Introduction
Laryngeal cancer is the most common malignancy of
the throat in China. At present, the incidence rate of laryngeal
cancer is increasing significantly, accounting for 5.7–7.6% of all
malignant tumors in the northeast areas of China (1). A number of hypotheses regarding the
pathogenesis of laryngeal carcinoma exist: Smoking, alcohol
consumption, air pollution and viral infection are considered the
main risk factors (2). Due to the
anatomical site of the larynx, patients often mistake hoarseness
and other clinical symptoms of the disease as an upper respiratory
tract infection or symptom of voice overuse, which results in
negligence of the illness (3).
Supraglottic cancer and subglottic cancer exhibit no specific
symptoms at the primary stages and thus, early diagnosis is
difficult, which results in the majority of cases being diagnosed
at late and terminal stages (4). Due
to recent advances in minimally invasive surgery, radiotherapy,
chemotherapy, concurrent radiochemotherapy, biological therapy and
other comprehensive treatment modalities, patient survival times
have increased and patient quality of life has improved (5). However, the high rates of relapse and
metastasis of laryngeal cancer in addition to chemotherapy
resistance lead to poor treatment outcomes (6,7). Thus, to
investigate and develop novel targeted treatments for cancer,
laryngeal cancer must be investigated from a novel perspective.
Effective cancer treatments aim to eradicate the
majority of differentiated tumor cells, as well as tumor stem
cells, which have potential to proliferate and differentiate
(8). However, traditional therapies,
including radiotherapy, chemotherapy and immunotherapy, kill
differentiated tumor cells but not tumor stem cells, which results
in the development of tumor cell resistance to treatment and
subsequent relapse (9). Ideally,
treatments should kill differentiated tumor cells without causing
damage to other cell types, which requires the identification of
specific cell markers, genes and signal transduction pathways
associated with cancer that may be used as therapeutic targets to
improve the efficacy of tumor treatment (10).
Xanthohumol is a flavonoid compound obtained from
hops resin (11). It exhibits
numerous biological properties, including anti-inflammation and
anti-infection effects and has been demonstrated to inhibit the
growth and proliferation of microorganisms (12). Recent studies investigating
xanthohumol have predominantly focused on the prevention and
treatment of cancer (13,14). Studies have demonstrated that
xanthohumol inhibits tumor cell growth of colon cancer, prostate
cancer, cervical cancer, liver cancer and leukemia cells (15–17).
Therefore, the aim of the present study was to investigate the
effect of xanthohumol on the proliferation of human laryngeal
squamous cell carcinoma. The results of the present study may
indicate whether xanthohumol presents a potential drug for the
treatment of human laryngeal squamous cell carcinoma and may also
provide information regarding potential molecular mechanisms of the
disease.
Materials and methods
Cell and reagents
The human laryngeal squamous cell carcinoma SCC4
cell line was obtained from Shanghai Jiao Tong University
Affiliated Sixth People's Hospital (Shanghai, China). Dulbecco's
modified Eagle's medium (DMEM), penicillin and streptomycin were
purchased from Gibco (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Fetal bovine serum (FBS) was obtained from Gibco (Thermo
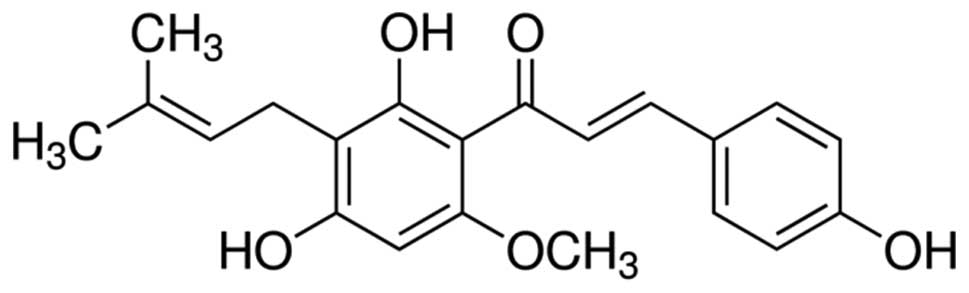
Fisher Scientific, Inc.). Xanthohumol (Fig. 1) and 3-(4,5-dimethylthiazol-2-yl)-2,
5-diphenyltetrazolium bromide (MTT) were purchased from
Sigma-Aldrich (Merck Millipore, Darmstadt, Germany). Annexin
V-fluorescein isothiocyanate (FITC)/propidium iodide (PI) staining
kit was obtained from BestBio (Shanghai, China).
Cell culture
The SCC4 cell line was cultured in DMEM supplemented
with 10% FBS, 100 U/ml penicillin and 100 U/ml streptomycin in a
humidified atmosphere with 5% CO2 at 37°C. The complete
medium was changed every 2–3 days.
Proliferation assay
SCC4 cells (1×105/well) in the
logarithmic growth phase were seeded in 96-well microplates. The
medium was replaced with DMEM containing 0, 10, 20, 30, 40 or 50 µM
xanthohumol and the cells were cultured for 72 h, after which 200
µl MTT (0.5 mg/ml; Sigma-Aldrich) was added to each well. Following
incubation at 4°C, 150 µl DMSO was added and the absorbance was
measured using a spectrophotometer (Infinite® 200 PRO;
Tecan, San Jose, CA, USA) at a wavelength of 540 nm.
Annexin V-FITC/PI staining
SCC4 cells (2.4×106/well) in the
logarithmic growth phase were seeded in 6-well microplates. The
medium was replaced with DMEM containing 20, 30 or 40 µM
xanthohumol and cells were cultured for 48 h at 4°C. SCC4 cells
(1×106) were harvested, washed with PBS and resuspended
in binding buffer (BestBio). Next, 5 µl Annexin V-FITC and 5 µl PI
were added to each well and cultured for 20 min at 4°C. Apoptotic
rate was determined using a flow cytometer (FACSCalibur™; BD
Biosciences, San Jose, CA, USA).
