Introduction
Mantle cell lymphoma (MCL) is a mature B-cell
non-Hodgkin lymphoma (NHL) (1). MCL
is characterized by atypical small lymphoid cells with wide mantles
around benign germinal centers, therefore MCL is known as a mantle
zone lymphoma (2). MCL accounts for
3–10% of adult NHLs (3). The median
age of patients presenting with MCL is in the seventh decade of
life, and there is a striking male predominance (2:1). MCL is
associated with the chromosomal translocation t(11,14)(q13;q32), which leads to cyclin D1
overexpression (3). Patients may
present with pancytopenia or leukemia with extensive leukocytosis
(4). Patients generally have Ann
Arbor stage III/IV disease at the time of diagnosis, and commonly
present with extensive lymphadenopathy and extranodal involvement,
particularly bone marrow, gastrointestinal tract, liver, spleen or
Waldeyer's ring involvement (5). MCL
is particularly involved in the gastrointestinal system and may
mimic other diseases (6), which may
cause a delay in diagnosis and treatment. In past years, MCL had a
poor prognosis, with a 5-year overall survival rate of 27%
(7). Due to the introduction of novel
drugs and therapeutic options, the effect of treatment for MCL has
shown significant improvements, with a current 5-year survival rate
of 50–75% (8). To the best of our
knowledge, no cases of primary bone MCL, involving either single or
multiple bones, have been reported in the literature thus far. The
clinical features of the disease may mimic other diseases, which
can cause a delay in the diagnosis and treatment. The current study
presents a rare case of primary MCL of the spine in a 75-year-old
Chinese male patient.
Case report
A 75-year-old Chinese male patient presented to the
The First Affiliated Hospital of Soochow University (Suzhou, China)
on September, 18 2014 with lower back pain for 1 year, without a
history of traumatic events. The pain was described as moderately
pricking but not radiating, and it was exacerbated by movement and
coughing. B symptoms were not observed in the patient's medical
history. Furthermore, there was no history of bleeding
manifestations, or respiratory, cardiovascular, urinary or
gastrointestinal symptoms. The patient had undergone an
appendectomy 40 years prior. He did not suffer from diabetes, and
did not smoke or consume excess alcohol. The findings of the
physical examination were not significant: No lymphadenopathy,
petechiae or ecchymoses were identified; the patient's spleen and
liver were not palpable; and pain was only felt upon spinal
percussion of the T10 and T12 vertebrae. In addition, the
examination revealed no hypoesthesia, weakness or spasticity. Blood
test results were as follows: Hemoglobin, 10.5 g/dl (normal range,
13.0–17.5 g/dl); white blood cells, 7,430/µl (normal range,
3,500–9,500/µl); neutrophils, 4,930/µl (normal range,
1,800–6,300/µl); lymphocytes, 1,860/µl (normal range,
1,100–3,200/µl); and platelets, 246,000/µl (normal range,
125,000–350,000/µl). Slightly increased C-reactive protein levels
(13 mg/l; normal range, 0–8 mg/l), erythrocyte sedimentation rate
(38 mm/h; normal range, 0–15 mm/h) and ferritin levels (412.1
ng/ml; normal range, 22.0–322.0 ng/ml) were observed. Lactic
dehydrogenase (LDH) was 133 U/l (normal range, 100–225 U/l).
