Introduction
Gastroenteropancreatic neuroendocrine neoplasms
(GEP-NENs) constitute a heterogeneous group of neoplasms, with
various clinical presentations and biological behaviors that
present diagnostic and therapeutic challenges. As 60–80% of
patients present with metastatic disease at the time of diagnosis,
they are treated with multidisciplinary approaches for symptom
control and inhibition of tumor growth (1).
Neuroendocrine tumors are known to overexpress
somatostatin receptors (SSTRs), a family of G
protein-coupled-receptors, most commonly SSTR2 and SSTR5 (2). In previous studies, SSTRs have been
extensively mapped in neuroendocrine tumors, using reverse
transcription-polymerase chain reaction, autoradiography and
immunoblotting (2–4). To date, few studies have examined the
expression of SSTRs in GEP-NEN by means of immunohistochemistry
(IHC), which allows precise SSTR cellular localization (5,6).
Furthermore, whether or not differences in the expression of SSTR
subtypes in GEP-NEN are associated with tumor characteristics
remains to be elucidated, as does the potential prognostic role
played by the expression of SSTRs in these tumors.
The expression of SSTRs on tumor cells forms the
basis for somatostatin analog (SSA) treatment of patients with NEN
(7). SSAs are an important means of
biotherapy. They are a class of artificially synthesized peptides
that have multiple biological effects similar to natural
somatostatin (8). SSA is able to
either inhibit the release of hormones and neurotransmitters by
binding SSTRs to improve symptoms caused by excessive secretion of
hormones (9), or inhibit tumor growth
directly by regulating the signaling pathways of tumor cell
proliferation/apoptosis and angiogenesis directly or indirectly
(10). At present, clinical treatment
of gastroenteropancreatic neuroendocrine tumor (GEP-NET) is mainly
focused on long-acting SSAs, including octreotide long-acting
release (LAR) and lanreotide sustained-release (Somatuline
Autogel). The results of a phase III prospective, randomized and
double-blind study (PROMID) proved that octreotide LAR
significantly prolonged the time to progression (TTP; 14.3 vs. 6
months) in patients with unresectable, well-differentiated
metastatic midgut neuroendocrine tumors, as compared with the
placebo (11). The results of the
CLARINET study, which included 204 patients with non-functional,
metastatic NET (including those with a Ki67 <10% for tumors in
the gastrointestinal tract and the pancreas), showed that the
median progression-free survival was not reached in the lanreotide
group and was 18.0 months in the placebo group, with the difference
between the two groups being statistically significant (P<0.001)
(12).
However, patients in the above-mentioned studies
were all from Western countries rather than Asian countries.
Previous studies revealed that GEP-NEN is a type of tumor with high
heterogeneity; the primary site and symptoms of the tumor vary from
patients of various races in differing regions (13–16). In
addition, the response and tolerance of GEP-NEN patients to
anti-tumor drug treatment also varied between different races
(17,18). Therefore, although numerous studies in
Western population reported that SSA had anti-tumor activity
against GEP-NET, considering the clear heterogeneity, whether SSA
has the similar efficacy in GEP-NET patients in Asian countries is
worth investigation.
To address some of these issues in the present
study, the expression of SSTR2 and SSTR5 was determined in a large
cohort of GEP-NEN using immunohistochemistry, and findings were
associated with clinicopathological variables and patient
prognosis. In addition, the present study investigated the efficacy
and safety of long-acting SSA octreotide LAR in Chinese GEP-NET
patients by conducting a multicenter retrospective analysis on the
data of 54 Chinese patients with unresectable, well-differentiated
advanced or metastatic GEP-NET treated by octreotide LAR.
Materials and methods
Patient information
A total of 143 patients with histologically
confirmed sporadic GEP-NEN at The First Affiliated Hospital, Sun
Yat-sen University (Guangzhou, China) from January 1995 to December
2014 were enrolled in the present study to determine the expression
of SSTR2 and SSTR5. A total of 54 patients with advanced GEP-NET,
who received octreotide LAR treatment in four centers across China
with high-quality medical care between November 2009 and December
2015, were included in the present study to investigate the
efficacy and safety of octreotide LAR in Chinese GEP-NET patients.
Data from the following centers were included in the validation
analysis: The First Affiliated Hospital, Sun Yat-Sen University
(n=31), Sun Yat-sen University Cancer Center (Guangzhou, China;
n=12), Fudan University Shanghai Cancer Center (Shanghai, China;
n=10), Changzheng Hospital, Second Military Medical University
(Shanghai, China; n=1). Electronic datasheets were provided for all
participating centers. All de-identified data were reviewed and
cross-checked for inconsistencies by YH Wang. Patient
clinicopathological characteristics were summarized in Tables I and II.
 | Table I.Clinicopathological characteristics
of gastroenteropancreatic neuroendocrine neoplasm patients with
somatostatin receptor immunohistochemical detection. |
Table I.
Clinicopathological characteristics
of gastroenteropancreatic neuroendocrine neoplasm patients with
somatostatin receptor immunohistochemical detection.
| Demographic and
clinical characteristics (n=143) | n | % |
|---|
| Gendera |
|
|
|
Male | 87 | 60.8 |
|
Female | 56 | 39.2 |
| Age at diagnosis
(years) |
|
|
|
≤50 | 71 | 49.7 |
|
>50 | 72 | 50.3 |
| Median
(range) | 51 (18–85) |
|
| Functional
status |
|
|
|
Nonfunctional | 113 | 79.0 |
|
Functional | 30 | 21.0 |
|
Insulinoma | 24 | 16.8 |
|
Vasoactive intestinal
polypeptidoma | 4 | 2.8 |
|
Somatostatinoma | 1 | 0.7 |
|
Carcinoid syndrome | 1 | 0.7 |
| Tumor location |
|
|
|
Gastrointestinal tract | 79 | 55.2 |
|
Rectum | 34 | 23.8 |
|
Stomach | 19 | 13.3 |
|
Duodenum | 15 | 10.5 |
|
Jejunum/ileum | 7 | 4.9 |
|
Appendix | 4 | 2.8 |
|
Pancreas | 64 | 44.8 |
| Tumor grade |
|
|
| G1 | 69 | 48.3 |
| G2 | 39 | 27.3 |
| G3 | 35 | 24.5 |
| Tumor type |
|
|
|
NET | 110 | 76.9 |
| NET
G1 | 69 | 48.3 |
| NET
G2 | 39 | 27.3 |
| NET
G3 | 2 | 1.4 |
|
NEC | 31 | 21.7 |
|
MANEC | 2 | 1.4 |
| Tumor stage |
|
|
| I | 43 | 30.1 |
| II | 28 | 19.6 |
|
III | 16 | 11.2 |
| IV | 56 | 39.2 |
 | Table II.Clinicopathological characteristics
of gastroenteropancreatic neuroendocrine tumor patients with
octreotide long-acting release treatment. |
Table II.