Measurement of caspase-3, −8 and −9
activity
SCC4 cells (2.4×106/well) in the
logarithmic growth phase were seeded in 6-well microplates. The
medium was replaced with DMEM containing 20, 30 or 40 µM
xanthohumol and cells were cultured for 48 h at 4°C. SCC4 cells
(1×106) were harvested, washed with PBS and resuspended
in binding buffer. A total of 1 µl fluorescent substrate
[Ac-DEVD-pNA for caspase-3, Ac-IETD-pNA for caspase-8 and
Ac-LEHD-pNA for caspase-9 (BestBio, Shanghai, China)] was added to
each well and incubated for 1 h at 4°C. Cells were then centrifuged
at 500 × g for 10 min at room temperature and the
supernatant was removed. The cells were resuspended in 100 µl wash
buffer (BestBio), and the fluorescence intensity was measured at
485 nm (excitation wavelength) and 535 nm (emission wavelength)
using a spectrophotometer.
Western blot analysis
SCC4 cells (2.4×106/well) in the
logarithmic growth phase were seeded in 6-well microplates. The
medium was replaced with DMEM containing 20, 30 or 40 µM
xanthohumol and cells were cultured for 48 h at 4°C. SCC4 cells
(1×106) were harvested, washed with PBS, and lysed with
cold RIPA buffer (BestBio) containing protease inhibitors. Protein
concentrations were quantified using the bicinchonic acid assay
method (BestBio). A total of 10 µg protein was boiled in water
prior to separation by 12% SDS-PAGE for 10 min then transferred
onto polyvinylidene difluoride membranes at 100 V for 1.5 h.
Membranes were blocked with 5% skimmed milk in Tris-buffered saline
with 0.1% Tween 20 for 2 h followed by incubation with anti-B-cell
lymphoma 2 (Bcl-2; cat. no. sc-7382; 1:1,000), anti-myeloid cell
leukemia 1 (Mcl-1; cat. no. sc-377487; 1:1,000), anti-poly ADP
ribose polymerase (PARP; cat. no. sc-56197; 1:2,000), anti-p53
(sc-393031; 1:1,000), anti-apoptosis-inducing factor (AIF; cat. no.
sc-390619; 1:1,000) and anti-β-actin (cat. no. sc-47778; 1:1,000)
antibodies (Santa Cruz Biotechnology Inc., Dallas, TX, USA)
overnight 4°C. The membranes were then incubated with mouse
secondary antibodies (cat. no. sc-358914; 1:15,000; Santa Cruz
Biotechnology, Inc.) for 2 h at 4°C. The proteins were visualized
using BeyoECL Star (cat. no. P0018A; Beyotime Institute of
Biotechnology, Jiangsu, China) and quantified using the Molecular
Imager ChemiDoc XRS+ System with Image Lab™ software (Bio-Rad
Laboratories, Inc., Hercules, CA, USA).
Statistical analysis
All data are presented as the mean ± standard error
of the mean. Data was analyzed by one-way analysis of variance
followed by Dunnett's t-test using SPSS version 22 statistical
software (SPPS, Inc., Chicago, IL, USA). P<0.05 was considered
to indicate a statistically significant difference.
Results
Xanthohumol inhibits proliferation of
laryngeal squamous cell carcinoma cells
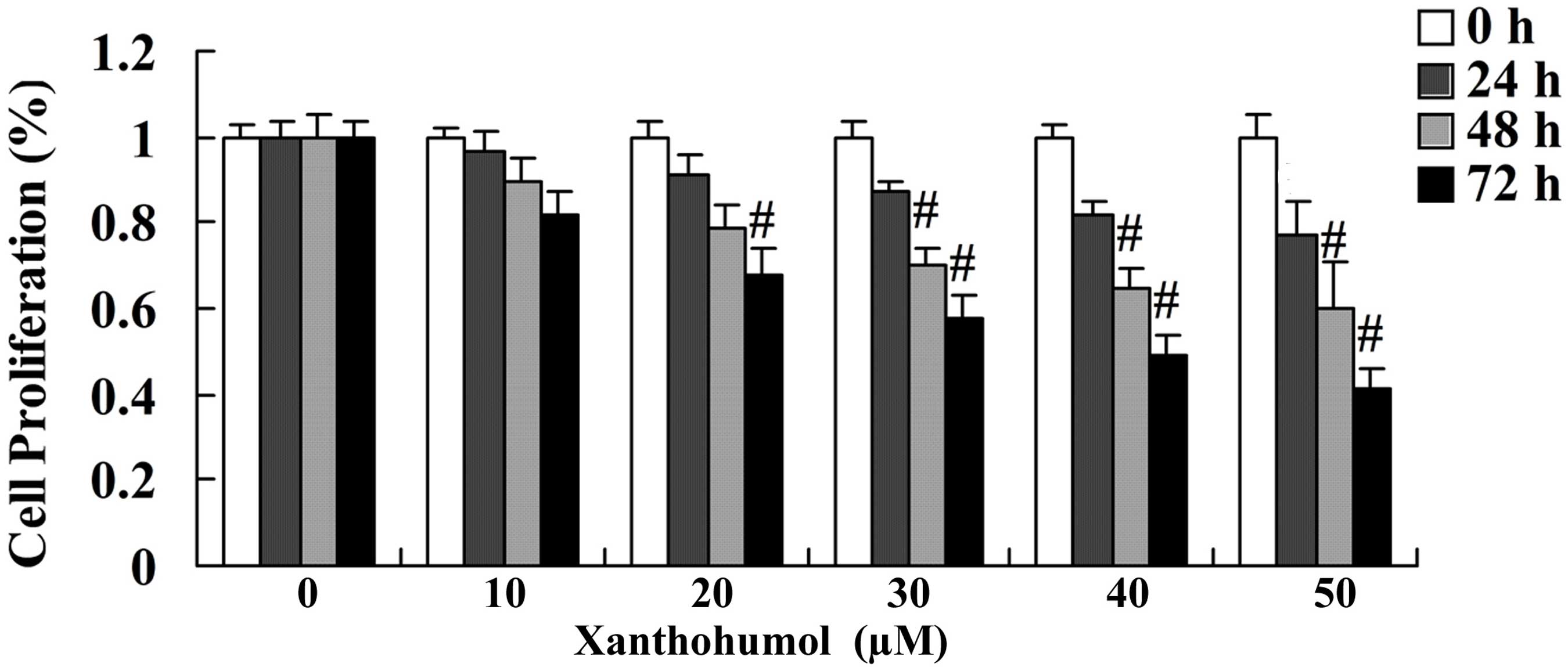
MTT assay was performed to determine the effect of
xanthohumol on the proliferation of SCC4 cells following treatment
with 30, 40 and 50 µM xanthohumol for 24, 48 and 72 h. The results
revealed that xanthohumol inhibited the proliferation of SCC4 cells
in a concentration- and time-dependent manner when compared with
that of control group (Fig. 2).
