Introduction
Lung cancer is the most common and lethal type of
cancer worldwide, and ovarian cancer is a leading cause of
cancer-associated mortality in women (1,2). In 2012,
the estimated global incidence rates for lung and ovarian cancer
were 23.1 and 6.1 cases per 100,000 individuals, respectively, and
the estimated global mortality rates were 19.7 and 3.7 mortalities
per 100,000 individuals, respectively (3). Platinum-based antitumor drugs, including
cisplatin (DDP) and oxaliplatin (L-OHP), are typical first-line
agents that are used for the treatment of various types of cancer,
including lung and ovarian cancer, as well as head and neck
squamous cell carcinoma and lymphoma (4,5). It is
generally accepted that the efficacy of platinum-based therapy is
modulated by drug uptake and efflux, cellular proliferation, DNA
adduct formation and DNA repair (6).
Platinum-based agents are able to form DNA intra- and inter-strand
crosslinks, and DNA-protein complexes, which interfere with DNA
synthesis, RNA transcription, the cell cycle and apoptosis
(7,8).
L-OHP is a 1,2-diaminocyclohexane-containing platinum-based
compound that is known to induce a reduced number of DNA
double-strand breaks and possess decreased cytotoxicity compared
with DDP (9,10). However, certain patients do not
respond to treatment with platinum-based agents, and even those who
initially benefit from the treatment will eventually demonstrate
resistance to these drugs (11,12).
Clinically, drug resistance is divided into natural
and acquired resistance (13).
Acquired resistance to platinum develops during extended periods of
treatment with platinum-based agents, while natural platinum
resistance occurs in cells that have not previously been treated
with platinum-based drugs (6).
Therefore, predictive factors for improved management of lung and
ovarian cancer patients with natural and acquired platinum
resistance are urgently required. Although certain predictive
markers, including excision repair cross-complementation group 1
(ERCC1) and Tau, have been investigated for their potential to
predict platinum resistance in lung and ovarian cancer patients,
more effective predictors require development and investigation
(12,14).
In a previous study, we cloned a novel gene from the
Tca8113/pingyangmycin (Tca8113/PYM) tongue cancer multi-drug
resistant cell line, which was termed tongue cancer
resistance-associated protein 1 (TCRP1) (15). Notably, it was observed that TCRP1
contributed to DDP resistance in human oral squamous cell carcinoma
(OSCC) cells (15–17). TCRP1 was reported to interact with
Akt, and activation of the phosphoinositide 3-kinase/Akt/nuclear
factor (NF)-κB signaling pathway was involved in the functioning of
TCRP1 in OSCC cells (16,18). It was additionally demonstrated that
TCRP1 has a significant role in the mediation of DDP resistance via
increased cellular proliferation and decreased apoptosis (15). In another previous study, we reported
that TCRP1 contributes to DDP resistance in A549 lung cancer cells,
and that DNA repair protein polymerase β (Pol β) is involved in
TCRP1-mediated DDP resistance (19).
A previous study investigated the tissue-specific expression of
TCRP1, and it was observed that TCRP1 expression levels in samples
of lung and ovarian cancer were significantly increased compared
with normal controls (data not published). However, the
participation of TCRP1 in the resistance to platinum-based agents
in human lung and ovarian cancer cells remains to be
elucidated.
In the present study, the association between the
expression of TCRP1 and the chemoresistance to DDP and L-OHP was
analyzed in human lung and ovarian cancer cells. It was
demonstrated that TCRP1 contributed to the resistance to DDP and
L-OHP in lung and ovarian cancer cells, and knockdown of TCRP1
resensitized the cells to platinum-based agents. In addition, the
present study identified that the Akt/NF-κB signaling pathway had a
significant role in TCRP1-mediated platinum resistance. In summary,
the results of the present study suggested that TCRP1 may be a
potential predictor of platinum resistance in the treatment of lung
and ovarian cancer.
Materials and methods
Reagents
Platinum-based agents DDP and L-OHP were purchased
from Sigma-Aldrich (St. Louis, MO, USA). The NF-κB inhibitor BAY
11–7082 was obtained from EMD Millipore (Billerica, MA, USA).
Cell culture
The A549 human lung adenocarcinoma epithelial, A549
DDP-resistant (A459/DDP), COC1 human ovarian cancer, COC1
DDP-resistant (COC1/DDP), MCF-7 human breast cancer, MCF-7
5-fluorouracil (5-Fu)-resistant (MCF7/5-Fu) and Tca8113 human OSCC
cell lines were obtained from the China Center for Type Culture
Collection (Wuhan, China). The PYM-induced Tca8113/PYM multidrug
resistant cell line was previously established by Dr Guopei Zheng
at the Cancer Research Institute of Central South University
(Changsha, China) (20). The cells
were maintained in RPMI 1640 medium (Invitrogen; Thermo Fisher
Scientific, Inc., Waltham, MA, USA) containing 10% fetal calf serum
(Invitrogen; Thermo Fisher Scientific, Inc.) at 37°C in a
humidified atmosphere containing 5% CO2. To maintain
drug resistance, A549/DDP and COC1/DDP cells were routinely
cultured with an additional 2 µg/ml DDP, and Tca8113/PYM cells were
cultured with an additional 100 ng/ml PYM (Harbin Bolai
Pharmaceutical Co., Ltd., Harbin, China) at 37°C in a humidified
atmosphere containing 5% CO2. The cells were
additionally cultured in drug-free RPMI 1640 medium 1 week prior to
starting the experiments at 37°C in a humidified atmosphere
containing 5% CO2. Histopathological subtypes of 8 human
lung cancer cell lines, H1299, H1975, H460, H446, SK-MES-1,
SPC-A-1, LTEP-a-2 and 95D, were obtained from the China Center for
Type Culture Collection and cultured in RPMI 1640 medium containing
10% fetal calf serum at 37°C in a humidified atmosphere containing
5% CO2.