Clinicopathological characteristics
of gastroenteropancreatic neuroendocrine tumor patients with
octreotide long-acting release treatment.
| Demographic and
clinical characteristics (n=54) | n | % |
|---|
| Gendera |
|
|
|
Male | 32 | 59.3 |
|
Female | 22 | 40.7 |
| Age at diagnosis
(years) |
|
|
|
≤50 | 28 | 51.9 |
|
>50 | 26 | 48.1 |
| Median
(range) | 50 (18–72) |
|
| ECOG PS |
|
|
| 0 | 36 | 66.7 |
| 1 | 16 | 29.6 |
| 2 | 2 | 3.7 |
| Functional
status |
|
|
|
Nonfunctional | 41 | 75.9 |
|
Functional | 13 | 24.1 |
|
Vasoactive intestinal
polypeptidoma | 8 | 14.8 |
|
Carcinoid syndrome | 2 | 3.7 |
|
Gastrinoma | 2 | 3.7 |
|
Insulinoma | 1 | 1.9 |
| Tumor location |
|
|
|
Gastrointestinal tract | 13 | 24.1 |
|
Rectum | 6 | 11.1 |
|
Jejunum/ileum | 4 | 7.4 |
|
Duodenum | 3 | 5.6 |
|
Pancreas | 41 | 75.9 |
| Ki67 index (%) |
|
|
| ≤2 | 11 | 20.4 |
|
3–10 | 33 | 61.1 |
|
>10 | 10 | 18.5 |
| Tumor grade |
|
|
| G1 | 11 | 20.4 |
| G2 | 42 | 77.8 |
| G3 | 1 | 1.9 |
| Tumor stage |
|
|
| IV | 54 | 100.0 |
| Combined
treatment |
|
|
|
Monotherapy | 31 | 57.4 |
| With
targeted drug therapy | 9 | 16.7 |
| With
interventional therapy | 5 | 9.3 |
| With
chemotherapy | 2 | 3.7 |
| With
palliative surgery | 2 | 3.7 |
| With
>2 therapies | 5 | 9.3 |
| Previous
treatment |
|
|
|
None | 16 | 29.6 |
|
Surgical therapy | 13 | 24.1 |
|
Targeted drug therapy | 3 | 5.6 |
|
Interventional therapy | 3 | 5.6 |
|
Chemotherapy | 2 | 3.7 |
| >2
therapies | 17 | 31.5 |
| SSTR2
expressionb |
|
|
|
Positive | 19 | 86.4 |
|
Negative | 3 | 13.6 |
| SSTR5
expressionb |
|
|
|
Positive | 18 | 81.8 |
|
Negative | 4 | 18.2 |
A functional tumor was defined as overproducing a
hormone such as 5-hydroxytryptamine, gastrin, glucagon, insulin,
somatostatin and vasoactive intestinal peptide, which causes
clinical symptoms. The pathology of each patient was reviewed
according to the latest World Health Organization classification of
tumors of the digestive system (19).
Tumor-Node-Metastasis (TNM) stage was adopted according to the
European Neuroendocrine Tumor Society Consensus Guidelines
(20,21). Treatment responses were evaluated
according to Response Evaluation Criteria in Solid Tumors (RECIST,
version 1.1) (22).
The study was conducted in accordance with
Declaration of Helsinki and in compliance with good clinical
practice guidelines. The trial protocol was approved by the
institutional review board of each institution. Written informed
consent was obtained from each patient.
IHC
SSTR2 and SSTR5 IHC stains were performed in all 143
cases. Sections of tumor specimens (4-µm thick) from formalin-fixed
paraffin-embedded sections were used for IHC examinations. The
slides were dewaxed with xylene and rehydrated in a graded series
of ethanol. Heat-induced epitope retrieval was performed using a
microwave oven at 600 W for 30 min in preheated 10 mmol/l citric
acid (pH 6.0). Endogenous peroxidase activity was blocked by
incubating the slides in 3% hydrogen peroxide for 20 min at room
temperature. The slides were transferred to phosphate-buffered
saline and subsequently incubated at 4°C with rabbit monoclonal
anti-SSTR2 (1:100; ab134152; Epitomics, Burlingame, CA, USA) and
anti-SSTR5 (1:100; ab109495; Epitomics) overnight at 4°C. The
following day, sections were incubated in secondary antibody (Real
EnVision Detection kit, ready-to-use; K5007; Dako; Agilent
Technologies, Inc., Santa Clara, CA, USA) for 1 h at room
temperature. The substrate chromogen, 3,3′-diaminobenzidine,
enabled visualization of the complex via a brown precipitate.
Hematoxylin (blue) counterstaining enabled the visualization of the
cell nuclei with a light microscope (4500; Olympus Corporation,
Tokyo, Japan). Omission of primary antibody served as a negative
control.
Histological interpretation
For evaluation of SSTR2 and SSTR5 immunopositivity,
a scoring system standardized and proposed by Volante et al
(6) was used, at is has been reported
to have a good correlation with Octreoscan findings. The scoring
system was as follows: 0, absence of immunoreactivity; 1, pure
cytoplasmic immunoreactivity, either focal or diffuse; 2,
membranous reactivity in <50% of tumor cells, irrespective of
the presence of cytoplasmic staining; and 3, circumferential
membranous reactivity in >50% of tumor cells, irrespective of
the presence of cytoplasmic staining. Cases with a score of 2–3
were considered as positive, and 0–1 were considered as
negative.
All slides were evaluated independently by two
investigators (Y.L. and L.X.) who were blinded to the patient
clinical data. Any discordant results were subsequently reviewed
together to reach agreement or determine an average value for
disputed sections.
Treatment
The 54 patients were administered octreotide LAR
from a starting dose of 20–40 mg, administered by intramuscular
injection once every 28 days, and the treatment continued until
disease progression, evidenced by imaging or occurrence of adverse
reactions that rendered further drug administration impossible. The
treatment was suspended or the therapeutic dose was adjusted
(increasing or reducing the dose, or shortening the interval
between injections) depending on tumor control or functional
symptoms (carcinoid syndrome and diarrhea) and the severity of
adverse reactions. In the present study, there were six patients
whose dose was increased to 30–40 mg during the period of
treatment, and a single patient's dose was increased to 60 mg, with
the interval between injections being shortened to 21 days. The
reasons for adjustment of therapeutic dose or interval of
injections for the seven patients were exacerbation of the
functional symptoms. Tumors in the chest, abdomen and pelvic cavity
were measured prior to treatment and once every 4–12 weeks
following treatment by using three-dimension spiral computed
tomography or magnetic resonance imaging, and the size of tumor was
evaluated by the imaging experts. The patient clinicopathological
data, as well as the data of imaging examination following
octreotide LAR treatment, were collected.