Following 24, 48 and 72 h treatment with 30, 40 and 50 µM
xanthohumol significantly inhibited the proliferation of SCC4 cells
(Fig. 2). In addition, following
treatment with 20 µM xanthohumol for 72 h proliferation of SCC4
cells was significantly inhibited compared with the control group
(Fig. 2).
Xanthohumol induces cell apoptosis of
laryngeal squamous cell carcinoma cells
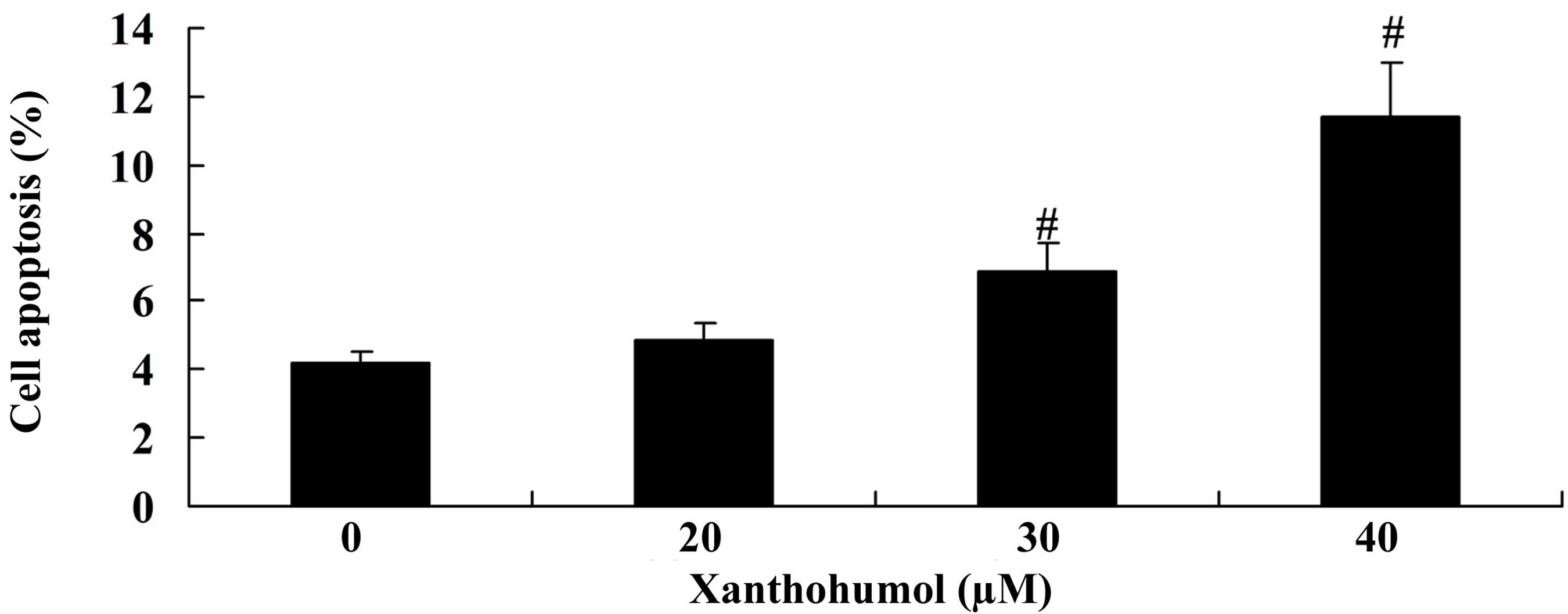
To evaluate the effect of xanthohumol on SCC4 cell
apoptosis, flow cytometry analysis was performed. The results
demonstrated that treatment with 30 and 40 µM xanthohumol for 48 h
significantly induced apoptosis of SCC4 cells compared with the
control group (Fig. 3).