However, the bone mineral density test T-score was −3.5 (normal
range, −1.0–1.0), indicating severe osteoporosis. The Bence-Jones
protein urine test was negative. A computed tomography (CT) scan of
the neck, chest and abdomen showed no abnormalities. Spinal X-ray
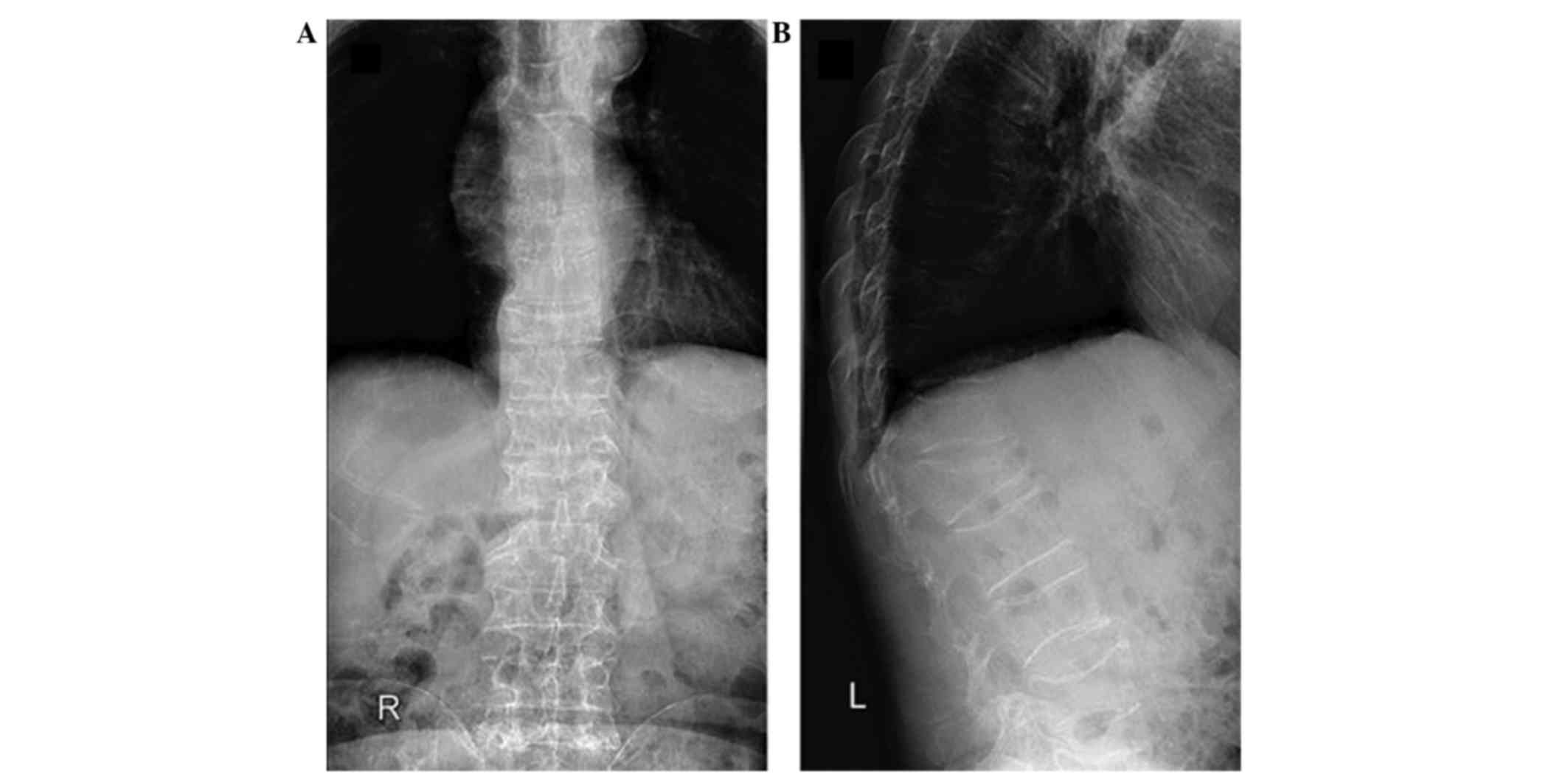
(Ysio Digital X-ray system; Siemens AG, Munich, Germany) revealed
osteoporosis (Fig. 1A and B), and
mixed osteolytic and osteosclerotic lesions in T10 and L1 with
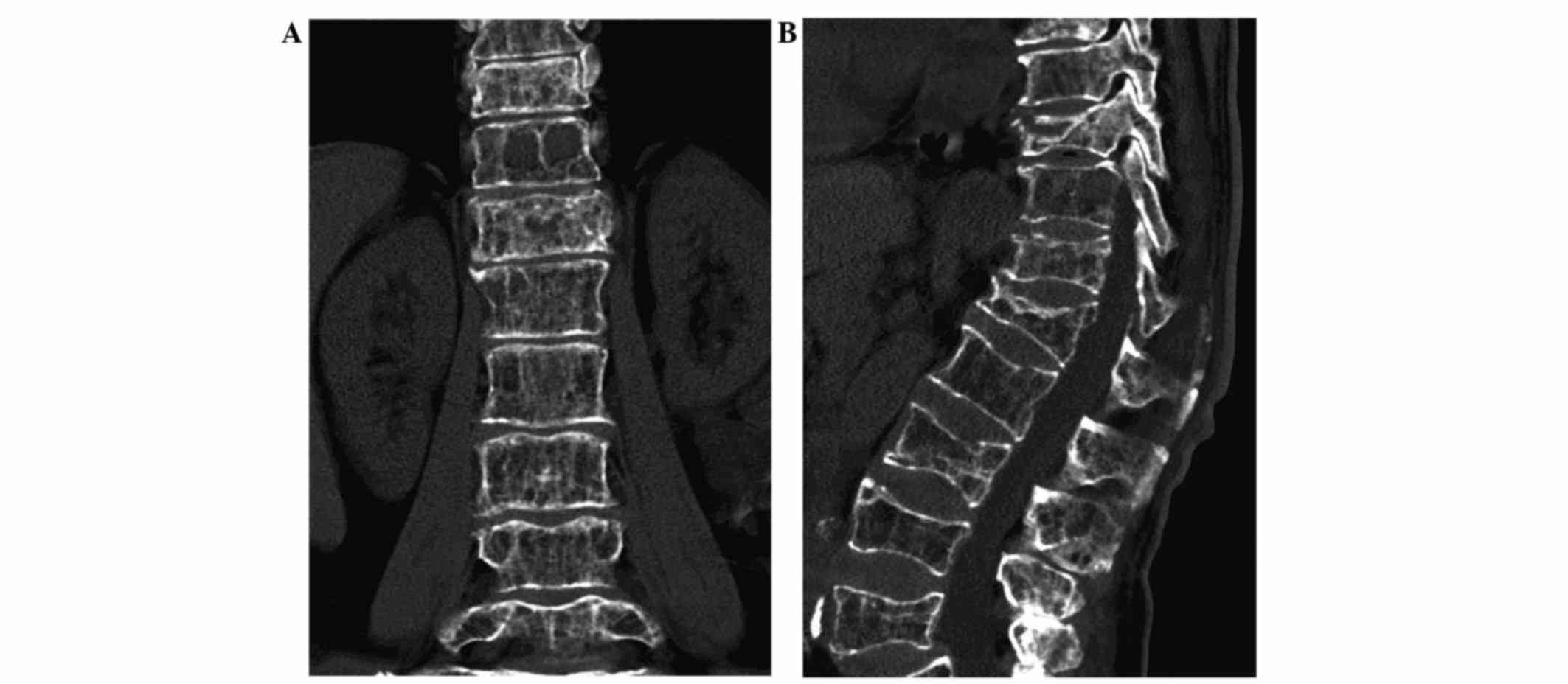
decreased height. A spinal CT scan (SOMATOM Sensation 64; Siemens
AG) identified multiple vertebral compression fractures,
particularly in T10 and L1 (Fig. 2A and
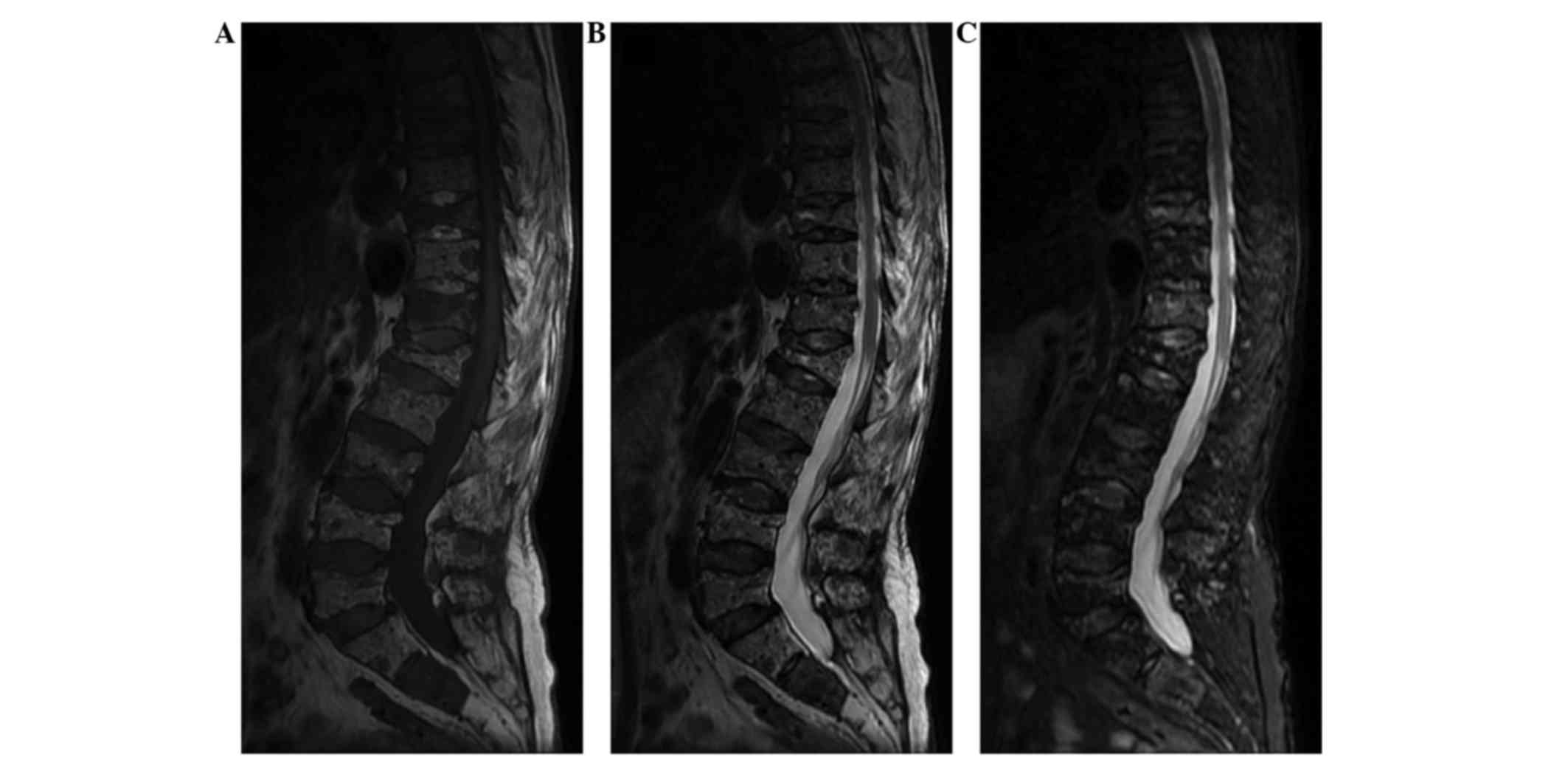
B). Sagittal magnetic resonance imaging (MRI; Signa HDxt 3.0T;
GE Healthcare Life Sciences, Little Chalfont, UK) demonstrated a
diffuse homogeneous abnormal signal with multiple vertebral
compression fractures (Fig. 3A-C).