Efficacy and safety assessments
The primary study endpoint was TTP. The secondary
endpoints included overall survival (OS), objective response rate
(ORR) and stable disease (SD) rate. The adverse reactions were
evaluated according to Common Terminology Criteria for Adverse
Events (version 4.0) published by the U.S. National Cancer
Institute (23).
Statistical analysis
SPSS version 16.0 software (SPSS, Inc., Chicago, IL,
USA) was employed for statistical analysis of the data. Descriptive
statistics of qualitative data such as patient's general data,
positive expression rates, treatment evaluation and adverse
reactions, were expressed as numbers and percentages. The results
of SSTR2 and SSTR5 expression analysis were compared in terms of
various clinicopathological data, including functional status,
tumor site, grade, type and stage. Statistical evaluation was
performed by means of the χ2 tests. OS and TTP analyses
were performed using Kaplan-Meier survival plots and comparisons
between groups were made with the log-rank test. ORR and SD rate
were described using percentage, and 95% confidence intervals (CIs)
were calculated. P<0.05 was considered to indicate a
statistically significant difference.
Results
Immunohistochemical expression of
SSTR2 and SSTR5 in GEP-NEN
As shown in Fig. 1,
SSTR2 was positively immunostained in the membrane of tumor cells,
and varied from weak-incomplete to strong-complete staining. The
overall expression rate of SSTR2 was 67.8% (97/143). Membranous
SSTR5 immunopositivity was noted in 56.6% (81/143) of tumors. No
nuclear immunostaining was observed.
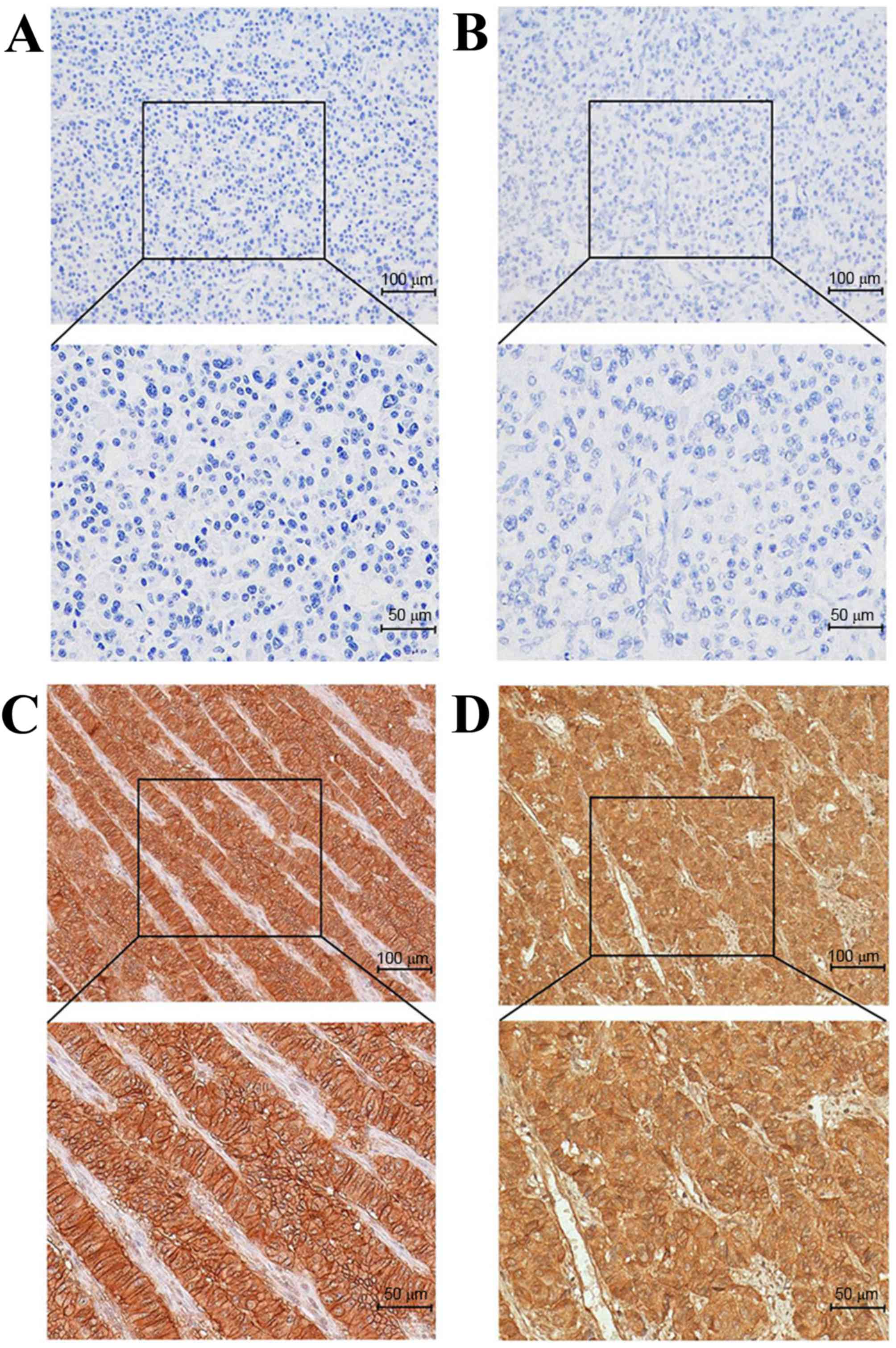 | Figure 1.Immunohistochemical staining of SSTR2
and SSTR5 in gastroenteropancreatic neuroendocrine neoplasm (using
the EnVision method). (A) Pancreatic NET, G2, SSTR2-negative
staining. (B) Pancreatic NET, G2, SSTR5-negative staining. (C)
Pancreatic NET, G2, strong SSTR2-positive staining. (D) Pancreatic
NET, G2, strong SSTR5-positive staining. For each panel: Upper
panel magnification, ×20; lower panel magnification, ×40. SSTR,
somatostatin receptor; NET, neuroendocrine tumor. |
Association of SSTR2 and SSTR5
expression with clinicopathological variables
SSTR2 expression was increased in tumors with
hormonal syndrome (P=0.041). Patients with pancreatic tumors had a
significantly increased SSTR2 expression compared with
gastrointestinal (GI) tumors (79.7 vs. 58.2%; P=0.006). Poorly
differentiated tumors [G3 tumors and neuroendocrine carcinoma (NEC)
+ mixed adenoneuroendocrine carcinoma (MANEC)] had lower SSTR2
expression compared with well- and moderately-differentiated tumors
[G1, G2 tumors and neuroendocrine tumor (NET); P<0.001]. The
expression rate of SSTR2 in tumors of stage I and II was 77.5%,
which was markedly increased compared with tumors of stage III and
IV (58.3%; P=0.014). Similarly, SSTR5 was significantly increased
in pancreatic and well-differentiated tumors compared with in
gastrointestinal and poorly differentiated tumors (P=0.022, P=0.008
and P=0.002, respectively). The expression rates and statistical
data are summarized in Table
III.
 | Table III.Association of SSTR2 and SSTR5
expression with clinicopathological variables (n=143). |
Table III.
