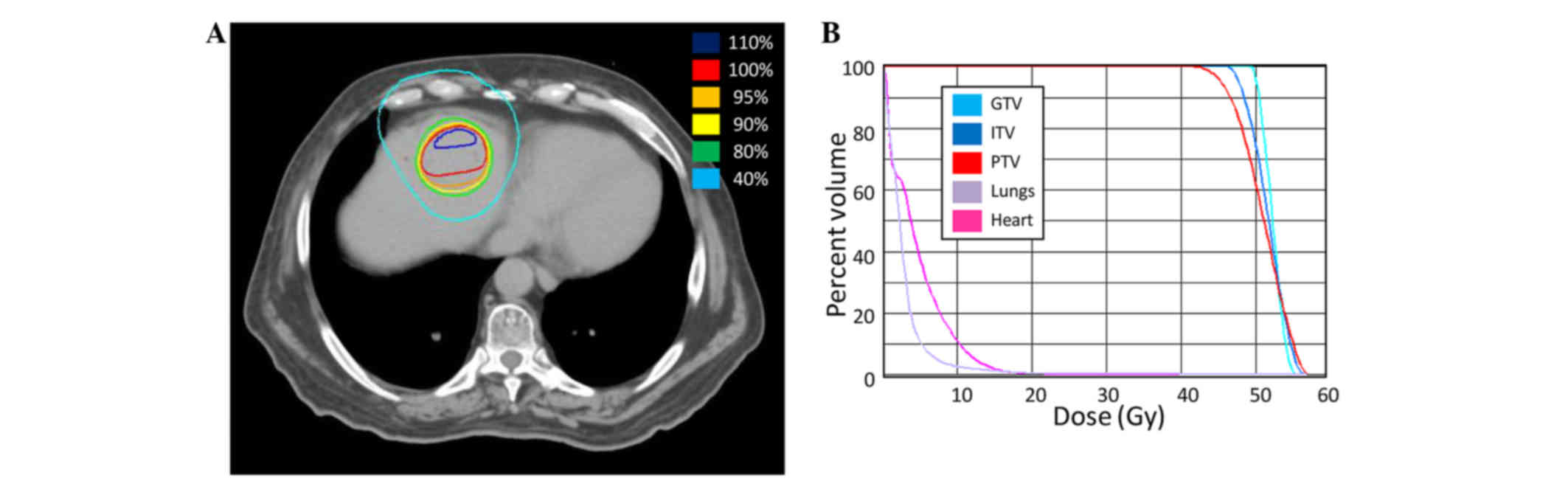
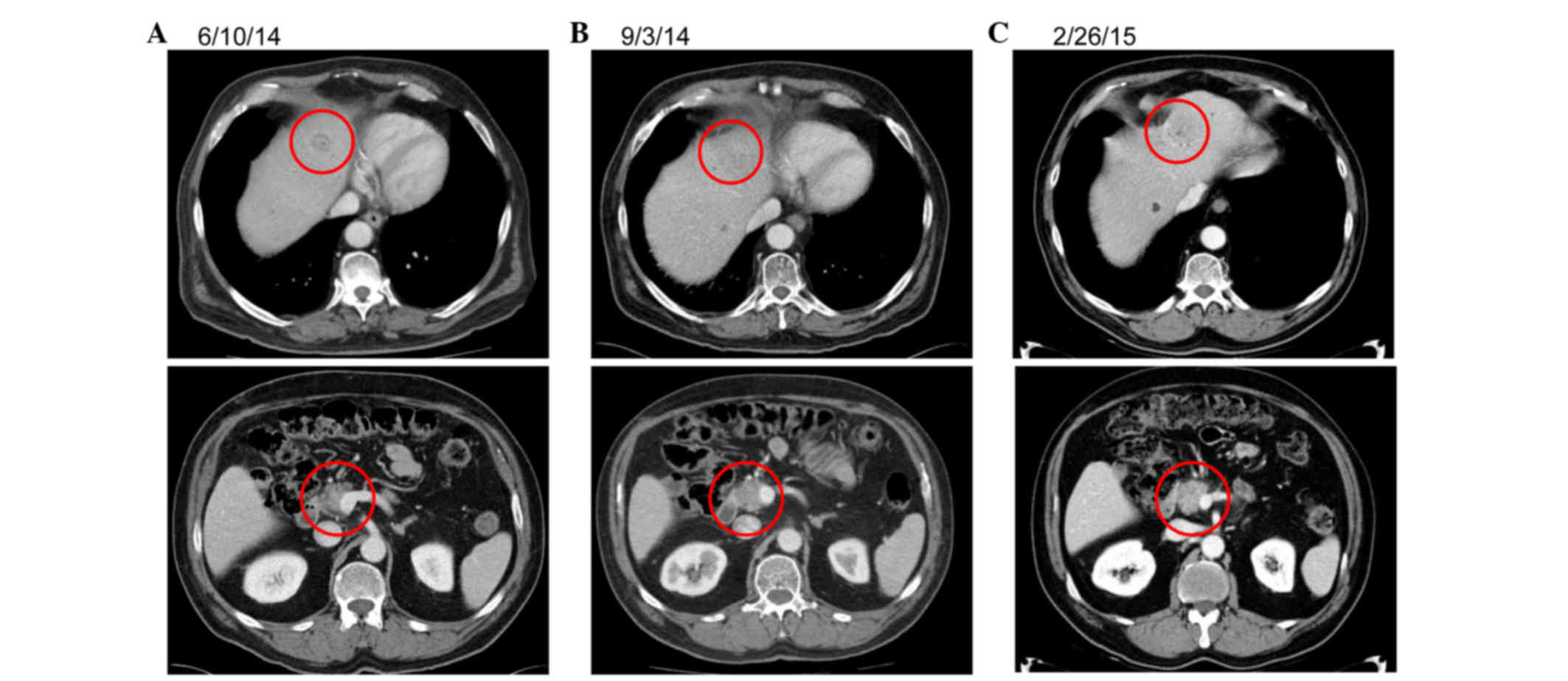
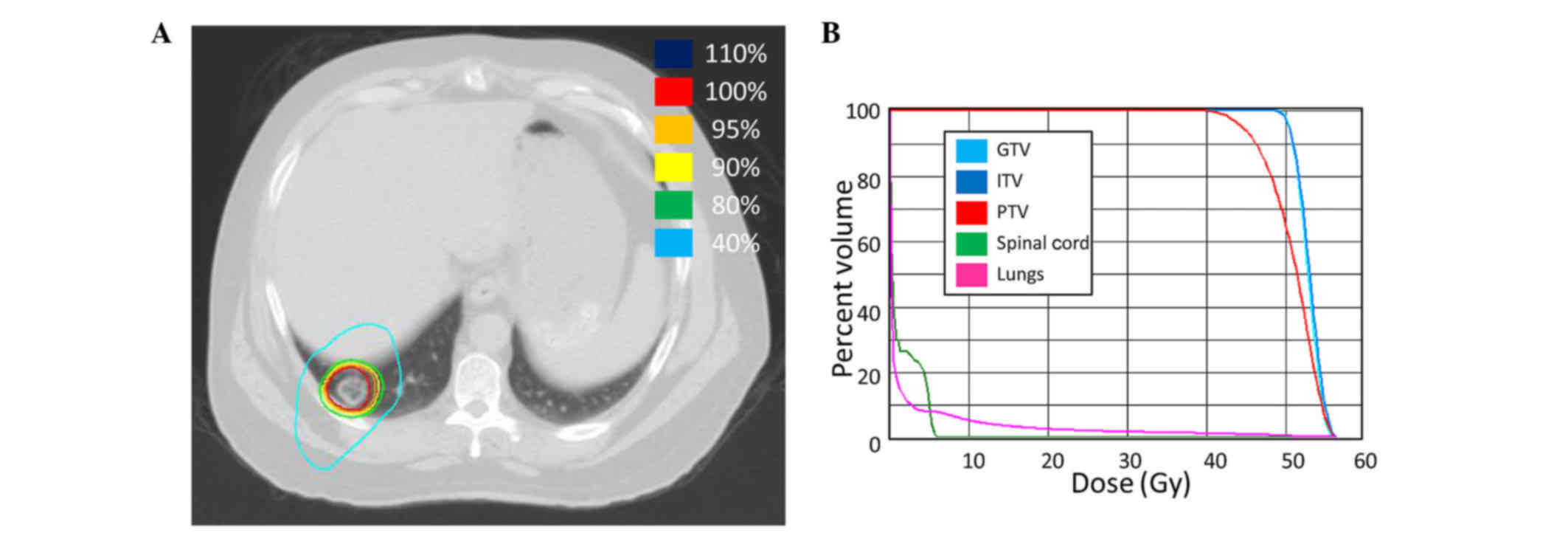
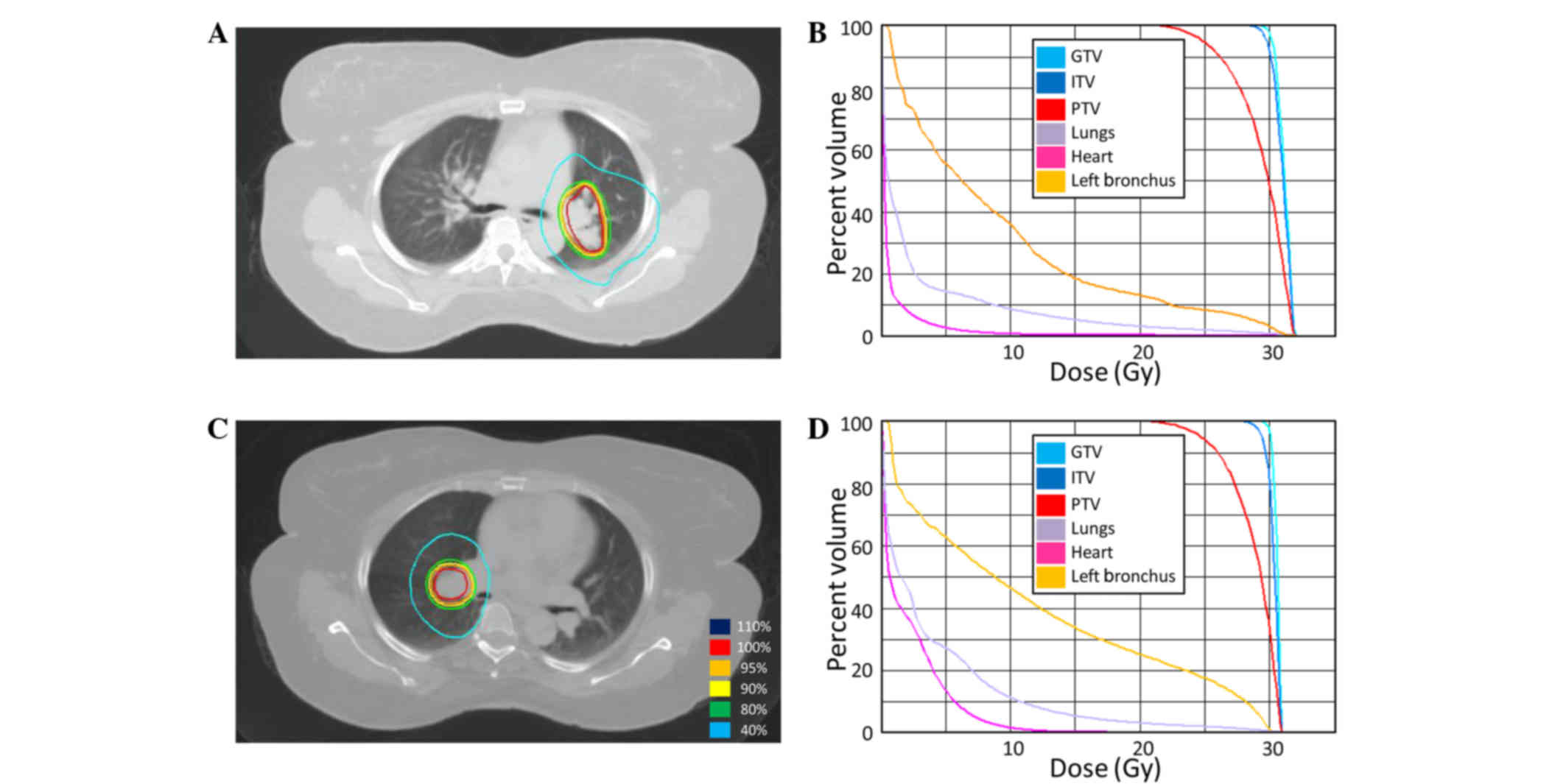
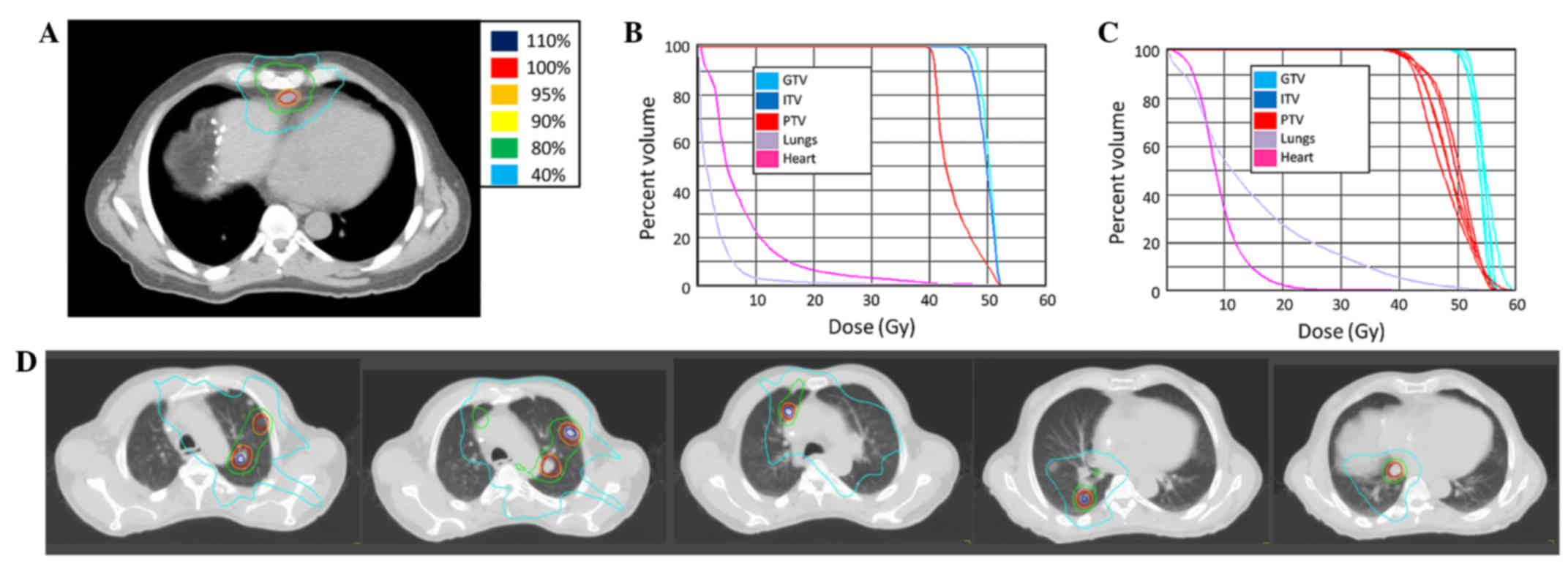
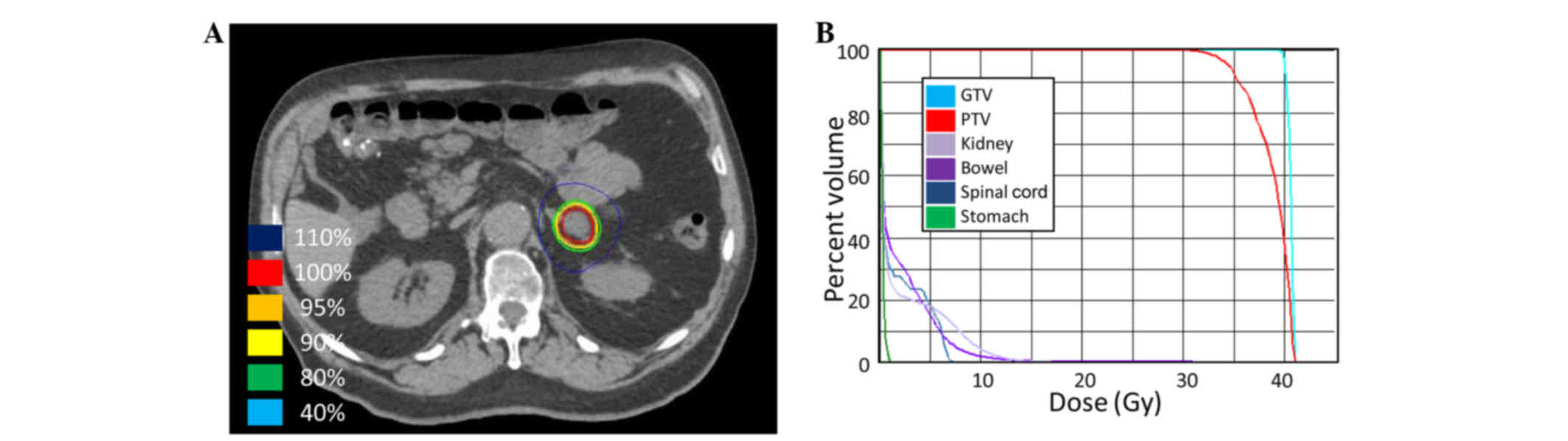
Spandidos Publications style
Wray J, Hawamdeh RF, Hasija N, Dagan R, Yeung AR, Lightsey JL, Okunieff