Similarly, T1-weighted MRI showed multiple vertebral compression
fractures with a low signal intensity. T10 and L1 appeared to
harbor the most severe fractures. T2-weighted MRI also indicated
multiple vertebral fractures with a mildly high signal intensity.
On the short tau inversion recovery sequences, MRI revealed a
diffuse homogeneous abnormal signal. A mildly high signal intensity
was detected in T10 and T12, suggesting that T10 and T12 were
responsible for the pain. The initial clinical diagnosis was
pathological vertebral compression fractures and osteoporosis.
Differential diagnosis typically includes osteoporotic vertebral
compression fractures, multiple myeloma, osteomyelitis, lymphoma,
metastasis and primary bone sarcoma. To confirm the proposed
diagnosis, the patient underwent vertebral biopsy on September, 22
2014, and percutaneous balloon kyphoplasty (PKP) of T10 and T12.
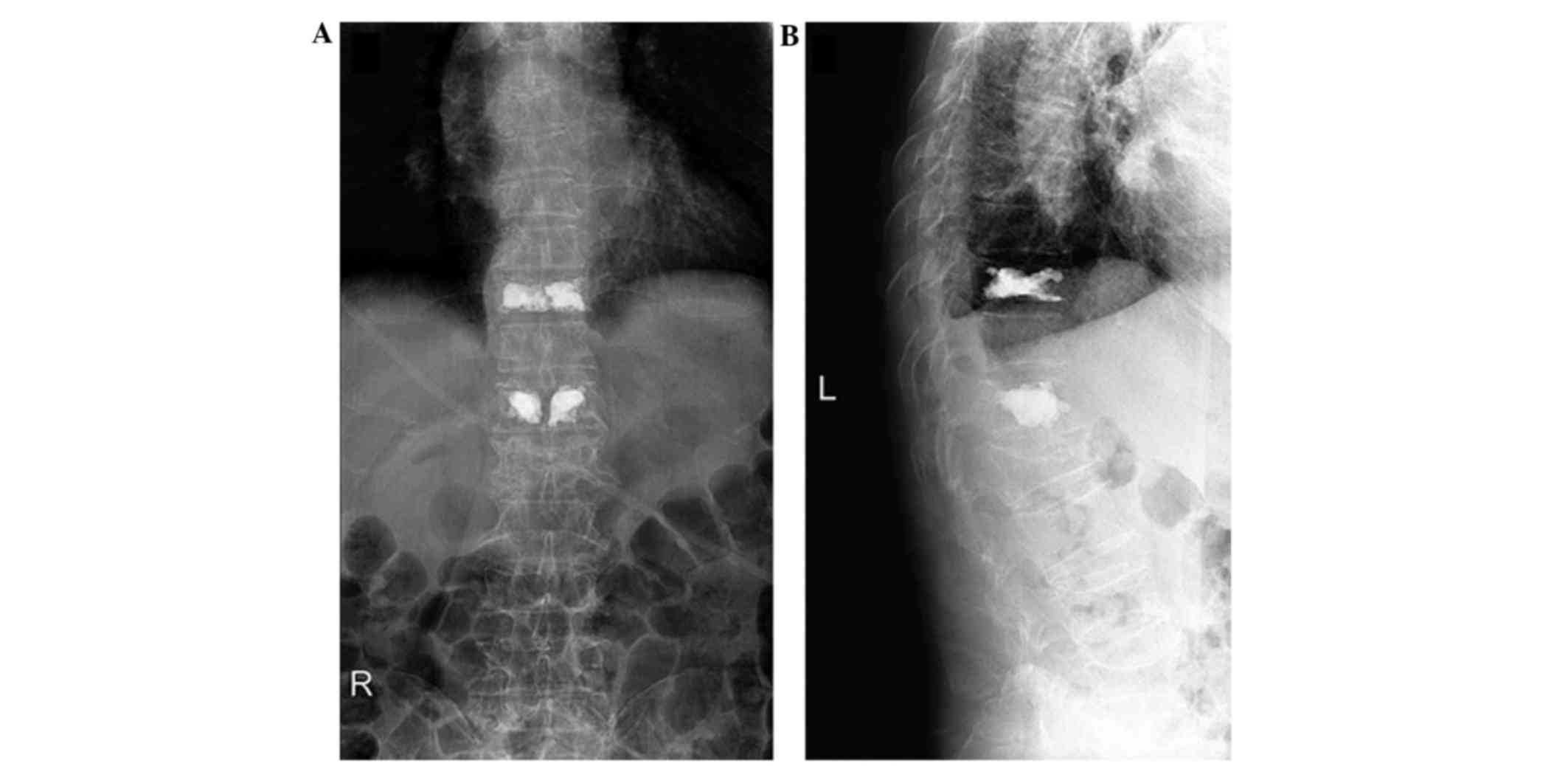