Western blot analysis
Immunoblotting was performed as described previously
(16). Briefly, whole cell lysates
were prepared with radioimmunoprecipitation assay lysis buffer [50
mM Tris-Cl (pH 7.4), 150 mM NaCl, 1% Triton X-100, 1% sodium
deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 1 mM sodium
orthovanadate, 10 mM sodium fluoride and 1% protease inhibitor
cocktail; Thermo Fisher Scientific, Inc.]. The proteins were
separated by 10% SDS-polyacrylamide gel electrophoresis and
transferred onto a polyvinylidene difluoride membrane (Thermo
Fisher Scientific, Inc.). The membrane was blocked with 5% v/w
non-fat milk in 1X phosphate-buffered saline with Tween 20 to
prevent non-specific binding, incubated with polyclonal rabbit
anti-human TCRP1 (cat. no. sc-138365; 1:200; Santa Cruz
Biotechnology, Inc., Dallas, TX, USA), monoclonal mouse anti-human
β-actin (cat. no. sc-843; 1:500; Santa Cruz Biotechnology, Inc.),
polyclonal rabbit anti-human p-Akt (cat. no. 9275; 1:1,000; Cell
Signaling Technology, Inc., Danvers, MA, USA), monoclonal rabbit
anti-human p-IκB (cat. no. 2859; 1:1,000; Cell Signaling
Technology, Inc.) and polyclonal rabbit anti-human B-cell lymphoma
(Bcl)-2 (cat. no. 2876; 1:1,000; Cell Signaling Technology, Inc.)
primary antibodies and horseradish peroxidase-conjugated goat
anti-rabbit (cat. no. 7074; 1:2,000; Cell Signaling Technology,
Inc.) and horse anti-mouse (cat. no. 7076; 1:2,000; Cell Signaling
Technology, Inc.) IgG secondary antibodies and detected with
chemiluminescent substrate (ECL Western Blotting Detection kit; GE
Healthcare Life Sciences, Chalfont, UK).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Cellular RNA was extracted from cells using
TRIzol® reagent (Thermo Fisher Scientific, Inc.). The
RNA was treated with 2 units DNase I (Thermo Fisher Scientific,
Inc.). Complementary (c)DNA was synthesized using a First Strand
cDNA Synthesis kit (Thermo Fisher Scientific, Inc.) according to
the manufacturer's protocols. The expression levels of
glyceraldehyde-3-phosphate dehydrogenase, which served as an
internal control, and TCRP1 were verified by RT-qPCR using the
Applied Biosystems 7500 Real-Time PCR System (Thermo Fisher
Scientific, Inc.) with the SYBR Green Master Mix (Thermo Fisher
Scientific, Inc.). PCR was performed under the following
conditions: 95°C for 10 min, followed by 40 cycles of 95°C for 15
sec and 60°C for 1 min. β-actin was used as an internal control.
The fold change in relative expression of the target gene relative
to β-actin was calculated as described previously (16). The following primers were designed
using Primer3 (primer3.ut.ee/): TCRP1 forward,
5′-CCAATAGTCCCAGTTATGCTCCA-3′ and reverse,
5′-TGCTTGGTAAGTTCGGTTCTCG-3′; and β-actin forward,
5′-CTCACCATGGATGATGATATCGC-3′ and reverse,
5′-AGGAATCCTTCTGACCCATGC-3′. Primers were synthesized by Invitrogen
(Thermo Fisher Scientific, Inc.). Experiments were performed in
triplicate.
Cytotoxicity assay
The cytotoxicity of DDP and L-OHP in various cell
lines was determined by 3-(4,5-
dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfo
phenyl)-2H-tetrazolium assay, as previously described (16). The absorbance was recorded at 490 nm
using a Synergy 2 Multi-Mode plate reader (Bio-Tek Instruments,
Inc., Winooski, VT, USA). Cell proliferation in the presence of DDP
or L-OHP was detected based on the half maximal inhibitory
concentration (IC50) values determined by the
dose-response curve.
RNA interference
The cells were transiently transfected with the
small interfering (si)RNA sequence against TCRP1
(5′-CCGAGAACCGAACUUACCA-3′) and control oligonucleotides, as
described previously (19).
Lipofectamine® 2000 (Thermo Fisher Scientific, Inc.) was
used to transfect the siRNA into the cells according to the
manufacturer's protocols. The expression of TCRP1 was determined by
western blot analysis.
Statistical analysis
Quantitative values are expressed as the mean ±
standard deviation. Spearman's rank correlation was calculated
using SPSS version 13.0 (SPSS Inc., Chicago, IL, USA). Student's
t-test was utilized to compare corresponding data. P<0.05 was
considered to indicate a statistically significant difference.
Results
TCRP1 expression is associated with
sensitivity to DDP in lung and ovarian cancer cells with acquired
platinum resistance
To examine the role of TCRP1 in the acquired
platinum resistance to DDP in lung and ovarian cancer cells, 4 cell
lines and their drug-resistant equivalents were treated with
various concentrations of DDP (2.5, 5.0, 10.0, 15.0, 25.0 µg/ml).
IC50 values were determined by dose-response curve, and
the mRNA and protein levels of TCRP1 were analyzed. As shown in
Fig. 1A, the IC50 values
of A549/DDP, COC1/DDP and Tca8113/PYM cells to DDP were
significantly increased compared with parental cells (P=0.015,
P-0.021 and P=0.039, respectively). The results of RT-qPCR and
western blot analysis revealed that A549/DDP, COC1/DDP and
Tca8113/PYM cells expressed a significantly increased level of
TCRP1 compared with the parental controls (P=0.016, P=0.035 and
P=0.041, respectively), in terms of the mRNA and protein levels
(Fig. 1B and C). The present study
demonstrated that the MCF7 and MCF7/5-Fu cells were sensitive to
DDP treatment, and the expression levels of TCRP1 were relatively
low in these two cell lines (Fig.
1A-C).
