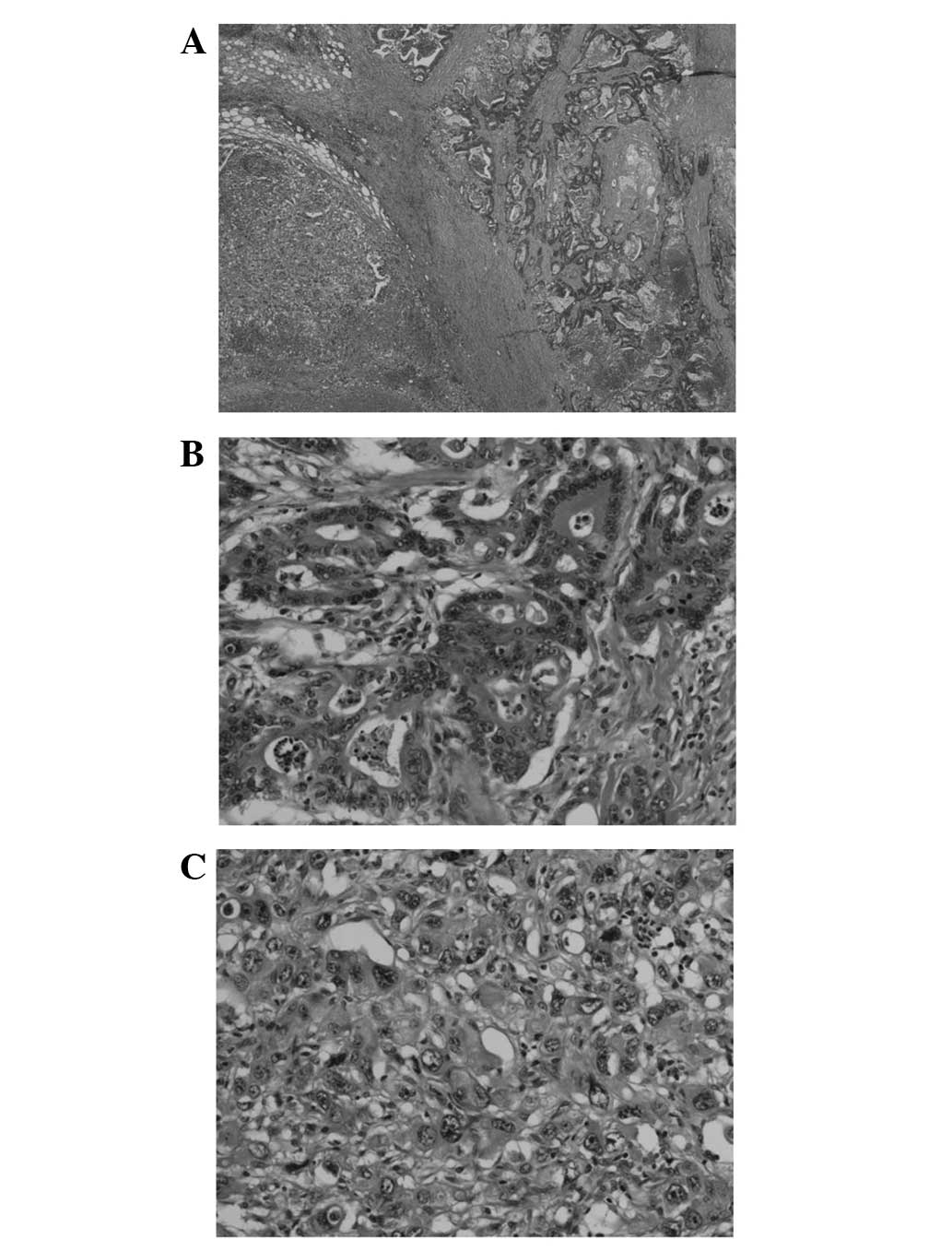
The resected specimen revealed a circumferential
type 2 tumor, based on the Japanese Classification of Colorectal
Carcinoma (8th edition) (10), 95×50
mm in size (Fig. 3). Following
histopathological examination, the patient was diagnosed with an
admixture of poorly- and moderately-differentiated adenocarcinoma,
accompanied by advanced venous-lymphatic infiltration (Fig. 4A). Histological examination revealed a
mixture of mucinous adenocarcinoma, with significant mucus
production at the periphery of the tumor cell nest. Metastasis was
observed in 12/19 of the dissected lymph nodes. Minimal eosinophil
infiltration into the tumor tissue was observed (Fig. 4B and C). Metastases of the inferior
mesenteric lymph nodes were predominantly composed of
moderately-differentiated adenocarcinoma cells.