Postoperative radiographs (Fig. 4A and
B) revealed satisfying fracture reduction and cement
distribution. Tumor samples were fixed in formalin (Sangon Biotech
Co. Ltd., Shanghai, China). Subsequently, the samples were
decalcified with 20% ethylenediamine tetra-acetic acid (Sangon
Biotech Co. Ltd), dehydrated using a graded ethanol (Sangon Biotech
Co. Ltd) series and paraffin (Sangon Biotech Co. Ltd.) embedded.
Tumor tissue sections (4-µm thick) were sliced, deparaffinized in
xylene (Sangon Biotech Co. Ltd.) and stained with hematoxylin and
eosin (Sangon Biotech Co. Ltd.). The tissue sections were
visualized by microscopy (Eclipse E600; Nikon Corporation, Tokyo,
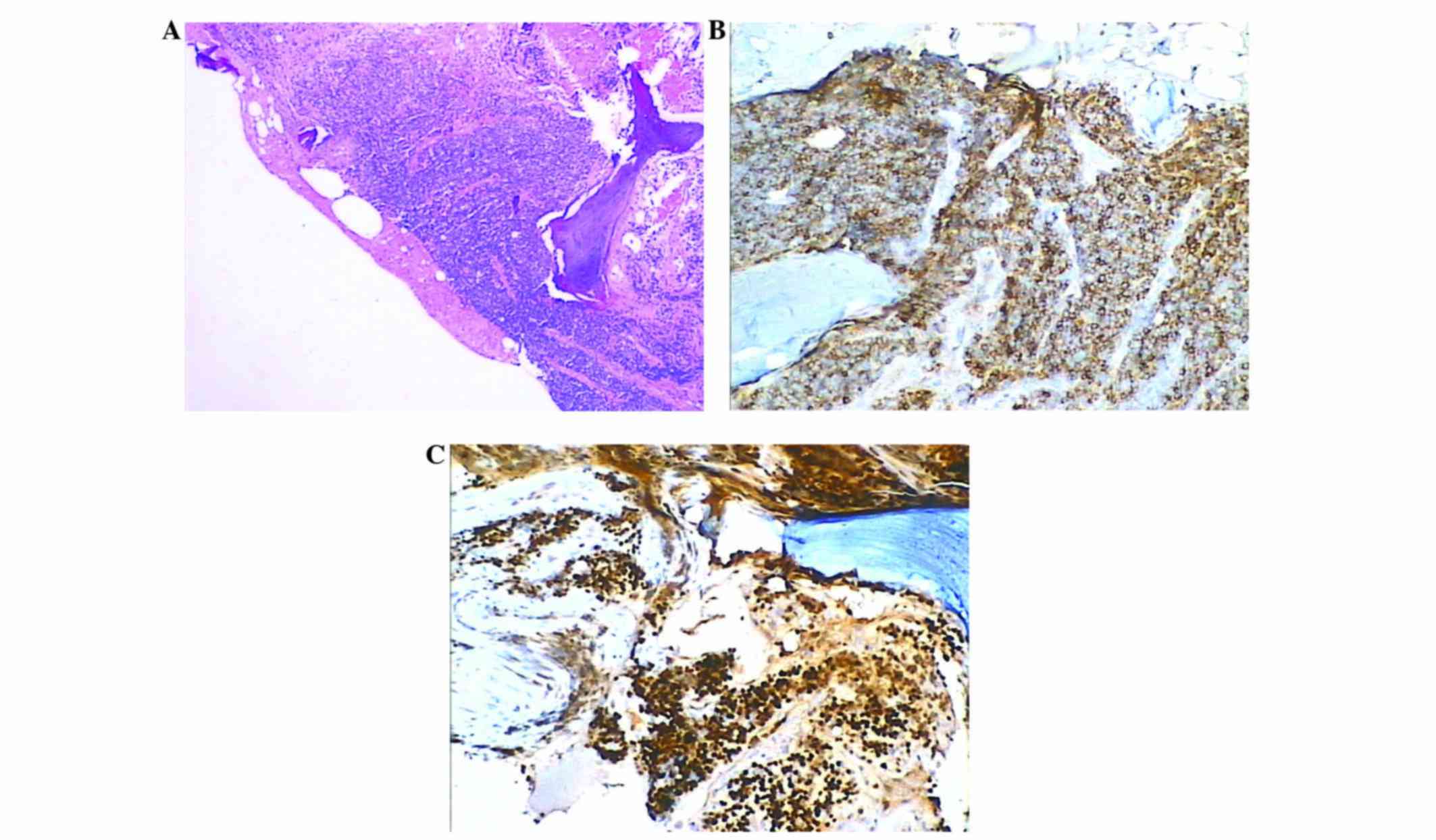
Japan). Histopathological examination of the vertebral biopsy
showed abnormal, diffuse, atypical, small-sized, slightly irregular
lymphocytes with condensed chromatin (Fig. 5A). Immunohistochemical staining was