Xanthohumol increases caspase-3, −8
and −9 activity in laryngeal squamous cell carcinoma
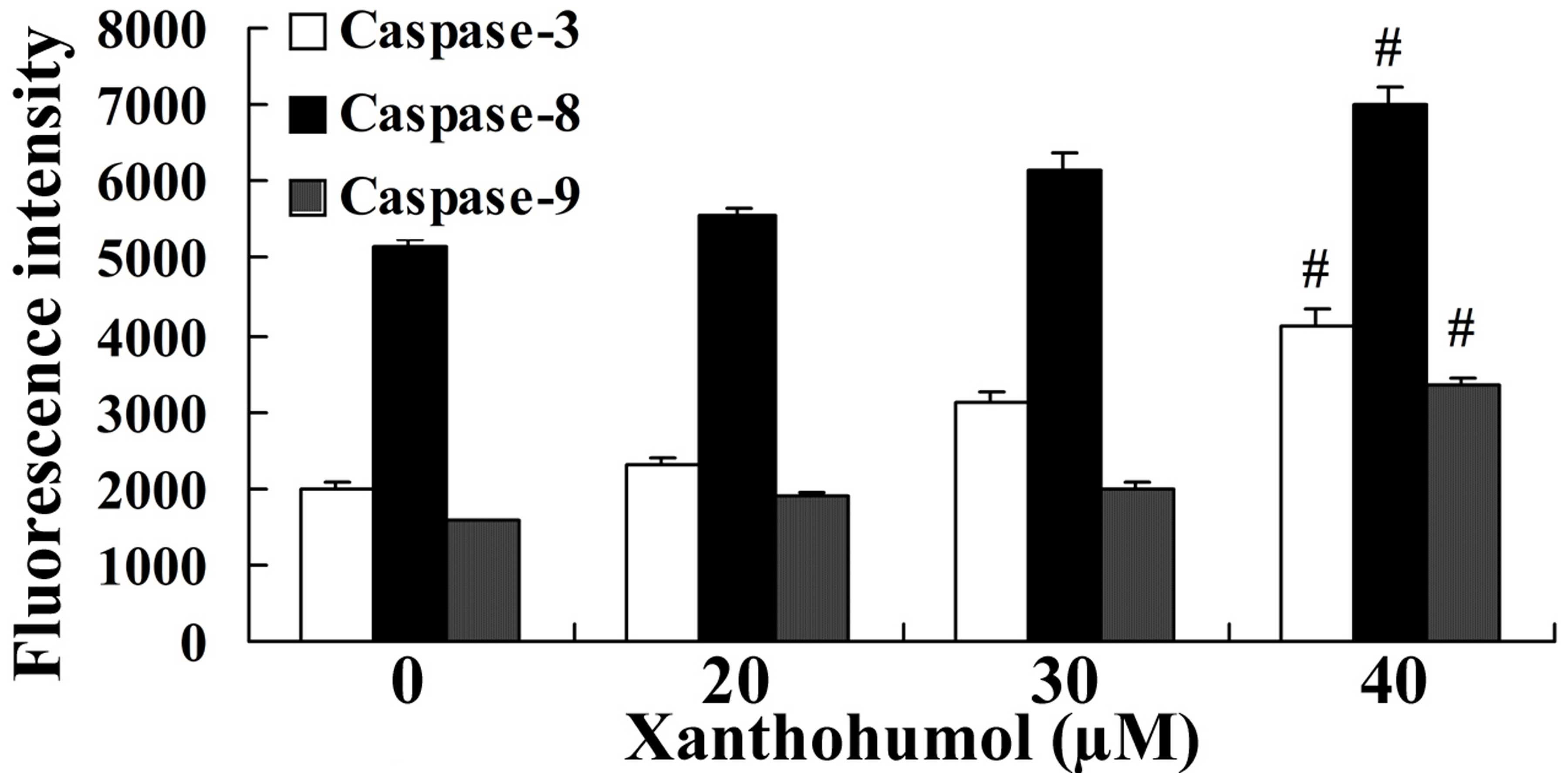
To further investigate the effect of xanthohumol on
caspase activity in laryngeal squamous cell carcinoma, the
florescence intensities of caspase-3, −8 and −9 were measured in
SCC4 cells following 48 h treatment with 20, 30 and 40 µM
xanthohumol. The results revealed that caspase-3, −8 and −9
activity was significantly increased following treatment with 30
and 40 µM xanthohumol when compared with the control group
(Fig. 4).
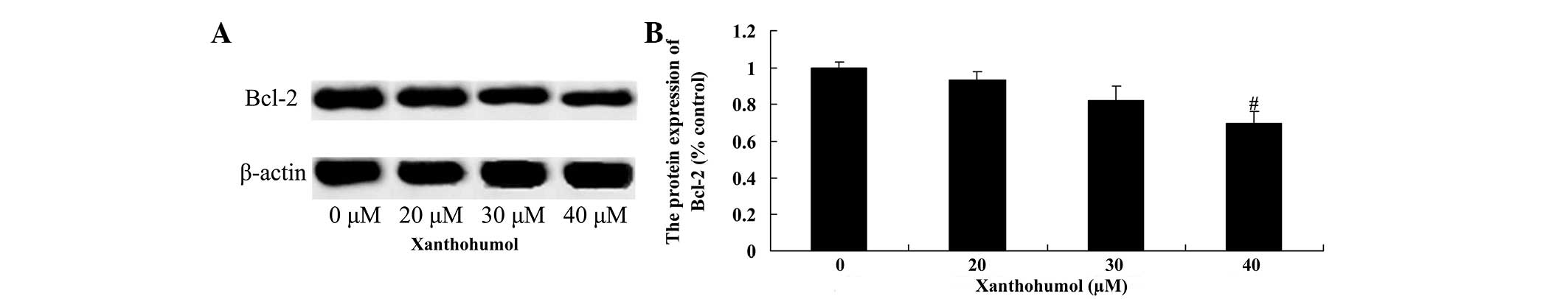
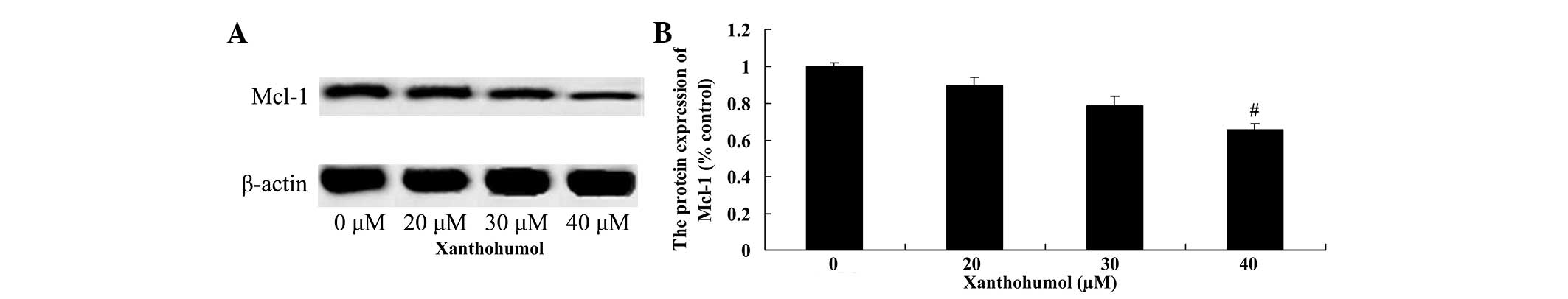
Xanthohumol decreases Bcl-2 and Mcl-1
protein expression in laryngeal squamous cell carcinoma cells