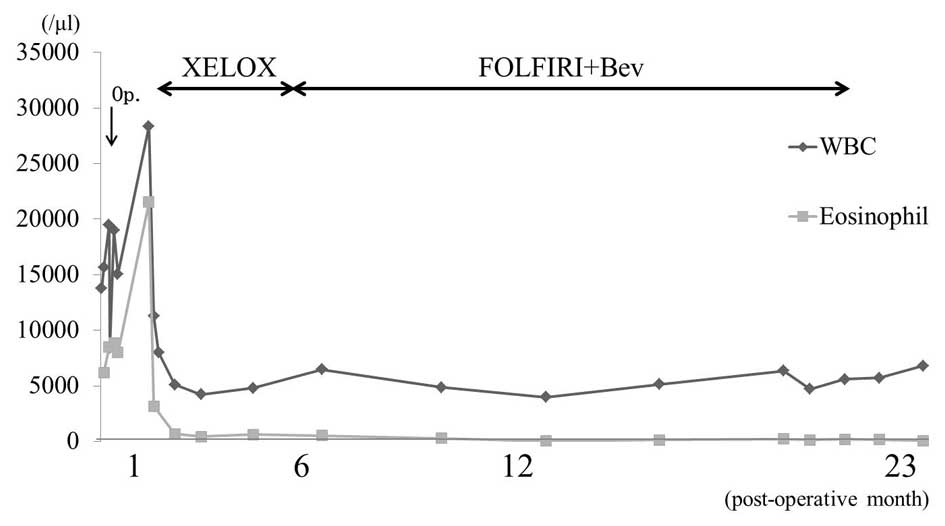
Following surgery, the eosinophil count in the
peripheral blood was immediately reduced, however, by
post-operative day 21, it had increased to 21,000/µl. Interleukin
(IL)-2 receptor levels were mildly elevated (1,464 U/ml; normal
range, 127–582 U/ml)), bone marrow examination did not reveal any
leukemic blasts and fluorescence in situ hybridization
analysis, for the identification of the factor interacting with
PAPOLA and CPSF1-platelet derived growth factor receptor α
(FIP1L1-PDGFRA) fusion gene, revealed no chromosomal abnormalities.
Therefore, the possibility of chronic eosinophilic leukemia was
excluded. Blood concentrations of granulocyte macrophage
colony-stimulating factor, IL-3 and IL-5 were also measured, and
were all within the reference values. The initiation of
chemotherapy was delayed by 1 month due to the complication of a
deep surgical site infection. On post-operative day 32, the patient
was administered oxaliplatin (130 mg/m2, day 1) plus
capecitabine (1,000 mg, twice daily, days 1–14) (XELOX) therapy.
Following 1 3-week cycle of chemotherapy, the eosinophil count
decreased to 666/µl (Fig. 5). After
the completion of 3 cycles of XELOX therapy, the lymph nodes,
including the para-aortic lymph nodes, exhibited a marked reduction
in size (Fig. 6), and the
carcinoembryonic antigen and carbohydrate antigen 19–9 the tumor
marker levels normalized to 4.1 ng/ml and 36.7 U/ml, respectively.
However, following the completion of 6 cycles of XELOX therapy,
enlargement of the iliac lymph nodes was observed. Subsequently,
the chemotherapy regimen was changed to the following: FOLFIRI
[Leucovorin 200 mg/m2 intravenous (i.v.) prior to
fluorouracil (5-FU) on day 1 and 5-FU 500 mg/m2 i.v.
bolus on day 1; then 2,400 mg/m2 i.v. over 46 h and
irinotecan 180 mg/m2 i.v. over 90 min on day 1; in a
2-week cycle) plus Bev (5 mg/kg i.v. bolus on day 1). A total of 29
cycles of chemotherapy (FOLFIRI+Bev) were administered. Stable
disease was then maintained until peritoneal and pleural
dissemination were observed at 22 months post-surgery. The
patient's condition deteriorated rapidly and multiple skin nodules
developed, which were composed primarily of poorly-differentiated
adenocarcinoma. The patient succumbed to the disease at 23 months
post-surgery.