performed using the EnVision two-step staining method (Dako,
Glostrup, Denmark). Deparaffinization, rehydration and epitope
retrieval were performed using PT Link kit (Dako). The primary
antibodies used were monoclonal mouse anti-cluster of
differentiation (CD) 20 (catalog no., F0799; Dako) and monoclonal
rabbit anti-cyclin D1 (catalog no., IR083; Dako), at a dilution of
1:100. Diaminobenzidine and hematoxylin staining were achieved
using the autostainer system (Autostainer Link 48; Dako).
Immunohistochemistry demonstrated that the tumor cells expressed
CD20 and cyclin D1 (Fig. 5B and C).
The histopathological and immunohistochemical features of the
vertebral bone were concordant with MCL. Following PKP surgery, the
lower back pain was immediately relieved. The patient was then
referred to the Department of Hematology with the diagnosis of MCL
stage IV, according to the Ann Arbor classification (9), and was dicharged on September, 25 2014.
An R-CHOP chemotherapy regime was administered to the patient 4
weeks following surgery. In total, the patient received 8 cycles of
rituximab (700 mg on day 1), cyclophosphamide (1.4 g on day 2),
doxorubicin (140 mg on day 2), vincristine (4 mg on day 2) and
dexamethasone (15 mg on day 2). During 1 year follow-up, the
patient remained in good clinical condition, only complaining of
slight back pain.
Written informed consent was obtained from the
patient for the publication of the present study.
Discussion
In 1964, Lennert first described MCL. Lennert termed
the disease ‘centrocytic lymphoma’, as he considered these tumors
to be the neoplastic counterpart of centrocytes located in germinal
centers of lymphoid follicles (10).
Various other terms were used to describe the condition in the
following decades, until, in 1992, Banks et al (11) proposed the term MCL for this neoplasm.