The effect of xanthohumol on the expression of Bcl-2
and Mcl-1 proteins in laryngeal squamous cell carcinoma SCC4 cells
was examined by western blot analysis. Following treatment with 40
µM xanthohumol the expression of Bcl-2 (Fig. 5) and Mcl-1 (Fig. 6) proteins were significantly decreased
compared with the control group.
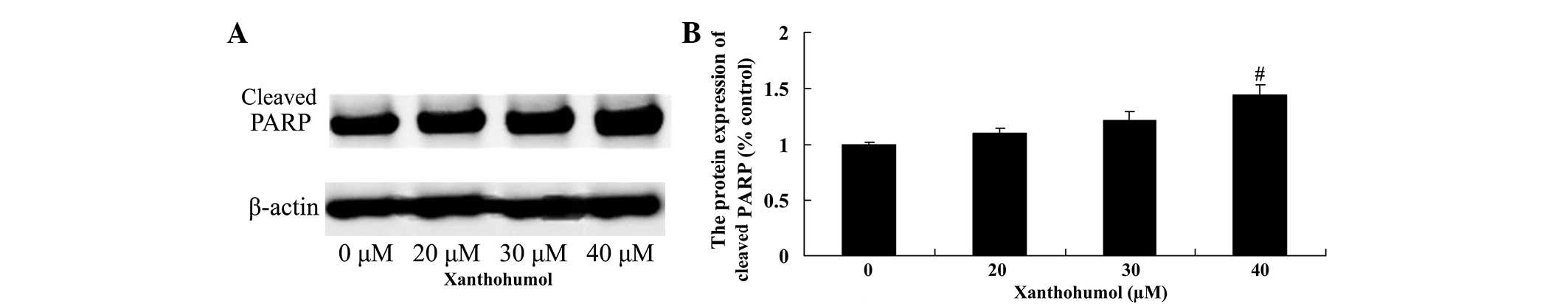
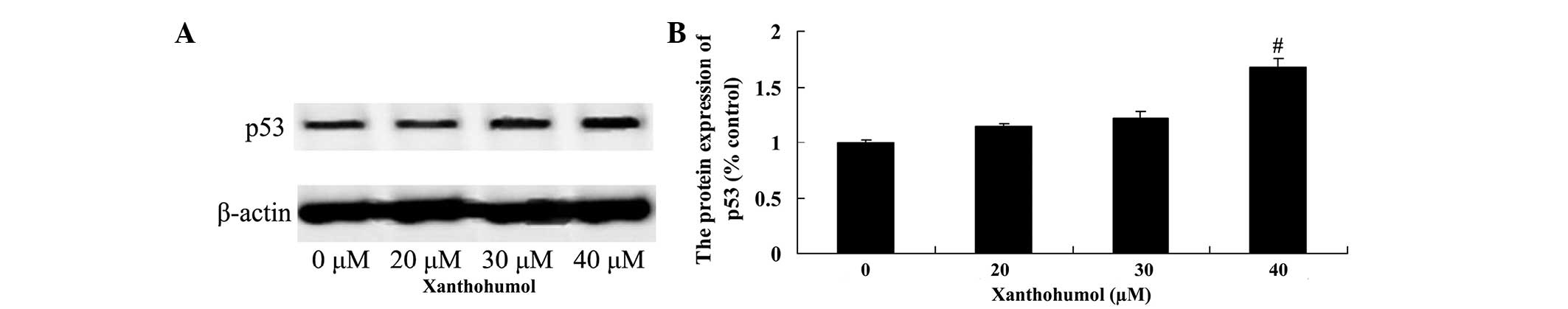
Xanthohumol increases PARP and p53
protein expression in laryngeal squamous cell carcinoma
The effect of xanthohumol on PARP and p53 protein
expression in SSC4 cells was examined by western blot analysis.
Following treatment with 40 µM xanthohumol for 48 h, the expression
of PARP (Fig. 7) and p53 (Fig. 8) proteins were significantly increased
compared with control group.