Association of SSTR2 and SSTR5
expression with clinicopathological variables (n=143).
| Characteristic | n | SSTR2 positive, n
(%) | χ2
value | P-value | SSTR5 positive, n
(%) | χ2
value | P-value |
|---|
| Functional
status |
|
| 4.181 | 0.041 |
| 0.692 | 0.406 |
|
Nonfunctional | 113 | 72 (63.7) |
|
| 62 (54.9) |
|
|
|
Functional | 30 | 25 (83.3) |
|
| 19 (63.3) |
|
|
| Site |
|
| 7.462 | 0.006 |
| 5.245 | 0.022 |
|
Gastrointestinal tract | 79 | 46 (58.2) |
|
| 38 (48.1) |
|
|
|
Pancreas | 64 | 51 (79.7) |
|
| 43 (67.2) |
|
|
| Tumor grade |
|
| 20.330 | <0.001 |
| 9.570 | 0.008 |
| G1 | 69 | 55 (79.7) |
|
| 45 (65.2) |
|
|
| G2 | 39 | 29 (74.4) |
|
| 24 (61.5) |
|
|
| G3 | 35 | 13 (37.1) |
|
| 12 (34.3) |
|
|
| Tumor type |
|
| 23.400 | <0.001 |
| 9.492 | 0.002 |
|
NET | 110 | 86 (78.2) |
|
| 70 (63.6) |
|
|
|
NEC+MANEC | 33 | 11 (33.3) |
|
| 11 (33.3) |
|
|
| Tumor stage |
|
| 5.996 | 0.014 |
| 0.070 | 0.792 |
|
I+II | 71 | 55 (77.5) |
|
| 41 (57.7) |
|
|
|
III+IV | 72 | 42 (58.3) |
|
| 40 (55.6) |
|
|
Association of SSTR2 and SSTR5
expression with survival
A total of 116/143 patients received long-term
follow up with a median duration of 3.36 years (range, 0.02–15.05
years). At the final follow-up, 36 patients (31.0%) had succumbed
to the disease. The major causes of mortality were tumor-associated
(34/36; 94.4%), and treatment-associated adverse events (2/36;
5.6%; both succumbed from surgical complications). Only
NEN-associated mortalities were considered as events for survival
analysis.
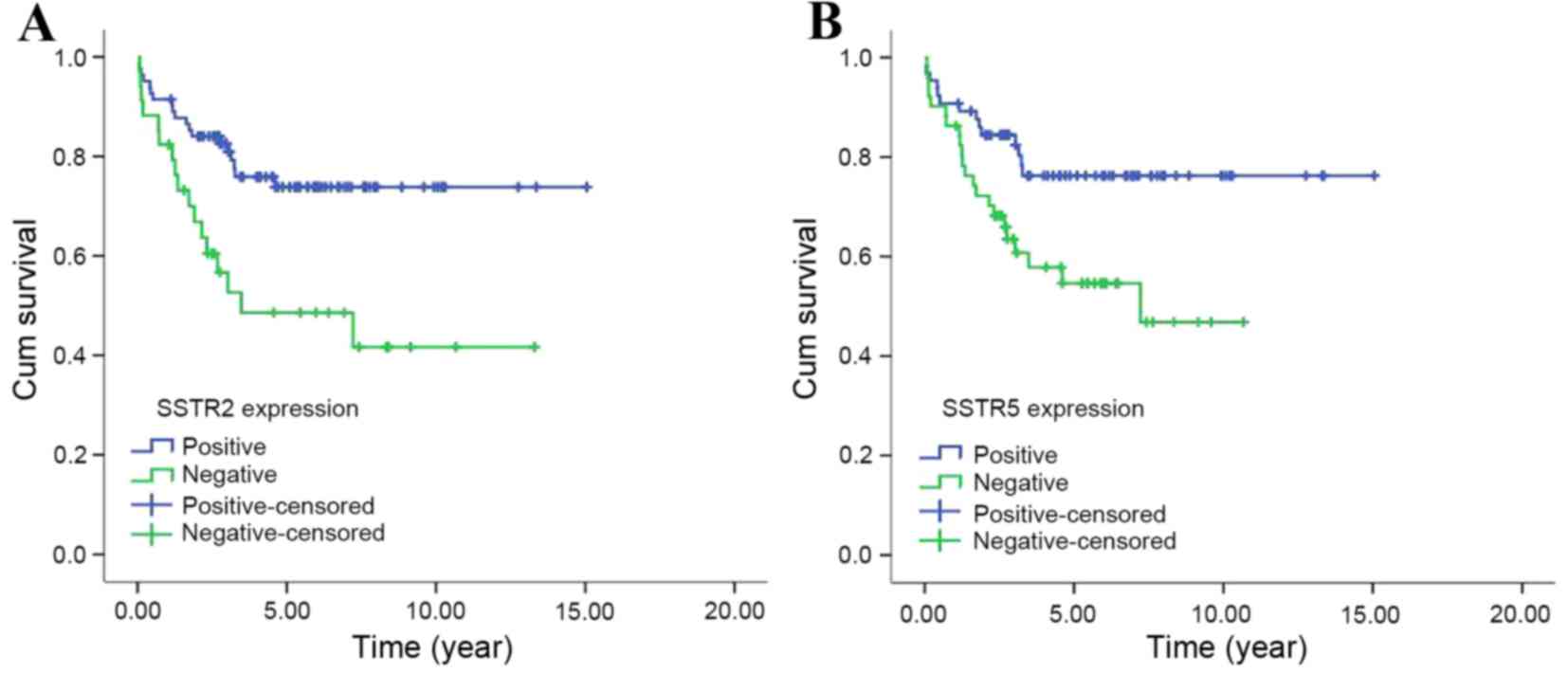
Kaplan-Meier survival curves revealed that the
median OS time of patients with positive expression of SSTR2 was
not reached (NR), while patients with negative expression had a
median OS of 3.48 years, which demonstrated a statistically
significant difference (χ2=8.758, P=0.003). Similarly,
SSTR5 positive expression also predicted improved survival compared
with negative expression (the median OS times were NR and 7.22
years, respectively; χ2=6.396, P=0.011) (Fig. 2).