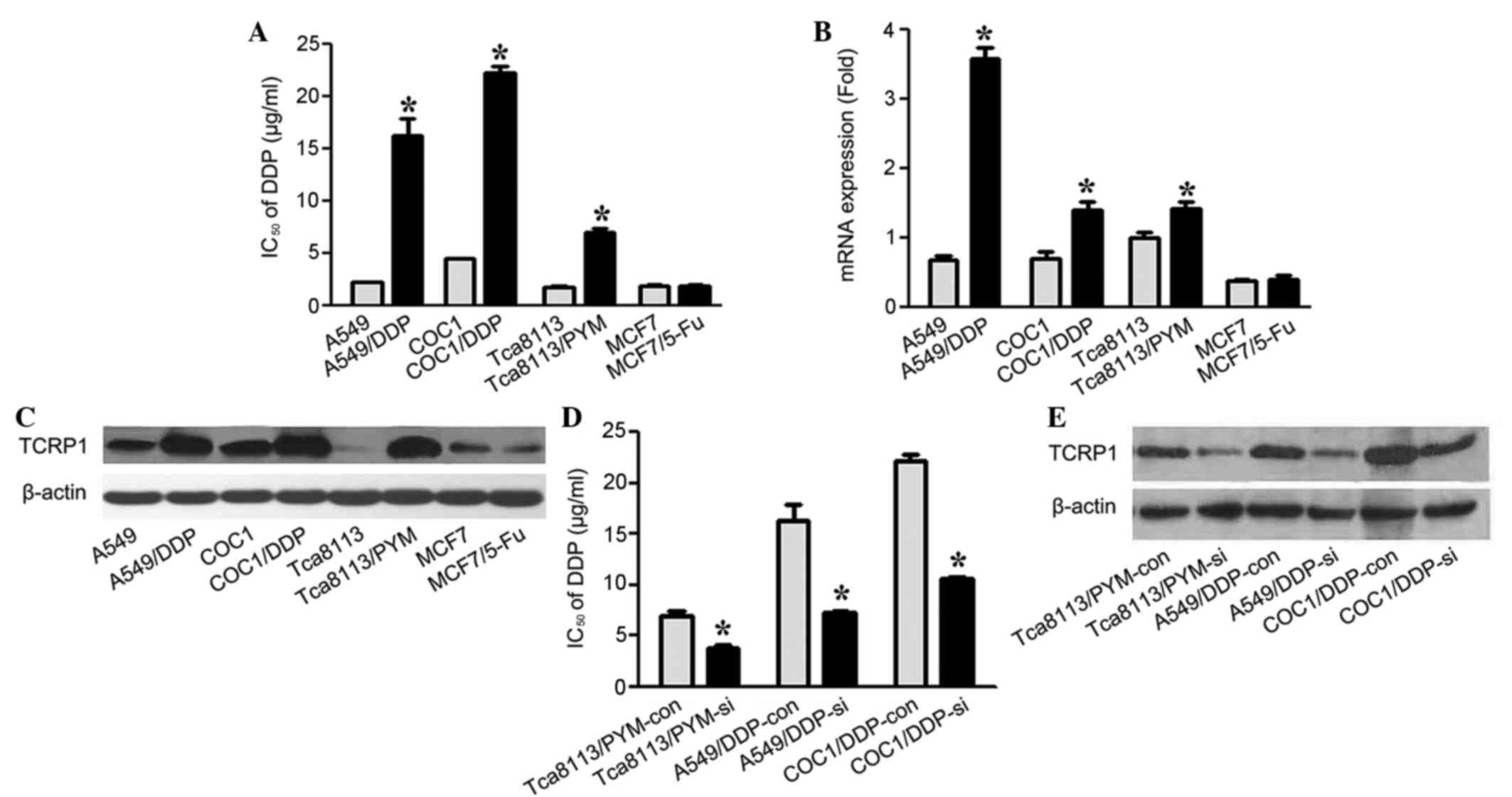 | Figure 1.TCRP1 contributes to DDP resistance in
lung and ovarian cancer cells with acquired platinum resistance.
(A) IC50 values for DDP were analyzed. A549, COC1,
Tca8113, MCF-7 and their relative drug-resistant cells were treated
with various concentrations of DDP (2.5, 5.0, 10.0, 15.0, 25.0
µg/ml), and IC50 values were determined by dose-response
curve. *P<0.05 vs. control. (B) mRNA expression levels of TCRP1
in A549, COC1, Tca8113, MCF-7 and their drug resistant cells were
detected by reverse transcription-quantitative polymerase chain
reaction. *P<0.05 vs. control. (C) Protein levels of TCRP1 in
the cell lines were analyzed by western blotting. (D) TCRP1 siRNA
or scramble control was transfected into Tca8113/PYM, A549/DDP and
COC1/DDP cells for 48 h. The IC50 values for DDP in the
indicated TCRP1 siRNA-treated cells and controls were investigated.
*P<0.05 vs. control. (E) Protein expression levels of TCRP1 were
detected by western blot analysis in the indicated TCRP1
siRNA-treated cells and controls. TCRP1, tongue cancer
resistance-associated protein 1; DDP, cisplatin; IC50,
half maximal inhibitory concentration; PYM, pingyangmycin; 5-Fu,
fluorouracil; siRNA, small interfering RNA; si, siRNA-treated; con,
control. |
Subsequently, the effect of the siRNA knockdown of
TCRP1 was detected in TCRP1-proficient cell lines, Tca8113/PYM,
A549/DDP and COC1/DDP, and the TCRP1 siRNA or control was
transfected into these three cell lines. The cells were treated
with the indicated concentrations of DDP and the dose-response
curves were analyzed. The IC50 values for sensitivity to
DDP following TCRP1-knockdown in Tca8113/PYM, A549/DDP and COC1/DDP
cells were 1.8-, 2.3- and 2.1-fold lower compared with the
controls, respectively (Fig. 1D).
These results showed that the knockdown of TCRP1 increased the
sensitivity to DDP in Tca8113/PYM, A549/DDP and COC1/DDP cells (P=
0.037, P=0.022 and P=0.030, respectively) (Fig. 1D and E). Therefore, this suggested
that the expression of TCRP1 may be correlated with DDP resistance
in lung and ovarian cancer cells with acquired platinum
resistance.
TCRP1 mediates resistance to L-OHP in
lung and ovarian cancer cells with acquired platinum
resistance
L-OHP is a third-generation platinum-based agent and
has a markedly different spectrum of activity compared with DDP
in vitro and in vivo (21). Clinical data has revealed that L-OHP
has reduced toxicity and is therapeutically beneficial in the
treatment of DDP-resistant tumors (10). To investigate whether TCRP1 mediated
resistance to L-OHP in lung and ovarian cancer cells, A549, COC1,
Tca8113, MCF-7 and their relative drug-resistant cells were
incubated with various concentrations of L-OHP and IC50
values were determined. Increased resistance to L-OHP was observed
in TCRP1-proficient cells A549/DDP, COC1/DDP and Tca8113/PYM
compared with their controls, and there was no significant change
in sensitivity to L-OHP in the MCF-7 TCRP1-deficient and MCF-7/5-Fu
drug-resistant cells (P=0.153) (Figs.
1C and 2A). To additionally
identify the role of TCRP1 in L-OHP resistance, the expression of
TCRP1 was knocked down in Tca8113/PYM, A549/DDP and COC1/DDP cells
and IC50 values for L-OHP were detected. As a result,
increased sensitivity to L-OHP was detected in cells treated with
TCRP1-knockdown, indicating that downregulation of TCRP1 may
sensitize cells to L-OHP treatment (Fig.
2B). Taken together, these results suggested that TCRP1 may
mediate resistance to L-OHP in lung and ovarian cancer cells with
acquired platinum resistance.