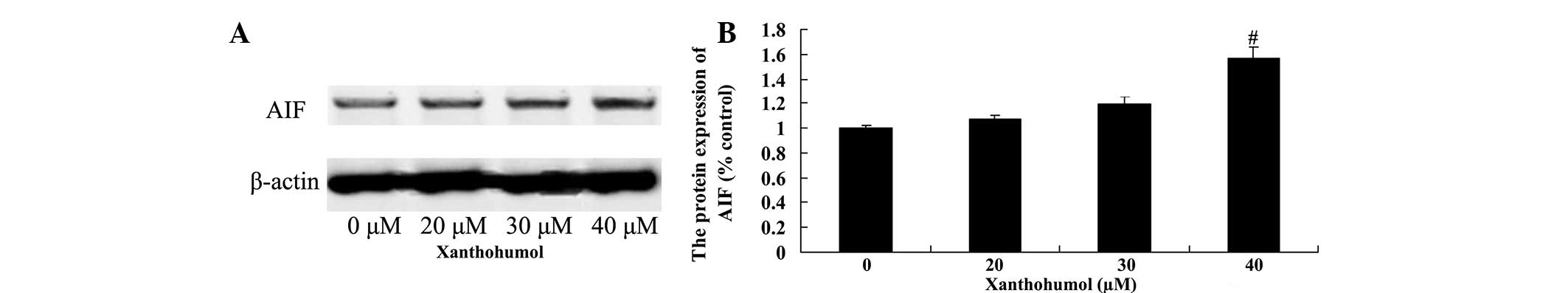
Xanthohumol increases AIF protein
expression in laryngeal squamous cell carcinoma cells
To determine whether the AIF pathway mediates the
anticancer effects of xanthohumol, AIF protein expression in SCC4
cells was measured using western blot analysis. Following treatment
with 40 µM xanthohumol for 48 h, the expression of AIF protein was
significantly increased compared with the control group (Fig. 9).
Discussion
Laryngeal squamous cell carcinoma is a common
malignant tumor derived from the laryngeal epithelium, which is the
third most common cause of head and neck cancer morbidity,
accounting for 1–5% of all malignant tumors (2). Notably, the incidence rate of laryngeal
cancer is increasing significantly among young individuals and
thus, better treatments are urgently required (18). Although the treatment of laryngeal
cancer has markedly improved in recent years due to advances in
surgical techniques and combined chemotherapy and radiotherapy
regimens, certain cases of squamous cell carcinoma of the larynx do
not respond to treatment (19).
Furthermore, due to the rapid development of molecular biology
technology, gene therapy is gaining increasing attention and is
considered to present a promising option for cancer patients
(20). Therefore, the identification
of specific genes that are involved in the development of laryngeal
squamous cell carcinoma may lead to further studies and the
clinical application of gene therapy for laryngeal cancer. Previous
studies have demonstrated that xanthohumol induces apoptosis in
hepatocellular carcinoma (21), human
cervical cancer cells (17) and
breast cancer (22). To the best of
our knowledge, the present study is the first to demonstrate that
xanthohumol inhibits cell proliferation and induces cell apoptosis
of laryngeal squamous cell carcinoma SCC4 cells.
Cancer is a disease caused by abnormal cell
proliferation, differentiation and apoptosis. It has been reported
that caspases are activated during tumor cell apoptosis (23). A series of novel caspase-related
apoptosis-inducing pathways have been identified that may be
targeted to control cancer, which have practical significance for
the majority of tumors and thus, may result in breakthroughs with
regard to the treatment of malignant tumors (24). The activation of caspase-9 and
caspase-3 may underlie the apoptosis of fibroblasts in keloids
(25). Furthermore, caspase-8
activates caspase-9 downstream of the apoptosis cascade to induce
apoptosis (26). In the current
study, xanthohumol promoted the activity of caspase-3, −8 and −9
and suppressed Bcl-2 and Mcl-1 protein expression in laryngeal
squamous cell carcinoma SCC4 cells. These results are in accordance
with those reported by Pan et al (27), which demonstrated that xanthohumol
induces apoptosis of human colon cancer cells via caspase-3, −8 and
−9. Furthermore, Zajc et al (15) reported that xanthohumol induces
different cytotoxic and apoptotic pathways via Bax/Bcl-2 in
malignant and normal astrocytes. Kunnimalaiyaan et al
(28) demonstrated that xanthohumol
inhibits induces apoptosis via the anti-apoptotic markers,
survivin, cyclin D1 and Mcl-1, in hepatocellular carcinoma.
PARP-1 is a member of the PARP family, which
initiates DNA damage repair via the modification of poly adenosine
diphosphate glycosylation receptor protein. PARP-1 has been shown
to trigger apoptosis signaling and activate caspase-3 to induce
cell apoptosis and DNA damage (24,29). Thus,
we hypothesize that treatment with xanthohumol promoted PARP
protein expression in laryngeal squamous cell carcinoma SCC4 cells.
Lust et al (11) demonstrated
that xanthohumol activates the proapoptotic pathway via PARP
activation in chronic lymphocytic leukemia.
The p53 gene is recognized as the most commonly
mutated tumor suppressor gene (30).
Recent studies have indicated that p53 gene mutations exhibit an
important function in the development of laryngeal squamous cell
carcinoma (31,32). The mutation rate of p53 is >90% in
certain tumors (lung, liver, colon and gastric cancer) and ranges
between 34 and 93% in head and neck tumor tissues and cell lines,
which is associated with early relapse of cancer (33). Xanthohumol activates PARP protein
expression in laryngeal squamous cell carcinoma cells. In the
present study, p53 expression increased significantly following
treatment with xanthohumol. Monteghirfo et al (12) reported that xanthohumol inhibits
leukemia cell invasion via p53 modulation in Bcr/Abl-transformed
cells.