Efficacy assessment
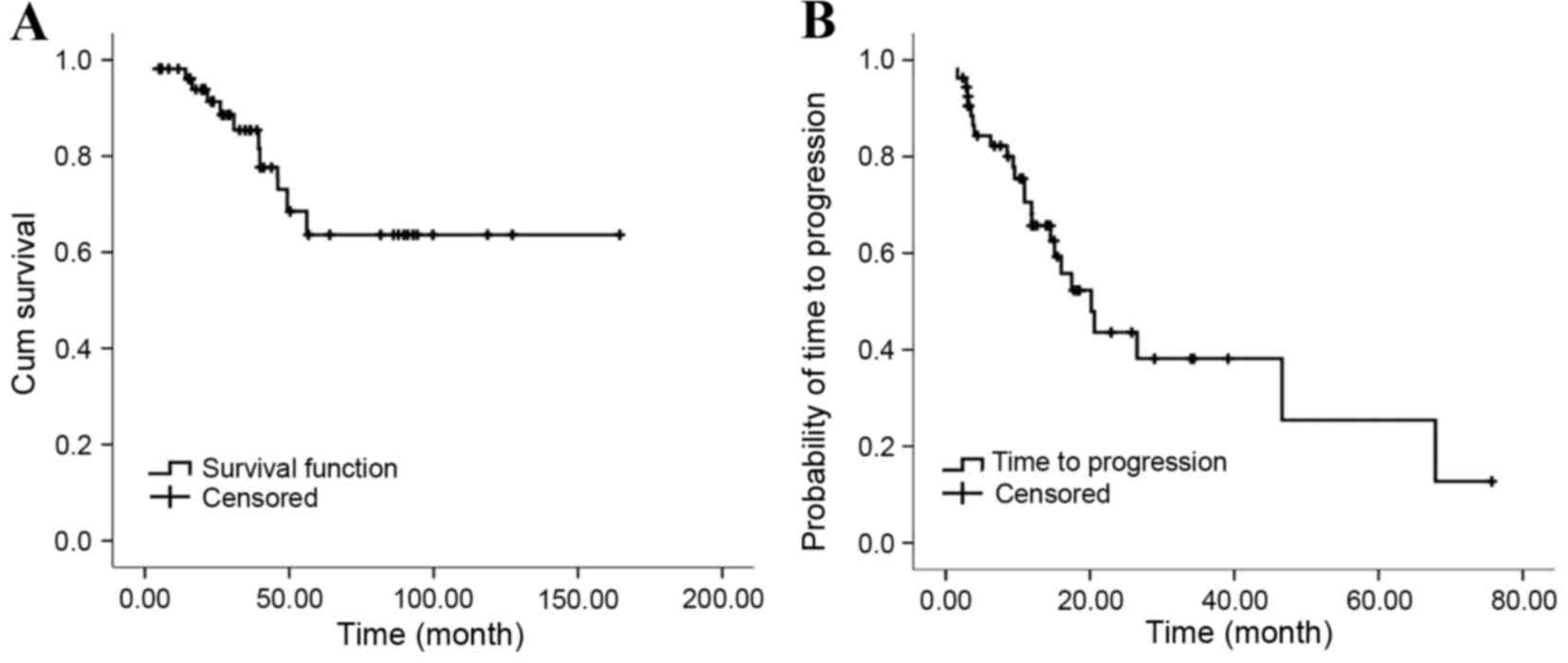
All 54 patients that received octreotide LAR were
followed up for a period of 3.2–164.5 months, with a median
follow-up period of 31.8 months. By the conclusion of follow-up, 11
of the patients died of progressive disease (PD) and 26 of the
patients were still receiving octreotide LAR treatment. The median
OS was not reached and the median TTP was 20.2 months (95% CI,
13.9–26.5%) (Fig. 3). Imaging
evaluation was performed for all patients according to RECIST, and
three patients achieved partial remission (PR), with the ORR being
5.6% (95% CI, 0.0–11.7%). A total of 43 patients achieved SD, with
the SD rate being 79.6% (95% CI, 68.9–90.4%) and 8 patients
demonstrated PD. At the conclusion of follow-up, there were still
three patients achieving PR, 26 patients achieving SD and 25
patients demonstrating PD.
The median TTP in all 54 patients treated with
octreotide LAR was not associated with the patient's functional
status, tumor site, Ki67 index and whether or not they received
other anti-tumor therapy prior to octreotide LAR treatment or
combined therapy (P=0.116, P=0.665, P=0.512, P=0.256 and P=0.817,
respectively). No associations between the expression of SSTR2 and
SSTR5 and median TTP were evident (P=0.352 and 0.575, respectively;
Table IV).
 | Table IV.Time to progression and its
association with the sub-groups (n=54). |
Table IV.
Time to progression and its
association with the sub-groups (n=54).
|
Characteristics | n | Median
(months) | 95% CI | χ2
value | P-value |
|---|
| Patients with
octreotide LAR treatment | 54 | 20.2 | 13.9–26.5 |
|
|
| Functional
status |
|
|
| 2.474 | 0.116 |
|
Non-functional | 41 | 17.5 | 11.0–23.9 |
|
|
|
Functional | 13 | 67.9 | NC |
|
|
| Tumor site |
|
|
| 0.188 | 0.665 |
|
Gastrointestinal tract | 13 | 17.5 | 0.0–43.7 |
|
|
|
Pancreas | 41 | 20.2 | 12.0–28.4 |
|
|
| Ki-67 index
(%) |
|
|
| 1.340 | 0.512 |
| ≤2 | 11 | 67.9 | NC |
|
|
|
3–10 | 33 | 20.6 | 15.0–26.2 |
|
|
|
>10 | 10 | 10.9 | 3.3–18.5 |
|
|
| Previous
treatment |
|
|
| 1.288 | 0.256 |
| No | 16 | NR | NC |
|
|
|
Yes | 38 | 16.0 | 5.6–26.5 |
|
|
| Combined
therapy |
|
|
| 0.053 | 0.817 |
| No | 31 | 17.5 | 4.5–30.5 |
|
|
|
Yes | 23 | 20.2 | 10.9–29.5 |
|
|
| SSTR2
expressiona |
|
|
| 0.867 | 0.352 |
|
Positive | 19 | 20.6 | 10.5–30.7 |
|
|
|
Negative | 3 | 9.4 | NC |
|
|
| SSTR5
expressiona |
|
|
| 0.314 | 0.575 |
|
Positive | 18 | 16.0 | 6.4–25.7 |
|
|
|
Negative | 4 | NR | NC |
|
|
Safety assessment
A total of 14/54 (25.9%) patients experienced
adverse drug reactions during the period of octreotide LAR
treatment, and the most common grade 1–2 adverse events (AEs) were
diarrhea (16.7%), abdominal distension (7.4%), abdominal pain
(7.4%) and elevation of blood glucose (1.9%). Octreotide
LAR-associated AEs occurred 1–4 weeks following administration of
the drug, primarily in the initial one or two weeks. All of the
above AEs were relieved or remedied following symptomatic
treatment. No serious adverse events (SAE) were observed during the
present study. None of the patients required dose reduction or drug
withdrawal due to AE.