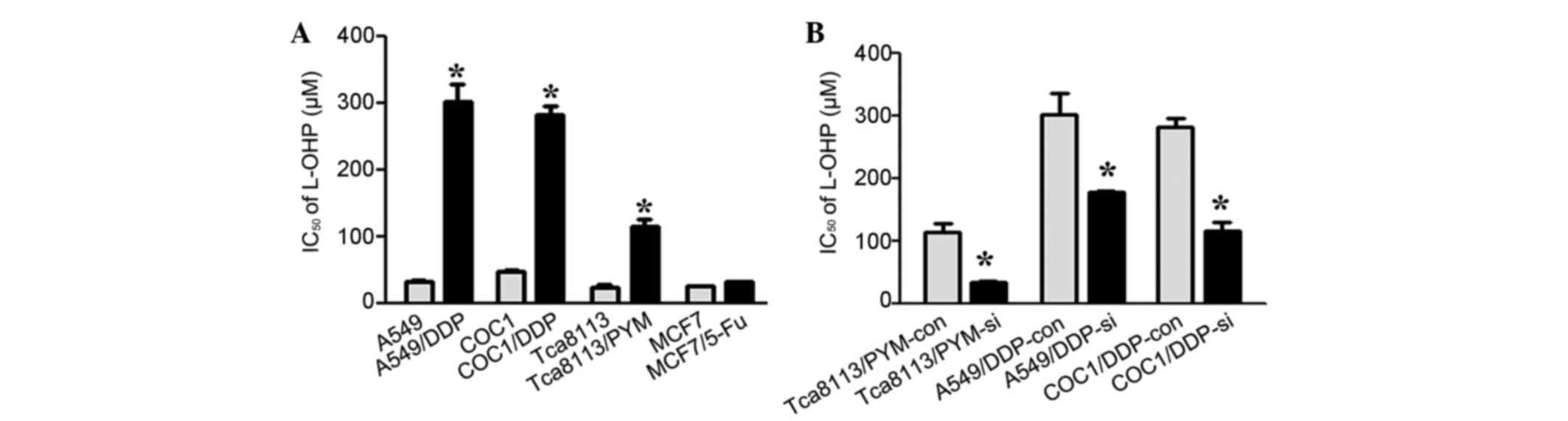 | Figure 2.TCRP1 mediates resistance to L-OHP in
lung and ovarian cancer cells with acquired platinum resistance.
(A) A549, COC1, Tca8113, MCF-7 and their drug-resistant cells were
treated with various concentrations of L-OHP, and IC50
values were determined by dose-response curve. *P<0.05 vs.
control. (B) Tca8113/PYM, A549/DDP and COC1/DDP cells were
transfected with TCRP1 siRNA or control siRNA, and the
IC50 values for L-OHP were determined by dose-response
curve. *P<0.05 vs. control. TCRP1, tongue cancer
resistance-associated protein 1; L-OHP, oxaliplatin; siRNA, small
interfering RNA; si, siRNA-treated; con, control; IC50,
half maximal inhibitory concentration. |
A positive correlation exists between
TCRP1 expression and primary resistance to platinum in lung cancer
cells
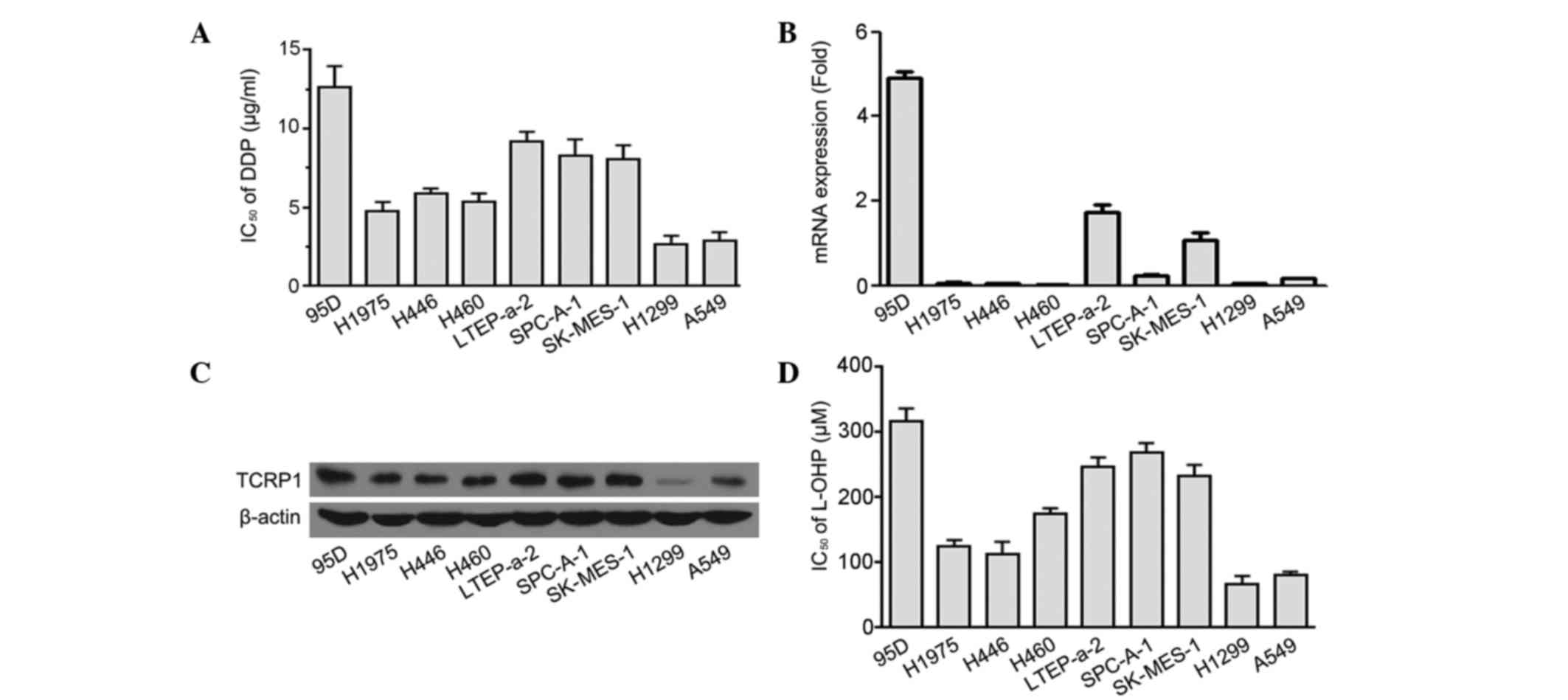
To assess the role of TCRP1 in the primary
resistance to platinum-based agents, the IC50 values for
DDP in 9 distinct histopathological subtypes of lung cancer cell
lines were analyzed, and the TCRP1 expression was detected. The
results of this analysis revealed that cell lines with higher
expression levels of TCRP1 mRNA and protein demonstrated increased
resistance to DDP (Fig. 3A-C). A
positive correlation was observed between TCRP1 mRNA levels and DDP
resistance in the 9 lung cancer cell lines (r=0.78; P=0.021). L-OHP
sensitivity was additionally detected in the aforementioned cell
lines and it was observed that the IC50 values for L-OHP
were increased in the cell lines with a higher expression level of
TCRP1 (Fig. 3D). A positive
correlation additionally existed between the mRNA expression of
TCRP1 and L-OHP resistance in the 9 lung cancer cell lines (r=0.7;
P=0.036). Thus, the results indicated that TCRP1 expression may be
associated with the primary resistance to DDP and L-OHP in lung
cancer cells.