Discussion
Paraneoplastic syndromes are characterized by
symptoms that are observed at sites distant from a primary tumor or
metastases, occurring in 1–7.4% of tumor-bearing patients (11). Paraneoplastic syndromes are clinically
significant, as in certain cases they may present as the first
symptoms of a cancer and thus, if patients consciously neglect to
seek diagnosis and treatment, a diagnosis may not be reached and
treatment may be delayed. Furthermore, the symptoms of
paraneoplastic syndromes may worsen in association with the
progression of the tumors (12).
Eosinophilia, which is considered a component of paraneoplastic
syndromes, has been reported not only in hematopoietic
malignancies, but also in solid tumors (1–3,5,13). In
addition, it has been hypothesized that IL-3, IL-5 and other
cytokines are involved in the differentiation and maturation of
eosinophils (1,5,13,14). An association between bone marrow
carcinogenesis and eosinophilia has been suggested (15), and although it is rare, previous
studies have reported cases of bone marrow carcinogenesis
accompanied by colon cancer (15,16). The
patient in the present case underwent a blood transfusion to
control bleeding caused by tumor hemorrhage and DIC, and then
underwent emergency surgery; therefore, it was not possible to
measure cytokine levels or perform bone marrow tests prior to
surgery. However, the levels of IL-3, IL-5 and
granulocyte-macrophage colony-stimulating factor in the blood were
all within the normal ranges when measured prior to the initiation
of chemotherapy, when the residual tumor was highly active. Bone
marrow examination did not identify leukemic blasts and thus, the
possibility of bone marrow carcinogenesis was excluded. The
possibility of a myeloid tumor accompanied by a genetic abnormality
was also considered, however, an examination to identify the
FIP1L1-PDGFRA fusion gene (17) did
not identify any chromosomal abnormalities. Therefore, the specific
factors involved in the eosinophilia were not identified in the
present case and the mechanism remains unknown. However, as the
eosinophil count was strongly associated with tumor activity, we
hypothesize that the tumor itself produced the factors required for
the development of eosinophilia.
In the present case, the administration of
coagulation factors to control venous thrombosis exacerbated the
patient's tendency to bleed, making it difficult to control the
bleeding from the tumor. The presence of a malignant tumor has been
considered to contribute to a thrombotic tendency, whereby
eosinophils produce granular proteins, such as the eosinophil
cationic protein, and induce vascular endothelial dysfunction and
inactivation of thrombomodulin, in order to enhance coagulation.
Therefore, patients with eosinophilia may experience severe
thrombosis complications (2,18). Furthermore, in the present case, the
possibility of adverse effects from the previous shoulder joint
surgery cannot be ignored as one of the causes; however, as a long
time had elapsed since the surgery, and rapid improvement of the
edema in the upper left extremity was observed following
anti-coagulation therapy and chemotherapy, we hypothesize that a
novel thrombus, formed as a result of eosinophilia and the tumor,
rather than a chronic thrombus, was involved
Reports of colon cancer accompanied by eosinophilia
are rare. Upon conducting a literature review of studies published
between 1946 and 2014 using PubMed, using the keywords
‘eosinophilia’ and ‘colon cancer’, only 5 cases were identified,
including the present case (4,13,18,19)
(Table I). The 4 previous studies
included poorly-differentiated adenocarcinomas, which were highly
malignant in 2 cases, including the present case. Distant
metastasis or disseminated disease are often observed at the time
of diagnosis and thus, patients may succumb due to DIC or embolism
prior to the initiation of chemotherapy, therefore the prognosis is
extremely poor (4,18,19). In
the present case, XELOX therapy significantly reduced the size of
the lymph node metastases, and the tumor marker levels and
eosinophil counts also normalized. The patient was administered
FOLFIRI + Bev for the exacerbated lesions 6 months later and a
stable disease state was maintained for 15 months. Overall, tumor
suppression was maintained for 21 months.
 | Table I.Previously reported cases of
colorectal cancer with eosinophilia. |
Table I.