MCL subsequently became widely accepted, and has been adopted in
the Revised European-American Classification of Lymphoid Neoplasms
(12) and the World Health
Organization classification of lymphoid neoplasms (13).
MCL is typically diagnosed in patients at an
advanced stage of the disease (Ann Arbor stage III/IV), and the
common symptoms include generalized lymphadenopathy, and
involvement of the spleen, liver and bone marrow. The majority of
MCLs develop within lymph nodes; the extranodal form is
considerably rare. The most frequently affected extra-lymphatic
sites are the gastrointestinal tract, Waldeyer's ring and
nasopharynx (14). In the current
case, the patient presented with pain in the lower back. No
lymphadenopathy or hepatosplenomegaly were identified.
Histopathological and immunohistochemical examination of the biopsy
revealed primary MCL of the spine.
Primary bone lymphoma is rare and primary bone MCL
is even more rare. To the best of our knowledge, no cases of MCL,
involving either single or multiple bones, have been reported in
the literature thus far. The patient in the present study exhibited
multiple lumbar vertebral bone involvement. Thus, this is the first
reported case of primary bone MCL with multiple vertebral
compression fractures.
MCL typically exhibits a proliferation of monotonous
small- or medium-sized lymphocytes with inconspicuous nucleoli
(7). The immunophenotypic analysis of
MCL is usually positive for CD5, CD20, CD43 and negative for CD10
and Bcl-6 (15). The chromosomal
translocation t(11;14)(q13;q32) is a genetic characteristic of MCL
that leads to the aberrant overexpression of cyclin D1. Cyclin D1
can be easily identified by immunohistochemistry and is considered
to be the hallmark of MCL. Only in rare cases is MCL cyclin
D1−; in these cases, the tumor exhibits overexpression
of cyclin D2 or D3 instead (16). The
typical immunohistochemical features of MCL are CD5+,
CD20+, cyclin D1+ and CD23−, with
which the present case was in accordance.
For the present patient, PKP and biopsy were
selected as the appropriate treatment methods. Kyphoplasty, which
uses a dilatation balloon to restore the height of the vertebra and
reduce angular kyphosis, was developed from vertebroplasty. PKP is
a minimally invasive technique that induces immediate pain relief
and permanent functional improvement. Despite the X-ray and CT scan
revealing that the L1 vertebra had the most severe compression
fractures, indicating that it was responsible for the pain,
meticulous physical examination and imageology eventually
determined that T10 and T12 were the source of the pain. Thus, PKP
was performed on T10 and T12. Pain relief was achieved immediately
following surgery, suggesting that the procedure may comprise a
good treatment option for bone MCLs with multiple, painful
vertebral compression fractures.
Indolent and aggressive MCL have the worst prognosis
among lymphomas, and they may have a gradually or rapidly
progressive course, respectively. The indolent subgroup accounts
for 10–15% of all MCL cases. Despite the fact that this MCL
subgroup shows no signs of progression for a long period of time,
the majority of patients face a relatively rapid disease
progression, short-term response to treatment, inevitable relapses
and a continuously declining survival curve, with a median survival
of only 3–5 years (17–19). The following clinical factors have
been associated with poor prognosis of MCL: Advanced age, poor
general condition, advanced stage of the disease, splenomegaly,
elevated LDH, low serum albumin, other tumors and anemia.
We propose a diagnosis of stage IV disease for the
current patient, due to multiple bone involvement and the Ann Arbor
classification (9). The question
remains as to whether primary osseous MCL has a better prognosis
than MCL with bone involvement. The patient in the present case
underwent PKP and received chemotherapy. During 1 year follow-up,
the patient remained in good clinical condition, only complaining
of slight back pain. Although patients with one or multiple bone
lesions usually respond well to combination therapy, including
chemotherapy and local radiotherapy, the present study proposes
that the current patient's prognosis will be poor considering the
low median overall survival.
To date, as no characteristic symptoms and
laboratory or radiological findings have been established for the
identification of primary bone MCLs, the diagnosis of osseous MCL
is challenging and may be delayed. Therefore, during differential
diagnosis, clinicians should consider that multiple vertebral
compression fractures without any systematic symptoms may be
associated with MCL. Early diagnosis and management may contribute
to the improved prognosis and survival of patients with osseous
MCL.
References
|
1
|
Weisenburger DD, Kim H and Rappaport H:
Mantle-zone lymphoma: A follicular variant of intermediate
lymphocytic lymphoma. Cancer. 49:1429–1438. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Berard CW and Dorfman RF: Histopathology
of malignant lymphomas. In: Clinics in HaematologyRosenburg S
(guest ed). 3. W.B. Suanders; London: pp. 39–76. 1974
|
|
3
|
Leonard JP, Williams ME, Goy A, Grant S,
Pfreundschuh M, Rosen ST and Sweetenham JW: Mantle cell lymphoma:
Biological insights and treatment advances. Clin Lymphoma Myeloma.