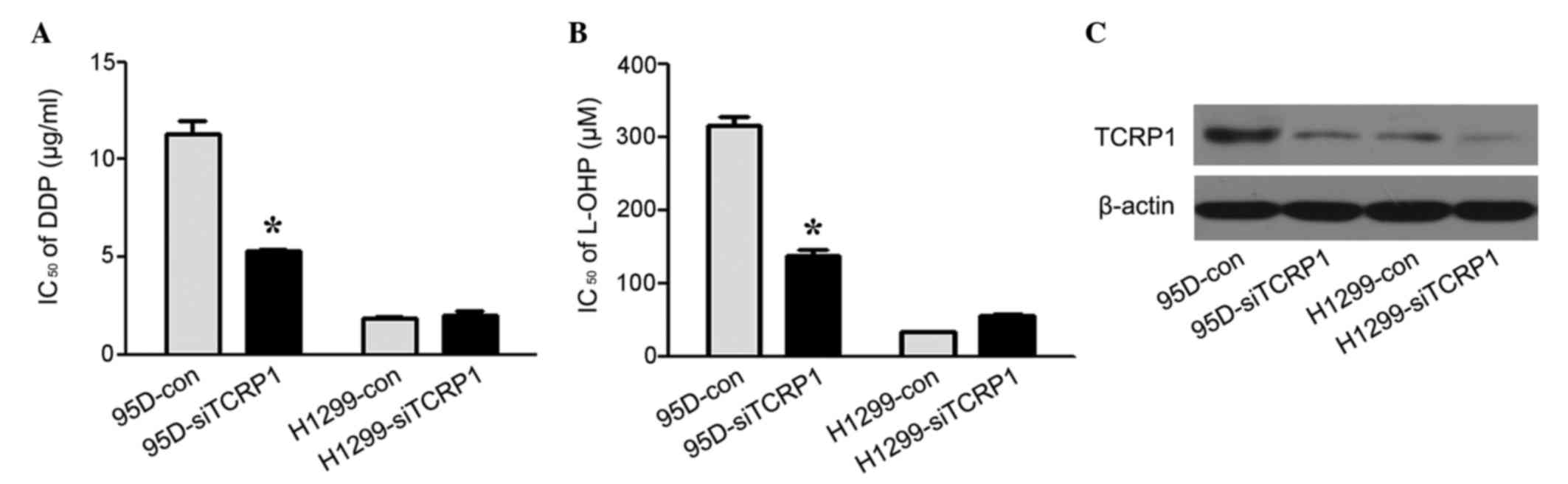
Primary platinum resistance in lung
cancer cells may be reversed by TCRP1-knockdown
To further investigate the role of TCRP1 in the
primary platinum resistance in lung cancer cells, the 95D and H1299
cell lines were selected as representatives of TCRP1-proficient and
TCRP1-deficient lung cancer cells, respectively. The
IC50 values for DDP and L-OHP in the 95D and H1299 cells
were determined following TCRP1-knockdown. As shown in Fig. 4A-C, knockdown of TCRP1 resulted in the
reversal of primary resistance to DDP and L-OHP in TCRP1-proficient
95D cells, while in TCRP1-deficient H1299 cells, no significant
changes in platinum resistance were observed with or without TCRP1
knockdown (P=0.210 and P=0.186, respectively). This suggested that
TCRP1 expression contributed to the primary resistance to platinum
in lung cancer cells.
Akt/NF-κB signaling pathway is
involved in TCRP1-mediated platinum resistance
Previously, we reported that the Akt/NF-κB signaling
pathway participated in TCRP1-associated DDP resistance in OSCC
cells (16). To provide additional
evidence that Akt/NF-κB mediated the platinum resistance of TCRP1
in lung and ovarian cancer cells, the expression of phosphorylated
(p)-Akt, p-NF-κB inhibitor α (IκBα) and Bclκ-2 was investigated
using western blotting following the knockdown of TCRP1 in COC1/DDP
and 95D cells (Fig. 5A). As the
activation of NF-κB requires removal of IκB by targeted
phosphorylation and subsequent degradation, the expression of
p-IκBα was assumed to represent the activity of NF-κB (22). Significant decreases in p-Akt, p-IκBα
and Bcl-2 were observed in TCRP1-knockdown cells compared with the
controls (Fig. 5A).