Discussion
The wide expression of SSTRs in neuroendocrine
tumors has been investigated by various methods (2–4).
Immunohistochemistry appears to be a reliable and reproducible
technique to detect the SSTRs in GEP-NEN with clear advantages,
including low cost, easy operation and allowing the SSTR profile
determination of GEP-NEN in the clinical setting (24). The expression rates of SSTR2 and SSTR5
with immunohistochemistry in GEP-NEN have been reported in previous
studies to be within the range of 60–93 and 38–83%, respectively
(2,5,24–28). In the present study, it was observed
that the overall expression rates of SSTR2 and SSTR5 were 67.8 and
56.6%, comprising a total of 143 samples from GEP-NEN patients,
which was comparable to previous studies.
Srirajaskanthan et al (29) reported that SSTR2 and SSTR5 expression
were inversely correlated with neuroendocrine tumor grade. Low to
intermediate-grade tumors, which were also well-differentiated, had
increased SSTR expression compared with high-grade tumors
(P<0.005) (29). In line with
previous findings, the present study demonstrated a gradual decline
in SSTR2 and SSTR5 expression of well-(G1, G2 and NET) and
poorly-differentiated tumors (G3 and NEC+MANEC; P<0.001,
P<0.001, P=0.008, P=0.002, respectively). The present study also
observed that SSTR2 and SSTR5 were significantly more likely to be
expressed in pancreatic tumors than GI tumors (P=0.006 and 0.022,
respectively). In addition, SSTR2 expression was significantly
increased in tumors with hormonal syndrome and TNM stage I and II
(P=0.041 and 0.014, respectively); however, SSTR5 was not. These
data are inconsistent with the results of previous studies
(2,25,30), which
revealed that no association was observed between SSTR expression
and tumor location, functional status and TNM stage. However, these
previous studies mainly focused on particular types of GEP-NENs,
including well-differentiated endocrine tumors or a single site of
tumor (pancreas). According to the above results in this study, it
was observed that SSTR subtype expression demonstrates marked
heterogeneity and differences in tumor sites and differentiation,
and a decrease in SSTR2 and SSTR5 expression with increasing
malignancy in GEP-NEN.
Previous studies investigating SSTR subtype
expression as a prognostic factor have shown conflicting results.
In a study of 60 patients with GEP-NEN, Kaemmerer et al
(31) showed that positive staining
for SSTR2 (n=54) was associated with significantly longer OS as
compared to negative staining (n=6; median OS, 49.5 vs. 16.5
months; P<0.001). Corleto et al (32) observed a significantly better survival
rate in patients with well-differentiated neuroendocrine tumors
expressing SSTR2, SSTR5 and Ki-67<2% simultaneously. However,
Papotti et al (2) reported no
statistically significant correlation between SSTR subtype
expression and clinical outcome in 54 cases. This discrepancy may
be due to the small number of a negligible SSTR2 expression cases,
and the differences in tumor origin and differentiation. Although
the present results concerning the association between SSTR
expression and survival were inconsistent, the current study
indicated that patients with SSTR2 and SSTR5 positive expression
had an improved prognosis.
SSAs have been proved in many clinical studies to be
able to inhibit the secretion of tumor-producing hormones by
binding with SSTRs on the surface of neuroendocrine neoplasm cells.
Placebo controlled PROMID and CLARINET studies have further
discovered that SSAs have anti-tumor activity along with inhibiting
hormone secretion (11,12). The present investigation conducted a
multicenter retrospective study of octreotide LAR in the treatment
of 54 Chinese patients with unresectable, well-differentiated
advanced or metastatic GEP-NETs, finding that the overall median
TTP was 20.2 months (95% CI, 13.9–26.5), with an ORR of 5.6% and an
SD rate of 79.6%. Analysis of the subgroups showed that differences
in the median TTP were not statistically significant regarding the
primary site of tumor (GI tract and pancreas) and functional status
(P=0.665 and P=0.116, respectively). The above results were similar
to the results of the studies in the Western population, indicating
that octreotide LAR is effective in Chinese GEN-NET patients,
regardless of whether the primary site is GI tract or pancreas and
whether the tumor is functional or not.
A retrospective study comprising 43 patients with
pancreatic NET treated with octreotide LAR conducted by Jann et
al (33) revealed that patients
with a Ki67 ≤10% showed a longer median TTP than those with a Ki67
>10%. In the present study, although no statistically
significant difference was observed (P=0.512), a tendency for
octreotide LAR to show improved efficacy in patients with Ki67 ≤10%
(the median TTP in patients with Ki67 ≤2%, Ki67 of 3–10% and Ki67
>10% was 67.9, 20.6 and 10.9 months, respectively) was
identified. The above results suggested that patients with lower
proliferation index appear to have a longer TTP and may be
candidates for octreotide LAR treatment.
In the present study, the therapeutic dose was
increased or the interval of injection was shortened for 7/54
patients during the period of octreotide LAR treatment, due to
exacerbation of the functional symptoms, and the patient symptoms
were thus improved. Previous studies showed that increases in the
dose or frequency of SSA may be considered for patients with poor
control of symptoms and tumors, particularly in cases where disease
was previously stabilized at a lower dose (34–37).
Therefore, efficacy can be obtained again by adjusting the dose of
SSA or the interval of treatment in clinical practice.
To the best of our knowledge, there have been few
studies focused on the predictive value of SSTR
immunohistochemistry in determining the treatment response to SSA.
In the present study, the differences between SSTR subtype
expression and median TTP were not statistically significant
(P=0.352 and P=0.575, respectively). Such an association was
limited in the present study because of heterogeneous biological
behavior of the disease and a small number of patients with SSTR
subtype detection (22 patients). Large clinical trials should be
designed to validate the role of somatostatin receptor
immunohistochemical profile in the prediction of clinical
response.
SSA is a therapeutic approach that has much fewer
side effects and higher safety than targeted drugs (18,38) or
cytotoxic drugs (39,40). In the PROMID study, 11 (12.9%) of the
85 patients experienced SAE, with the common adverse reactions in
the octreotide LAR group being diarrhea and abdominal distension,
and five of the patients discontinued the treatment due to AE
(11). In the CLARINET study, 50% of
the 101 patients in the lanreotide group experienced AEs, and three
(3.0%) of the patients experienced SAE, one of whom withdrew from
the study due to AE (12). Adverse
reactions observed in the present study were diarrhea, abdominal
distension and abdominal pain, being similar to those in the
aforementioned studies. However, octreotide LAR showed improved
safety in Chinese patients on the whole, with a lower incidence
(25.9%) of AE, and none of the patients experienced an SAE or
required dose reduction or drug withdrawal due to AE.