Previously reported cases of
colorectal cancer with eosinophilia.
| First author,
year | Age, years | Gender | Tumor
localization | Histological
type | Tumor stage | Outcome | (Ref.) |
|---|
| Anagnostopoulos et
al, 2005 | 47 | F | Cecum | tub2 | IV | DOD at 12 months
post-diagnosis | (4) |
| Tajima et al,
1998 | 76 | F | Rectum | tub2 | III | NR | (13) |
| Uemura et al,
2004 | 33 | M | Ascending,
transverse | NR | NR | DOD at 27 days
post-diagnosis, pulmonary emboli | (18) |
| Kato et al,
2010 | 72 | F | Ascending | por | IV | DIC, DOD at 1 month
post-diagnosis | (19) |
| Present study | 65 | M | Rectum | por + tub2 + muc | IV | DOD at 23 months
post-diagnosis |
|
Due to the highly malignant nature of
poorly-differentiated colon cancer, the mean post-operative
survival time is 8.3 months, representing a poor prognosis
(20), and to date, no standard
chemotherapy regimen has been established. In the present case,
equal proportions of moderately- and poorly-differentiated
adenocarcinomas were observed within the primary lesion, whereas
the lymph node metastases were primarily composed of
moderately-differentiated adenocarcinoma. No histopathological
analysis of the distant metastases was performed and thus, no
conclusions could be drawn; however, it is possible that the
chemotherapy was successful and contributed to improving the
prognosis, as the distant metastases were primarily composed of the
moderately-differentiated type. Among the previously reported
cases, the only patient with moderately-differentiated
adenocarcinoma who underwent chemotherapy survived for 12 months
(4). This indicates that although
colon cancer accompanied by eosinophilia exhibits a poor prognosis,
chemotherapy may be successful if the cancer is caused by a
moderately-differentiated adenocarcinoma.
In the present case, after pleural-peritoneal
dissemination developed, no enlargement of the lymph nodes or
increase in eosinophil counts was observed. We hypothesize that
eosinophilia was caused by the moderately-differentiated
adenocarcinoma in the present case, based on the following
observations: The skin metastasis was primarily composed of
poorly-differentiated adenocarcinoma; the eosinophil count was
normal despite the development of dissemination; the reduction in
eosinophil count coincided with the chemotherapy-induced reduction
of lymph node metastases; and the resected metastatic lymph nodes
were primarily composed of moderately-differentiated
adenocarcinoma. In the future, comprehensive analysis of the
factors that contribute to cancer-associated eosinophilia, such as
cytokines, is required.
In the present study, the administration of
chemotherapy successfully stabilized eosinophilia-associated
advanced rectal cancer following primary lesion resection. We
hypothesize that moderately-differentiated adenocarcinoma was
involved in the development of the eosinophilia. These observations
indicate that, although eosinophilia-associated colon cancer
exhibits a poor prognosis, early chemotherapy treatment may improve
the prognosis if the tumor is primarily composed of
moderately-differentiated adenocarcinoma cells.
Glossary
Abbreviations
Abbreviations:
|
IL
|
interleukin
|
|
CT
|
computed tomography
|
|
XELOX
|
oxaliplatin plus capecitabine
|
|
FOLFIRI
|
irinotecan/fluorouracil/folinic
acid
|
|
Bev
|
bevacizumab
|
|
DIC
|
disseminated intravascular
coagulopathy
|
References
|
1
|
Kundu S, Misra S, Haldar RK and Mitra R:
Severe paraneoplastic peripheral blood eosinophilia and
eosinophilic malignant pleural effusion as rare manifestation of
squamous cell carcinoma of lung. Int Med J. 20:34–35. 2013.
|
|
2
|
Takeda H, Nishikawa H, Tsumura T, Sekikawa
A, Maruo T, Okabe Y, Kimura T, Wakasa T and Osaki Y: Prominent
hypereosinophilia with disseminated intravascular coagulation as an
unusual presentation of advanced gastric cancer. Intern Med.
53:563–569. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Wei YB, Yan B, Yin Z and Yang JR:
Chromophobe renal cell carcinoma associated with eosinophilia: A
report of three cases. Exp Ther Med. 8:91–94. 2014.PubMed/NCBI
|
|
4
|
Anagnostopoulos GK, Sakorafas GH,
Kostopoulos P, Margantinis G, Tsiakos S, Terpos E, Pavlakis G,
Fortun P and Arvanitidis D: Disseminated colon cancer with severe
peripheral blood eosinophilia and elevated serum levels of
interleukine-2, interleukine-3, interleukine-5 and GM-CSF. J Surg
Oncol. 89:273–275. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Stefanini M, Claustro JC, Motos RA and
Bendigo LL: Blood and bone marrow eosinophilia in malignant tumors.