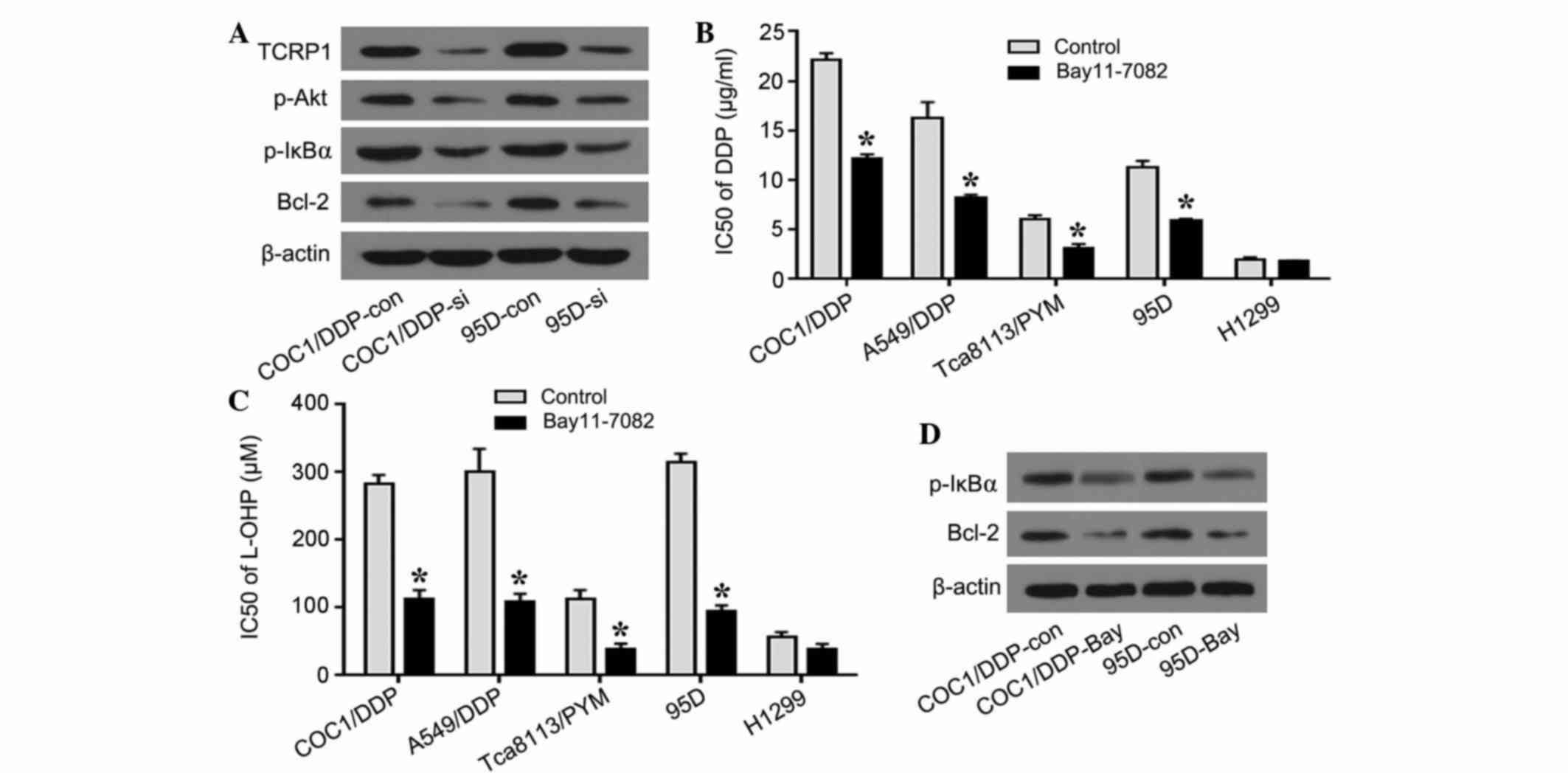 | Figure 5.Akt/NF-κB signaling pathway is
involved in TCRP1-mediated platinum resistance. (A) COC1/DDP and
95D cells were transfected with TCRP1 siRNA for 48 h and the
expression of p-Akt, p-IκBα and Bcl-2 was investigated using
western blotting analysis. (B) COC1/DDP, A549/DDP, Tca8113/PYM, 95D
and H1299 cells were treated with NF-κB inhibitor BAY 11–7082 for
24 h, and IC50 values for DDP were determined.
*P<0.05 vs. control. (C) Cells were treated with BAY 11–7082 and
the IC50 values for L-OHP were identified. *P<0.05
vs. control. (D) COC1/DDP and 95D cells were treated with BAY
11–7082 for 24 h and p-IκBα and Bcl-2 were tested using western
blotting analysis. NF, nuclear factor; TCRP1, tongue cancer
resistance-associated protein 1; DDP, cisplatin; L-OHP,
oxaliplatin; siRNA, small interfering RNA; si, siRNA-treated; con,
control; p, phosphorylated; IκBα, nuclear factor κB inhibitor α;
Bcl-2, B-cell lymphoma 2; PYM, pingyangmycin; IC50, half
maximal inhibitory concentration. |
To additionally investigate the functional effect of
NF-κB on platinum resistance, a platinum sensitivity assay was
performed by treatment with NF-κB inhibitor BAY 11–7082. In
acquired platinum resistance COC1/DDP, A549/DDP and Tca8113/PYM
cell lines, the cells treated with BAY 11–7082 exhibited increased
sensitivity to DDP, compared with control cells (P=0.043, P=0.036
and P=0.029, respectively). The IC50 value of 95D
primary platinum resistance cells for DDP was significantly reduced
following treatment with BAY 11–7082 compared with the controls
(P=0.033) (Fig. 5B). Similarly, as
shown in Fig. 5C, COC1/DDP, A549/DDP,
Tca8113/PYM, 95D and H1299 cell lines exhibited increased
sensitivity to L-OHP following treatment with BAY 11–7082, compared
with the controls (P=0.030, P=0.033, P=0.041 and P=0.037,
respectively). In addition, the expression of p-IκBα and Bcl-2 was
downregulated in the COC1/DDP and 95D cells following treatment
with NF-κB inhibitor, suggesting that the knockdown of TCRP1 and
the inhibition of NF-κB exhibited similar effects on the expression
of p-IκBα and Bcl-2 (Fig. 5D). The
results confirmed that TCRP1 was involved in the Akt/NF-κB
signaling pathway, and that the platinum resistance mediated by
TCRP1 may function at least partially via NF-κB and Bcl-2 in lung
and ovarian cancer cells.
Discussion
The present study reports that TCRP1 has a
significant role in platinum resistance in lung and ovarian cancer
cells. The results of the present study demonstrated that TCRP1
expression is associated with sensitivity to DDP and L-OHP in lung
and ovarian cancer cells with acquired platinum resistance, and
that the knockdown of TCRP1 increases the sensitivity to DDP and
L-OHP. It has additionally been identified that a positive
correlation exists between TCRP1 expression and primary resistance
to DDP and L-OHP in lung cancer cells. Furthermore, it was observed
that the Akt/NF-κB signaling pathway is involved in TCRP1-mediated
platinum resistance. Therefore, the results of the present study
suggest that TCRP1 may be a potential predictor for platinum
resistance in the treatment of lung and ovarian cancer.
Although DDP and L-OHP are two of the most commonly
used antitumor agents, resistance to platinum rapidly emerges
following commencement of treatment (23). Primary resistance and acquired
resistance are the two major forms of platinum resistance. A number
of studies have focused on various mechanisms of platinum
resistance (6). A serine/threonine
protein kinase p70 ribosomal S6 kinase was reported to contribute
to DDP resistance in lung cancer cells, and downregulation of this
gene may circumvent DDP resistance (24). Yamano et al (23) compared the gene expression profiles
between 3 DDP-sensitive OSCC cell lines and their relative
DDP-resistant cell lines, and identified 5 novel genes that may
have the potential for predicting the efficacy of DDP-based
chemotherapy against OSCC. ERCC1 has been reported to be positively
associated with the resistance to platinum-based chemotherapy in
various tumors (25,26). A microtubule-associated protein, Tau,
has additionally been identified as a potential predictive marker
in epithelial ovarian cancer patients treated with platinum and
paclitaxel (12). However, more
effective predictors of platinum resistance are required for the
improvement of the diagnosis and treatment of lung and ovarian
cancer.