In conclusion, the present study demonstrates that
SSTR2 and SSTR5 are heterogeneously expressed in GEP-NEN with
different tumor sites and differentiation. Both markers could serve
as potential prognostic factors to predict survival. Furthermore,
although the present retrospective study included only 54 cases,
the efficacy and safety of octreotide LAR in China was investigated
for the first time. It was observed that octreotide LAR is
effective in the treatment of Chinese patients with
well-differentiated advanced GEP-NET, with a low incidence of
adverse reactions.
Acknowledgements
The authors are grateful to Dr Yuan Gao (Department
of Gastroenterology, The First Affiliated Hospital, Sun Yat-Sen
University, Guangzhou, China) for skillful writing assistance.
Glossary
Abbreviations
Abbreviations:
|
SSTRs
|
somatostatin receptors
|
|
GEP-NENs
|
gastroenteropancreatic neuroendocrine
neoplasms
|
|
IHC
|
immunohistochemistry
|
|
SSA
|
somatostatin analog
|
|
GEP-NET
|
gastroenteropancreatic neuroendocrine
tumor
|
|
LAR
|
long-acting release
|
|
TTP
|
time to progression
|
|
GI
|
gastrointestinal
|
|
RECIST
|
response evaluation criteria in solid
tumors
|
|
OS
|
overall survival
|
|
ORR
|
objective response rate
|
|
SD
|
stable disease
|
|
CI
|
confidence interval
|
|
NEC
|
neuroendocrine carcinoma
|
|
MANEC
|
mixed adenoneuroendocrine
carcinoma
|
|
NR
|
not reached
|
|
PD
|
progressive disease
|
|
PR
|
partial remission
|
|
AEs
|
adverse events
|
|
SAE
|
serious adverse event
|
References
|
1
|
Modlin IM, Oberg K, Chung DC, Jensen RT,
de Herder WW, Thakker RV, Caplin M, Fave G Delle, Kaltsas GA,
Krenning EP, et al: Gastroenteropancreatic neuroendocrine tumours.
Lancet Oncol. 9:61–72. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Papotti M, Bongiovanni M, Volante M, Allìa
E, Landolfi S, Helboe L, Schindler M, Cole SL and Bussolati G:
Expression of somatostatin receptor types 1–5 in 81 cases of
gastrointestinal and pancreatic endocrine tumors. A correlative
immunohistochemical and reverse-transcriptase polymerase chain
reaction analysis. Virchows Arch. 440:461–475. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Kulaksiz H, Eissele R, Rössler D, Schulz
S, Höllt V, Cetin Y and Arnold R: Identification of somatostatin
receptor subtypes 1, 2a, 3 and 5 in neuroendocrine tumours with
subtype specific antibodies. Gut. 50:52–60. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Korner M, Eltschinger V, Waser B,
Schonbrunn A and Reubi JC: Value of immunohistochemistry for
somatostatin receptor subtype sst2a in cancer tissues: Lessons from
the comparison of anti-sst2a antibodies with somatostatin receptor
autoradiography. Am J Surg Pathol. 29:1642–1651. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Zamora V, Cabanne A, Salanova R, Bestani
C, Domenichini E, Marmissolle F, Giacomi N, O'Connor J, Méndez G
and Roca E: Buenos Aires and La Plata Argentina Argentum Working
Group: Immunohistochemical expression of somatostatin receptors in
digestive endocrine tumours. Dig Liver Dis. 42:220–225. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Volante M, Brizzi MP, Faggiano A, La Rosa
S, Rapa I, Ferrero A, Mansueto G, Righi L, Garancini S, Capella C,
et al: Somatostatin receptor type 2a immunohistochemistry in
neuroendocrine tumors: A proposal of scoring system correlated with
somatostatin receptor scintigraphy. Mod Pathol. 20:1172–1182. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Hofland LJ and Lamberts SW: The
pathophysiological consequences of somatostatin receptor
internalization and resistance. Endocr Rev. 24:28–47. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Oberg KE, Reubi JC, Kwekkeboom DJ and
Krenning EP: Role of somatostatins in gastroenteropancreatic
neuroendocrine tumor development and therapy. Gastroenterology.
139:742–753, 753.e1. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Oberg K: Future aspects of
somatostatin-receptor-mediated therapy. Neuroendocrinology.
80:(Suppl 1). S57–S61. 2004. View Article : Google Scholar
|
|
10
|
Appetecchia M and Baldelli R: Somatostatin
analogues in the treatment of gastroenteropancreatic neuroendocrine
tumours, current aspects and new perspectives. J Exp Clin Cancer
Res. 29:192010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Rinke A, Müller HH, Schade-Brittinger C,
Klose KJ, Barth P, Wied M, Mayer C, Aminossadati B, Pape UF, Bläker
M, et al: Placebo-controlled, double-blind, prospective, randomized
study on the effect of octreotide lar in the control of tumor
growth in patients with metastatic neuroendocrine midgut tumors: A
report from the promid study group. J Clin Oncol. 27:4656–4663.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Caplin ME, Pavel M, Ćwikła JB, Phan AT,
Raderer M, Sedláčková E, Cadiot G, Wolin EM, Capdevila J, Wall L,
et al: Lanreotide in metastatic enteropancreatic neuroendocrine
tumors. N Engl J Med. 371:224–233. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Lim T, Lee J, Kim JJ, Lee JK, Lee KT, Kim
YH, Kim KW, Kim S, Sohn TS, Choi DW, et al: Gastroenteropancreatic
neuroendocrine tumors: Incidence and treatment outcome in a single
institution in korea. Asia Pac J Clin Oncol. 7:293–299. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hauso O, Gustafsson BI, Kidd M, Waldum HL,
Drozdov I, Chan AK and Modlin IM: Neuroendocrine tumor
epidemiology: Contrasting norway and north america. Cancer.
113:2655–2664. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Yao JC, Hassan M, Phan A, Dagohoy C, Leary
C, Mares JE, Abdalla EK, Fleming JB, Vauthey JN, Rashid A and Evans
DB: One hundred years after ‘carcinoid’: Epidemiology of and
prognostic factors for neuroendocrine tumors in 35,825 cases in the
united states. J Clin Oncol. 26:3063–3072. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Wang YH, Lin Y, Xue L, Wang JH, Chen MH
and Chen J: Relationship between clinical characteristics and
survival of gastroenteropancreatic neuroendocrine neoplasms: A
single-institution analysis (1995–2012) in south china. BMC Endocr
Disord. 12:302012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Ito T, Okusaka T, Nishida T, Yamao K,
Igarashi H, Morizane C, Kondo S, Mizuno N, Hara K, Sawaki A, et al:
Phase ii study of sunitinib in japanese patients with unresectable
or metastatic, well-differentiated pancreatic neuroendocrine tumor.