P, Daily KC, George TJ, Zlotecki RA, Zlotecki RA, et al: Stereotactic body radiation therapy for oligoprogression of metastatic disease from gastrointestinal cancers: A novel approach to extend chemotherapy efficacy. Oncol Lett 13: 1087-1094, 2017.
APA
Wray, J., Hawamdeh, R.F., Hasija, N., Dagan, R., Yeung, A.R., Lightsey, J.L. ... Dang, L.H. (2017). Stereotactic body radiation therapy for oligoprogression of metastatic disease from gastrointestinal cancers: A novel approach to extend chemotherapy efficacy. Oncology Letters, 13, 1087-1094. https://doi.org/10.3892/ol.2016.5540
MLA
Wray, J., Hawamdeh, R. F., Hasija, N., Dagan, R., Yeung, A. R., Lightsey, J. L., Okunieff, P., Daily, K. C., George, T. J., Zlotecki, R. A., Trevino, J., Dang, L. H."Stereotactic body radiation therapy for oligoprogression of metastatic disease from gastrointestinal cancers: A novel approach to extend chemotherapy efficacy". Oncology Letters 13.3 (2017): 1087-1094.
Chicago
Wray, J., Hawamdeh, R. F., Hasija, N., Dagan, R., Yeung, A. R., Lightsey, J. L., Okunieff, P., Daily, K. C., George, T. J., Zlotecki, R. A., Trevino, J., Dang, L. H."Stereotactic body radiation therapy for oligoprogression of metastatic disease from gastrointestinal cancers: A novel approach to extend chemotherapy efficacy". Oncology Letters 13, no. 3 (2017): 1087-1094. https://doi.org/10.3892/ol.2016.5540