Previously, a high expression level of TCRP1 has
been shown to cause DDP resistance in OSCC cells (15). In the present study, it was observed
that TCRP1 was highly expressed in acquired DDP-resistant lung
(A549/DDP) and ovarian cancer (COC1/DDP) cells. As platinum-based
agents are first-line clinical therapies in lung cancer patients,
and 20% of tumors have been reported to exhibit primary resistance
in clinical trials, the present study collected 8 distinct
histopathological subtypes of human lung cancer cell lines to
investigate the association between TCRP1 expression and primary
platinum resistance (27,28). The lung cancer cells that exhibited
higher expression levels of TCRP1 showed increased resistance to
DDP, and repression of TCRP1 expression contributed to the reversal
of resistance to DDP and L-OHP. A positive correlation was observed
between the expression of TCRP1 and the resistance to DDP in the
investigated lung cancer cell lines. This suggested that TCRP1 may
be a potential predictor of platinum resistance and its expression
may assist with the selection of cancer patients who will benefit
from platinum-based chemotherapy. Additional research is required
to validate the results of the present study in a larger patient
cohort.
Akt is a serine-threonine protein kinase that is
involved in cell growth, survival and proliferation (29). Activation of the Akt pathway has been
identified to be one of the key mediators of platinum resistance
(30). A previous study revealed that
the expression of Akt and NF-κB is reduced in Tca8113 cells treated
with TCRP1 siRNA (18). In the
present study, knockdown of TCRP1 expression contributed to
increased sensitivity to platinum agents and downregulated
expression of p-Akt, p-IκBα and Bcl-2 in TCRP1 proficient cells,
including COC1/DDP, A549/DDP, Tca8113/PYM and 95D cell lines. This
suggested that the Akt/NF-κB/Bcl-2 signaling pathway is involved in
TCRP1-mediated platinum resistance in lung and ovarian cancer
cells.
NF-κB is a transcriptional regulator and has
significant roles in the cell cycle, apoptosis and stress responses
(21). The activity of NF-κB is
inhibited by IκBα in the cytoplasm. Various intra- and
extracellular stimuli lead to the phosphorylation of IκBα, followed
by subsequent polyubiquitination and degradation. Activated NF-κB
translocates into the nucleus and stimulates gene expression
(31). In addition, constitutive
NF-κB expression has been reported to exert an antiapoptotic effect
on chemoresistance in cancer cells (32). NF-κB binds the multidrug resistance
protein 1 (MDR1) promoter and induces drug resistance via MDR1
expression (33). It is additionally
known that NF-κB blocks the cytotoxicity induced by antitumor drugs
via suppression of Bcl-2 phosphorylation (34). In the present study, knockdown of
TCRP1 led to reversal of platinum resistance and the downregulation
of p-IκBα. Therefore, the present study subsequently aimed to
elucidate the mechanisms underlying this reversal and investigate
whether NF-κB is involved in TCRP1-associated platinum resistance.
The compound BAY 11–7082 selectively blocks tumor necrosis
factor-α-induced phosphorylation of IκBα and inhibits the
activation of NF-κB (35). The
present study observed that the sensitivity to DDP and L-OHP was
markedly increased following treatment with BAY 11–7082 in
platinum-resistant cancer cells. The results of the present study
indicated that NF-κB may function as a downstream target protein
involved in TCRP1-mediated platinum resistance. However, this
observation requires further investigation in future studies.
Although previous research has focused on the
functioning of TCRP1, the mechanisms of TCRP1 involvement in
platinum resistance remain to be elucidated. One of our previous
studies revealed that TCPR1 mediated DDP resistance via increasing
the repair of DDP-induced DNA damage by preventing Pol β
degradation in lung cancer cells (19). In the present study, it was reported
that TCRP1 expression is associated with the resistance to DDP and
L-OHP in lung and ovarian cancer cells and that the Akt/NF-κB
signaling pathway is involved in the functioning of TCRP1. The
present study hypothesizes that there may be a functional link
between the Akt/NF-κB signaling pathway and Pol β expression, and
this interaction is currently under investigation.
In conclusion, the present study has shown that
TCRP1 is associated with the resistance to DDP and L-OHP in lung
and ovarian cancer cells, and that the Akt/NF-κB signaling pathway
is involved in the functioning of TCRP1. The results of the present
study suggest that TCRP1 may be a potential predictor of platinum
resistance, and a novel target for the prevention and reversal of
platinum resistance in the treatment of lung and ovarian
cancer.
Acknowledgements
The present study was supported by research grants
from the Shenzhen Scientific and Technological Project (grant no.
JCYJ20150403100317057), National Natural Science Foundation of
China (grant nos. 30873088 and 81472184), the Natural Science
Foundation of Guangdong Province (grant no. S2012010008995) and the
Doctoral Fund of the Education Ministry of China (grant no.
20124423110003).
References
|
1
|
Liu C, Xu X and Zhou Y: Association
between EGFR polymorphisms and the risk of lung cancer. Int J Clin
Exp Pathol. 8:15245–15249. 2015.PubMed/NCBI
|
|
2
|
Narod S: Can advanced-stage ovarian cancer
be cured? Nat Rev Clin Oncol. Jan 20–2016.(Epub ahead of print).
View Article : Google Scholar
|
|
3
|
Ferlay J, Soerjomataram I, Ervik M,
Dikshit R, Eser S, Mathers C, Rebelo M, Parkin DM, Forman D and
Bray F: GLOBOCAN 2012 v1.0, Cancer Incidence and Mortality
Worldwide: IARC CancerBase No. 11 [Internet]International Agency
for Research on Cancer; Lyon, France: 2013, http://globocan.iarc.frAccessed May 9, 2016.
|
|
4
|
Shen DW, Pouliot LM, Hall MD and Gottesman
MM: Cisplatin resistance: A cellular self-defense mechanism
resulting from multiple epigenetic and genetic changes. Pharmacol
Rev. 64:706–721. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Macedo-Pérez EO, MoralesOyarvide V,
Mendoza-García VO, Dorantes-Gallareta Y, Flores-Estrada D and
Arrieta O: Long progression-free survival with first-line
paclitaxel plus platinum is associated with improved response and
progression-free survival with second-line docetaxel in advanced
non-small-cell lung cancer. Cancer Chemother Pharmacol. 74:681–690.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Siddik ZH: Cisplatin: Mode of cytotoxic
action and molecular basis of resistance. Oncogene. 22:7265–7279.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Fikrova P, Stetina R, Hrnciarik M,
Hrnciarikova D, Hronek M and Zadak Z: DNA crosslinks, DNA damage
and repair in peripheral blood lymphocytes of non-small cell lung
cancer patients treated with platinum derivatives. Oncol Rep.