Role and nature of blood and tissue eosinophil colony-stimulation
factor(s) in two patients. Cancer. 68:543–548. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Todenhöfer T, Wirths S, von Weyhern CH,
Heckl S, Horger M, Hennenlotter J, Stenzl A, Kanz L and Schwentner
C: Severe paraneoplastic hypereosinophilia in metastatic renal cell
carcinoma. BMC Urol. 12:72012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Chang WC, Liaw CC, Wang PN, Tsai YH and
Hsueh S: Tumor-associated hypereosinophilia: Report of four cases.
Changgeng Yi Xue Za Zhi. 19:66–70. 1996.PubMed/NCBI
|
|
8
|
Lowe D, Jorizzo J and Hutt MS:
Tumour-associated eosinophilia: A review. J Clin Pathol.
34:1343–1348. 1981. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Sobin LH, Gospodarowicz MK and Wittekind
C: TNM Classification of Malignant Tumours. 7th. Wiley-Blackwell;
New York, NY: pp. 100–109. 2009
|
|
10
|
Japanese Society for Cancer of the Colon
and Rectum, . Japanese Classification of Colorectal Carcinoma. 8th.
Kanehara; Tokyo: 2013
|
|
11
|
Baijens LW and Manni JJ: Paraneoplastic
syndromes in patients with primary malignancies of the head and
neck. Four cases and a review of the literature. Eur Arch
Otorhinolaryngol. 263:32–36. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Pelosof LC and Gerber DE: Paraneoplastic
syndromes: An approach to diagnosis and treatment. Mayo Clin Proc.
85:838–854. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Tajima K, Yamakawa M, Inaba Y, Katagiri T
and Sasaki H: Cellular localization of interleukin-5 expression in
rectal carcinoma with eosinophilia. Hum Pathol. 29:1024–1028. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Fridlender ZG, Simon HU and Shalit M:
Metastatic carcinoma presenting with concomitant eosinophilia and
thromboembolism. Am J Med Sci. 326:98–101. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Higashiyama A, Kudo M, Nagasako T,
Kawamura N, Abiko S, Yamamoto Y, Takano M, Gotoh J, Tamaki T,
Meguro J, et al: Successful chemotherapy of carcinomatosis of the
bone marrow with disseminated intravascular coagulation from a
rectal carcinoma found by eosinophilia. Nihon Shokakibyo Gakkai
Zasshi. 108:1244–1251. 2011.(In Japanese). PubMed/NCBI
|
|
16
|
Naito M, Yoshida Y, Aisu N, Tanimura S,
Hoshino S, Tanaka T, Nimura S, Tamura K and Yamashita Y: A report
of disseminated carcinomatosis of the bone marrow originating from
transverse colon cancer successfully treated with chemotherapy
using XELOX plus bevacizumab. Case Rep Oncol. 7:426–434. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Cools J, Deangelo DJ, Gotlib J, Stover EH,
Legare RD, Cortes J, Kutok J, Clark J, Galinsky I, Griffin JD, et
al: A tyrosine kinase created by fusion of the PDGFRA and FIP1L1
genes as a therapeutic target of imatinib in idiopathic
hypereosinophilic syndrome. N Engl J Med. 348:1201–1214. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Uemura K, Nakajim M, Yamauchi N, Fukayama
M and Yoshida K: Sudden death of a patient with primary
hypereosinophilia, colon tumours and pulmonary emboli. J Clin
Pathol. 57:541–543. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kato H, Kohata K, Yamamoto J, Ichikawa S,
Watanabe M, Ishizawa K, Ichinohasama R and Harigae H: Extreme
eosinophilia caused by interleukin-5-producing disseminated colon
cancer. Int J Hematol. 91:328–330. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Kosuge M, Ogawa M, Watanabe M, Eto K,
Yokoyama M and Yanaga K: A case of poorly differentiated
adenocarcinoma of the transverse colon in which MTX/5-FU therapy
was effective for disseminated intravascular coagulation syndrome
due to carcinomatosis of bone marrow. J Jpn Surg Assoc. 68:943–947.
2007.(In Japanese). View Article : Google Scholar
|