Invest New Drugs. 31:1265–1274. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Raymond E, Dahan L, Raoul JL, Bang YJ,
Borbath I, Lombard-Bohas C, Valle J, Metrakos P, Smith D, Vinik A,
et al: Sunitinib malate for the treatment of pancreatic
neuroendocrine tumors. N Engl J Med. 364:501–513. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Bosman F, Carneiro F, Hruban R and Theise
N: Who classification of tumours of the digestive system. 4th. IARC
Press; PA: 2010
|
|
20
|
Rindi G, Klöppel G, Alhman H, Caplin M,
Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M,
Komminoth P, et al: Tnm staging of foregut (neuro)endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
449:395–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Rindi G, Klöppel G, Couvelard A, Komminoth
P, Körner M, Lopes JM, McNicol AM, Nilsson O, Perren A, Scarpa A,
et al: Tnm staging of midgut and hindgut (neuro) endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
451:757–762. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Chen AP, Setser A, Anadkat MJ, Cotliar J,
Olsen EA, Garden BC and Lacouture ME: Grading dermatologic adverse
events of cancer treatments: The common terminology criteria for
adverse events version 4.0. J Am Acad Dermatol. 67:1025–1039. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Diakatou E, Kaltsas G, Tzivras M, Kanakis
G, Papaliodi E and Kontogeorgos G: Somatostatin and dopamine
receptor profile of gastroenteropancreatic neuroendocrine tumors:
An immunohistochemical study. Endocr Pathol. 22:24–30. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
van Adrichem RC, Kamp K, van Deurzen CH,
Biermann K, Feelders RA, Franssen GJ, Kwekkeboom DJ, Hofland LJ and
de Herder WW: Is there an additional value of somatostatin receptor
subtype 2a immunohistochemistry over somatostatin receptor
scintigraphy uptake in predicting gastroenteropancreatic
neuroendocrine tumor response? Neuroendocrinology. 103:560–566.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sclafani F, Carnaghi C, Di Tommaso L,
Rodari M, Destro A, Rimassa L, Giordano L, Chiti A, Roncalli M and
Santoro A: Detection of somatostatin receptor subtypes 2 and 5 by
somatostatin receptor scintigraphy and immunohistochemistry:
Clinical implications in the diagnostic and therapeutic management
of gastroenteropancreatic neuroendocrine tumors. Tumori.
97:620–628. 2011.PubMed/NCBI
|
|
27
|
Yerci O, Sehitoglu I, Ugras N, Cubukcu E,
Yuce S, Bedir R and Cure E: Somatostatin receptor 2 and 5
expressions in gastroenteropancreatic neuroendocrine tumors in
turkey. Asian Pac J Cancer Prev. 16:4377–4381. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Nasir A, Stridsberg M, Strosberg J, Su PH,
Livingston S, Malik HA, Kelley ST, Centeno BA, Coppola D, Malafa
ME, et al: Somatostatin receptor profiling in hepatic metastases
from small intestinal and pancreatic neuroendocrine neoplasms:
Immunohistochemical approach with potential clinical utility.
Cancer Control. 13:52–60. 2006.PubMed/NCBI
|
|
29
|
Srirajaskanthan R, Watkins J, Marelli L,
Khan K and Caplin ME: Expression of somatostatin and dopamine 2
receptors in neuroendocrine tumours and the potential role for new
biotherapies. Neuroendocrinology. 89:308–314. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Okuwaki K, Kida M, Mikami T, Yamauchi H,
Imaizumi H, Miyazawa S, Iwai T, Takezawa M, Saegusa M, Watanabe M
and Koizumi W: Clinicopathologic characteristics of pancreatic
neuroendocrine tumors and relation of somatostatin receptor type 2a
to outcomes. Cancer. 119:4094–4102. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaemmerer D, Trager T, Hoffmeister M,
Sipos B, Hommann M, Sänger J, Schulz S and Lupp A: Inverse
expression of somatostatin and cxcr4 chemokine receptors in
gastroenteropancreatic neuroendocrine neoplasms of different
malignancy. Oncotarget. 6:27566–27579. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Corleto VD, Falconi M, Panzuto F, Milione
M, De Luca O, Perri P, Cannizzaro R, Bordi C, Pederzoli P, Scarpa A
and Fave G Delle: Somatostatin receptor subtypes 2 and 5 are
associated with better survival in well-differentiated endocrine
carcinomas. Neuroendocrinology. 89:223–230. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Jann H, Denecke T, Koch M, Pape UF,
Wiedenmann B and Pavel M: Impact of octreotide long-acting release
on tumour growth control as a first-line treatment in
neuroendocrine tumours of pancreatic origin. Neuroendocrinology.
98:137–143. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Broder MS, Beenhouwer D, Strosberg JR,
Neary MP and Cherepanov D: Gastrointestinal neuroendocrine tumors
treated with high dose octreotide-lar: A systematic literature
review. World J Gastroenterol. 21:1945–1955. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Welin SV, Janson ET, Sundin A, Stridsberg
M, Lavenius E, Granberg D, Skogseid B, Oberg KE and Eriksson BK:
High-dose treatment with a long-acting somatostatin analogue in
patients with advanced midgut carcinoid tumours. Eur J Endocrinol.
151:107–112. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Ferolla P, Faggiano A, Grimaldi F, Ferone
D, Scarpelli G, Ramundo V, Severino R, Bellucci MC, Camera LM,
Lombardi G, et al: Shortened interval of long-acting octreotide
administration is effective in patients with well-differentiated
neuroendocrine carcinomas in progression on standard doses. J
Endocrinol Invest. 35:326–331. 2012.PubMed/NCBI
|
|
37
|
Baldelli R, Barnabei A, Rizza L, Isidori
AM, Rota F, Di Giacinto P, Paoloni A, Torino F, Corsello SM, Lenzi
A and Appetecchia M: Somatostatin analogs therapy in
gastroenteropancreatic neuroendocrine tumors: Current aspects and
new perspectives. Front Endocrinol (Lausanne). 5:72014.PubMed/NCBI
|
|
38
|
Yao JC, Shah MH, Ito T, Bohas CL, Wolin
EM, Van Cutsem E, Hobday TJ, Okusaka T, Capdevila J, de Vries EG,
et al: Everolimus for advanced pancreatic neuroendocrine tumors. N
Engl J Med. 364:514–523. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Kouvaraki MA, Ajani JA, Hoff P, Wolff R,
Evans DB, Lozano R and Yao JC: Fluorouracil, doxorubicin and
streptozocin in the treatment of patients with locally advanced and
metastatic pancreatic endocrine carcinomas. J Clin Oncol.
22:4762–4771. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Sun W, Lipsitz S, Catalano P, Mailliard JA
and Haller DG: Eastern Cooperative Oncology Group: Phase ii/iii
study of doxorubicin with fluorouracil compared with streptozocin
with fluorouracil or dacarbazine in the treatment of advanced
carcinoid tumors: Eastern cooperative oncology group study e1281. J
Clin Oncol. 23:4897–4904. 2005. View Article : Google Scholar : PubMed/NCBI
|