AIF protein, which is located in the mitochondrial
intermembrane space, exhibits apoptosis-inducing activity (34). In response to apoptotic stimuli, AIF
molecules are released from the mitochondria into the cytoplasm
followed by translocation to the nucleus and subsequent integration
with chromosomal DNA, which leads to chromosome condensation and
DNA breakage into large fragments (~50 kB) (35). AIF exhibits apoptosis-inducing
activity and oxidoreductase activity, which result in interlinkage
(36). Notably, AIF was the first
molecule to be identified that mediates cell apoptosis directly,
however, it has also been reported that caspases are involved in
AIF apoptotic activity (37). The
results of the present study also indicated that xanthohumol
increased AIF protein expression in laryngeal squamous cell
carcinoma cells. Yong and Abd Malek (17) reported that xanthohumol induces growth
inhibition and apoptosis via AIF protein expression in Ca Ski human
cervical cancer cells.
In conclusion, we postulate that xanthohumol
mediates growth suppression and induces caspase-dependent cell
death via the suppression of Bcl-2 and Mcl-1 and activation of
PARP, p53 and AIF signaling pathways. Therefore, future studies
that investigate xanthohumol as a potential therapeutic agent for
laryngeal squamous cell carcinoma are required.
References
|
1
|
Guibert M, Lepage B, Woisard V, Rives M,
Serrano E and Vergez S: Quality of life in patients treated for
advanced hypopharyngeal or laryngeal cancer. Eur Ann
Otorhinolaryngol Head Neck Dis. 128:218–223. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Tuomi L, Andréll P and Finizia C: Effects
of voice rehabilitation after radiation therapy for laryngeal
cancer: A randomized controlled study. Int J Radiat Oncol Biol
Phys. 89:964–972. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Robertson SM, Yeo JC, Sabey L, Young D and
MacKenzie K: Effects of tumor staging and treatment modality on
functional outcome and quality of life after treatment for
laryngeal cancer. Head Neck. 35:1759–1763. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Möhner M, Lindtner M and Otten H: Ionizing
radiation and risk of laryngeal cancer among German uranium miners.
Health Phys. 95:725–733. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Giotakis AI, Kontos CK, Manolopoulos LD,
Sismanis A, Konstadoulakis MM and Scorilas A: High BAX/BCL2 mRNA
ratio predicts favorable prognosis in laryngeal squamous cell
carcinoma, particularly in patients with negative lymph nodes at
the time of diagnosis. Clin Biochem. 49:890–896. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tae K, Jin BJ, Ji YB, Jeong JH, Cho SH and
Lee SH: The role of laryngopharyngeal reflux as a risk factor in
laryngeal cancer: A preliminary report. Clin Exp Otorhinolaryngol.
4:101–104. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Narwani V, Nalamada K, Lee M, Kothari P
and Lakhani R: Readability and quality assessment of internet-based
patient education materials related to laryngeal cancer. Head Neck.
38:601–605. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Xu CZ, Xie J, Jin B, Chen XW, Sun ZF, Wang
BX and Dong P: Gene and microRNA expression reveals sensitivity to
paclitaxel in laryngeal cancer cell line. Int J Clin Exp Pathol.
6:1351–1361. 2013.PubMed/NCBI
|
|
9
|
Yu D, Jin C, Liu Y, Yang J, Zhao Y, Wang
H, Zhao X, Cheng J, Liu X and Liu C: Clinical implications of
cancer stem cell-like side population cells in human laryngeal
cancer. Tumour Biol. 34:3603–3610. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wei X, Wang J, He J, Ma B and Chen J:
Biological characteristics of CD133(+) cancer stem cells
derived from human laryngeal carcinoma cell line. Int J Clin Exp
Med. 7:2453–2462. 2014.PubMed/NCBI
|
|
11
|
Lust S, Vanhoecke B, VAN Gele M, Boelens
J, VAN Melckebeke H, Kaileh M, Berghe W Vanden, Haegeman G,
Philippé J, Bracke M and Offner F: Xanthohumol activates the
proapoptotic arm of the unfolded protein response in chronic
lymphocytic leukemia. Anticancer Res. 29:3797–3805. 2009.PubMed/NCBI
|
|
12
|
Monteghirfo S, Tosetti F, Ambrosini C,
Stigliani S, Pozzi S, Frassoni F, Fassina G, Soverini S, Albini A
and Ferrari N: Antileukemia effects of xanthohumol in
Bcr/Abl-transformed cells involve nuclear factor-kappaB and p53
modulation. Mol Cancer Ther. 7:2692–2702. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen PH, Chang CK, Shih CM, Cheng CH, Lin
CW, Lee CC, Liu AJ, Ho KH and Chen KC: The miR-204-3p-targeted
IGFBP2 pathway is involved in xanthohumol-induced glioma cell
apoptotic death. Neuropharmacology. 110:362–375. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Dokduang H, Yongvanit P, Namwat N,
Pairojkul C, Sangkhamanon S, Yageta MS, Murakami Y and Loilome W:
Xanthohumol inhibits STAT3 activation pathway leading to growth
suppression and apoptosis induction in human cholangiocarcinoma
cells. Oncol Rep. 35:2065–2072. 2016.PubMed/NCBI
|
|
15
|
Zajc I, Filipič M and Lah TT: Xanthohumol
induces different cytotoxicity and apoptotic pathways in malignant
and normal astrocytes. Phytother Res. 26:1709–1713. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Colgate EC, Miranda CL, Stevens JF, Bray
TM and Ho E: Xanthohumol, a prenylflavonoid derived from hops
induces apoptosis and inhibits NF-kappaB activation in prostate
epithelial cells. Cancer Lett. 246:201–209. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yong WK and Abd Malek SN: Xanthohumol
induces growth inhibition and apoptosis in ca ski human cervical