9:267–277. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Ferrer A, Salaverria I, Bosch F, Villamor
N, Rozman M, Beà S, Giné E, López-Guillermo A, Campo E and
Montserrat E: Leukemic involvement is a common feature in mantle
cell lymphoma. Cancer. 109:2473–2480. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Tiemann M, Schrader C, Klapper W, Dreyling
MH, Campo E, Norton A, Berger F, Kluin P, Ott G, Pileri S, et al:
Histopathology, cell proliferation indices and clinical outcome in
304 patients with mantle cell lymphoma (MCL): A clinicopathological
study from the European MCL Network. Br J Haematol. 131:29–38.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Kelkitli E, Atay H, Yıldiz L, Bektaş A and
Turgut M: Mantle cell lymphoma mimicking rectal carcinoma. Case Rep
Hematol. 2014:6210172014.PubMed/NCBI
|
|
7
|
Doorduijn JK and Kluin-Nelemans HC:
Management of mantle cell lymphoma in the elderly patient. Clin
Interv Aging. 8:1229–1236. 2013.PubMed/NCBI
|
|
8
|
Alwasaidi TA, Hamadah A, Altouri S, Tay J,
McDiarmid S, Faught C, Allan D, Huebsch L, Bredeson C and
Bence-Bruckler I: Outcomes of both abbreviated hyper-CVAD induction
followed by autologous hematopoietic cell transplantation and
conventional chemotherapy for mantle cell lymphoma: A 10-year
single-centre experience with literature review. Cancer Med.
4:1817–1827. 2015. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Carbone PP, Kaplan HS, Musshoff K,
Smithers DW and Tubiana M: Report of the Committee on Hodgkin's
Disease Staging Classification. Cancer Res. 31:1860–1861.
1971.PubMed/NCBI
|
|
10
|
Lai R and Medeiros LJ: Pathologic
diagnosis of mantle cell lymphoma. Clin Lymphoma. 1:197–206. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Banks PM, Chan J, Cleary ML, Delsol G, De
Wolf-Peeters C, Gatter K, Grogan TM, Harris NL, Isaacson PG and
Jaffe ES: Mantle cell lymphoma. A proposal for unification of
morphologic, immunologic and molecular data. Am J Surg Pathol.
16:637–640. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Harris NL, Jaffe ES, Stein H, Banks PM,
Chan JK, Cleary ML, Delsol G, De Wolf-Peeters C, Falini B and
Gatter KC: A revised European-American classification of lymphoid
neoplasms: A proposal from the international lymphoma study group.
Blood. 84:1361–1392. 1994.PubMed/NCBI
|
|
13
|
Harris NL, Jaffe ES, Diebold J, Flandrin
G, Muller-Hermelink HK, Vardiman J, Lister TA and Bloomfield CD:
The World Health Organization Classification of hematological
malignancies report of the Clinical Advisory Committee Meeting,
Airlie House, Virginia, November 1997. Mod Pathol. 13:193–207.
2000. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lai R and Medeiros LJ: Pathologic
diagnosis of mantle cell lymphoma. Clin Lymphoma. 1:197–206;
discussion 207–208. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Vose JM: Mantle cell lymphoma: 2013 Update
on diagnosis, risk-stratification and clinical management. Am J
Hematol. 88:1082–1088. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rosenwald A, Wright G, Wiestner A, Chan
WC, Connors JM, Campo E, Gascoyne RD, Grogan TM, Muller-Hermelink
HK, Smeland EB, et al: The proliferation gene expression signature
is a quantitative integrator of oncogenic events that predicts
survival in mantle cell lymphoma. Cancer Cell. 3:185–197. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Chandran R, Gardiner SK, Simon M and
Spurgeon SE: Survival trends in mantle cell lymphoma in the United
States over 16 years 1992–2007. Leuk Lymphoma. 53:1488–1493. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Nygren L, Wennerholm S Baumgartner,
Klimkowska M, Christensson B, Kimby E and Sander B: Prognostic role
of SOX11 in a population-based cohort of mantle cell lymphoma.
Blood. 119:4215–4223. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ghielmini M and Zucca E: How I treat
mantle cell lymphoma. Blood. 114:1469–1476. 2009. View Article : Google Scholar : PubMed/NCBI
|