cancer cells. Evid Based Complement Alternat Med. 2015:9213062015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Wakisaka N, Kondo S, Endo K, Murono S and
Yoshizaki T: Adjuvant chemotherapy with an oral fluoropyrimidine,
S-1, following reduced RADPLAT in advanced laryngeal cancer. Ann
Otol Rhinol Laryngol. 121:555–562. 2012.PubMed/NCBI
|
|
19
|
Melinceanu L, Sarafoleanu C, Lerescu L,
Tucureanu C, Caras I and Sălăgeanu A: Impact of smoking on the
immunological profile of patients with laryngeal carcinoma. J Med
Life. 2:211–218. 2009.PubMed/NCBI
|
|
20
|
Wan G, Zhou L, Xie M, Chen H and Tian J:
Characterization of side population cells from laryngeal cancer
cell lines. Head Neck. 32:1302–1309. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Qu X, Xu C, Wang H, Xu J, Liu W, Wang Y,
Jia X, Xie Z, Xu Z, Ji C, et al: Hippocampal glutamate level and
glutamate aspartate transporter (GLAST) are up-regulated in senior
rat associated with isoflurane-induced spatial learning/memory
impairment. Neurochem Res. 38:59–73. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Yoshimaru T, Komatsu M, Tashiro E, Imoto
M, Osada H, Miyoshi Y, Honda J, Sasa M and Katagiri T: Xanthohumol
suppresses oestrogen-signalling in breast cancer through the
inhibition of BIG3-PHB2 interactions. Sci Rep. 4:73552014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Zhang H, Li X, Zhang Y and Luan X:
Luteolin induces apoptosis by activating Fas signaling pathway at
the receptor level in laryngeal squamous cell line Hep-2 cells. Eur
Arch Otorhinolaryngol. 271:1653–1659. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Corbiere C, Liagre B, Terro F and
Beneytout JL: Induction of antiproliferative effect by diosgenin
through activation of p53, release of apoptosis-inducing factor
(AIF) and modulation of caspase-3 activity in different human
cancer cells. Cell Res. 14:188–196. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Chen X, Deng M, Ma L, Zhou J, Xiao Y, Zhou
X, Zhang C and Wu M: Inhibitory effects of forkhead box L1 gene on
osteosarcoma growth through the induction of cell cycle arrest and
apoptosis. Oncol Rep. 34:265–271. 2015.PubMed/NCBI
|
|
26
|
Akagi T, Shimizu K, Takahama S, Iwasaki T,
Sakamaki K, Endo Y and Sawasaki T: Caspase-8 cleavage of the
interleukin-21 (IL-21) receptor is a negative feedback regulator of
IL-21 signaling. FEBS Lett. 585:1835–1840. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Pan L, Becker H and Gerhäuser C:
Xanthohumol induces apoptosis in cultured 40–16 human colon cancer
cells by activation of the death receptor- and mitochondrial
pathway. Mol Nutr Food Res. 49:837–843. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kunnimalaiyaan S, Sokolowski KM,
Balamurugan M, Gamblin TC and Kunnimalaiyaan M: Xanthohumol
inhibits Notch signaling and induces apoptosis in hepatocellular
carcinoma. PLoS One. 10:e01274642015. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Kang N, Zhang JH, Qiu F, Tashiro S,
Onodera S and Ikejima T: Inhibition of EGFR signaling augments
oridonin-induced apoptosis in human laryngeal cancer cells via
enhancing oxidative stress coincident with activation of both the
intrinsic and extrinsic apoptotic pathways. Cancer Lett.
294:147–158. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kropveld A, Slootweg PJ, van Mansfeld AD,
Blankenstein MA and Hordijk GJ: Radioresistance and p53 status of
T2 laryngeal carcinoma. Analysis by immunohistochemistry and
denaturing gradient gel electrophoresis. Cancer. 78:991–997. 1996.
View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Pei SG, Wang JX, Wang XL, Zhang QJ and
Zhang H: Correlation of survivin, p53 and Ki-67 in laryngeal cancer
Hep-2 cell proliferation and invasion. Asian Pac J Trop Med.
8:636–642. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Simsek H, Han U, Onal B and Simisek G: The
expression of EGFR, cerbB2, p16, and p53 and their relationship
with conventional parameters in squamous cell carcinoma of the
larynx. Turk J Med Sci. 44:411–416. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Ogawa T, Shiga K, Tateda M, Saijo S,
Suzuki T, Sasano H, Miyagi T and Kobayashi T: Protein expression of
p53 and Bcl-2 has a strong correlation with radiation resistance of
laryngeal squamous cell carcinoma but does not predict the
radiation failure before treatment. Oncol Rep. 10:1461–1466.
2003.PubMed/NCBI
|
|
34
|
Mendivil-Perez M, Velez-Pardo C and
Jimenez-Del-Rio M: TPEN induces apoptosis independently of zinc
chelator activity in a model of acute lymphoblastic leukemia and ex
vivo acute leukemia cells through oxidative stress and mitochondria
caspase-3- and AIF-dependent pathways. Oxid Med Cell Longev.
2012:3132752012. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Cho SY, Lee JH, Ju MK, Jeong EM, Kim HJ,
Lim J, Lee S, Cho NH, Park HH, Choi K, et al: Cystamine induces
AIF-mediated apoptosis through glutathione depletion. Biochim
Biophys Acta. 1853:619–631. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Yang R, Cui HJ, Wang H, Wang Y, Liu JH, Li
Y and Lu Y: N-stearoyltyrosine protects against glutamate-induced
oxidative toxicity by an apoptosis-inducing factor (AIF)-mediated
caspase-independent cell death pathway. J Pharmacol Sci.
124:169–179. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Hunter TB, Manimala NJ, Luddy KA, Catlin T
and Antonia SJ: Paclitaxel and TRAIL synergize to kill
paclitaxel-resistant small cell lung cancer cells through a
caspase-independent mechanism mediated through AIF. Anticancer Res.
31:3193–3204. 2011.PubMed/NCBI
|