31:391–396. 2014.PubMed/NCBI
|
|
8
|
Galluzzi L, Senovilla L, Vitale I, Michels
J, Martins I, Kepp O, Castedo M and Kroemer G: Molecular mechanisms
of cisplatin resistance. Oncogene. 31:1869–1883. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Raymond E, Faivre S, Chaney S, Woynarowski
J and Cvitkovic E: Cellular and molecular pharmacology of
oxaliplatin. Mol Cancer Ther. 1:227–235. 2002.PubMed/NCBI
|
|
10
|
Montagnani F, Turrisi G, Marinozzi C,
Aliberti C and Fiorentini G: Effectiveness and safety of
oxaliplatin compared to cisplatin for advanced, unresectable
gastric cancer: A systematic review and meta-analysis. Gastric
Cancer. 14:50–55. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Lowery WJ, Lowery AW, Barnett JC,
LopezAcevedo M, Lee PS, Secord AA and Havrilesky L:
Cost-effectiveness of early palliative care intervention in
recurrent platinum-resistant ovarian cancer. Gynecol Oncol.
130:426–430. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Smoter M, Bodnar L, Grala B, Stec R,
Zieniuk K, Kozlowski W and Szczylik C: Tau protein as a potential
predictive marker in epithelial ovarian cancer patients treated
with paclitaxel/platinum first-line chemotherapy. J Exp Clin Cancer
Res. 32:252013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao H, Bi T, Qu Z, Jiang J, Cui S and
Wang Y: Expression of miR-224-5p is associated with the original
cisplatin resistance of ovarian papillary serous carcinoma. Oncol
Rep. 32:1003–1012. 2014.PubMed/NCBI
|
|
14
|
Kuhlmann JD, Wimberger P, Bankfalvi A,
Keller T, Schöler S, Aktas B, Buderath P, Hauch S, Otterbach F,
Kimmig R and Kasimir-Bauer S: ERCC1-positive circulating tumor
cells in the blood of ovarian cancer patients as a predictive
biomarker for platinum resistance. Clin Chem. 60:1282–1289. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gu Y, Fan S, Xiong Y, Peng B, Zheng G, Yu
Y, Ouyang Y and He Z: Cloning and functional characterization of
TCRP1, a novel gene mediating resistance to cisplatin in an oral
squamous cell carcinoma cell line. FEBS Lett. 585:881–887. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Peng B, Gu Y, Xiong Y, Zheng G and He Z:
Microarray-assisted pathway analysis identifies MT1X & NFκB as
mediators of TCRP1-associated resistance to cisplatin in oral
squamous cell carcinoma. PLoS One. 7:e514132012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Peng B, Yi S, Gu Y, Zheng G and He Z:
Purification and biochemical characterization of a novel
protein-tongue cancer chemotherapy resistance-associated protein1
(TCRP1). Protein Expr Purif. 82:360–367. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Gu Y, Fan S, Liu B, Zheng G, Yu Y, Ouyang
Y and He Z: TCRP1 promotes radioresistance of oral squamous cell
carcinoma cells via Akt signal pathway. Mol Cell Biochem.
357:107–113. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Liu X, Wang C, Gu Y, Zhang Z, Zheng G and
He Z: TCRP1 contributes to cisplatin resistance by preventing Pol β
degradation in lung cancer cells. Mol Cell Biochem. 398:175–183.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zheng G, Zhou M, Ou X, Peng B, Yu Y, Kong
F, Ouyang Y and He Z: Identification of carbonic anhydrase 9 as a
contributor to pingyangmycin-induced drug resistance in human
tongue cancer cells. FEBS J. 277:4506–4518. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Voland C, Bord A, Péleraux A, Pénarier G,
Carrière D, Galiègue S, Cvitkovic E, Jbilo O and Casellas P:
Repression of cell cycle-related proteins by oxaliplatin but not
cisplatin in human colon cancer cells. Mol Cancer Ther.
5:2149–2157. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jobin C and Sartor RB: The IκB/NF-κB
system: A key determinant of mucosal inflammation and protection.
Am J Physiol Cell Physiol. 278:C451–C462. 2000.PubMed/NCBI
|
|
23
|
Yamano Y, Uzawa K, Saito K, Nakashima D,
Kasamatsu A, Koike H, Kouzu Y, Shinozuka K, Nakatani K, Negoro K,
et al: Identification of cisplatin-resistance related genes in head
and neck squamous cell carcinoma. Int J Cancer. 126:437–449. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Dhar R and Basu A: Constitutive activation
of p70 S6 kinase is associated with intrinsic resistance to
cisplatin. Int J Oncol. 32:1133–1137. 2008.PubMed/NCBI
|
|
25
|
Lee SH, Noh KB, Lee JS, Lee EJ, Min KH,
Hur GY, Lee SH, Lee SY, Kim JH, Lee SY, et al: Thymidylate synthase
and ERCC1 as predictive markers in patients with pulmonary
adenocarcinoma treated with pemetrexed and cisplatin. Lung Cancer.
81:102–108. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Steffensen KD, Waldstrøm M and Jakobsen A:
The relationship of platinum resistance and ERCC1 protein
expression in epithelial ovarian cancer. Int J Gynecol Cancer.
19:820–825. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Florea AM and Büsselberg D: Cisplatin as
an anti-tumor drug: Cellular mechanisms of activity, drug
resistance and induced side effects. Cancers (Basel). 3:1351–1371.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Haslehurst AM, Koti M, Dharsee M, Nuin P,
Evans K, Geraci J, Childs T, Chen J, Li J, Weberpals J, et al: EMT
transcription factors snail and slug directly contribute to
cisplatin resistance in ovarian cancer. BMC Cancer. 12:912012.
View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Cao H, Zhu K, Qiu L, Li S, Niu H, Hao M,
Yang S, Zhao Z, Lai Y, Anderson JL, et al: Critical role of AKT
protein in myeloma-induced osteoclast formation and osteolysis. J
Biol Chem. 288:30399–30410. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Stronach EA, Chen M, Maginn EN, Agarwal R,
Mills GB, Wasan H and Gabra H: DNA-PK mediates AKT activation and
apoptosis inhibition in clinically acquired platinum resistance.
Neoplasia. 13:1069–1080. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Oh H and Ghosh S: NF-κB: Roles and
regulation in different CD4(+) T-cell subsets. Immunol Rev.
252:41–51. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Arlt A, Gehrz A, Müerköster S, Vorndamm J,
Kruse ML, Fölsch UR and Schäfer H: Role of NF-κB and Akt/PI3K in
the resistance of pancreatic carcinoma cell lines against
gemcitabine-induced cell death. Oncogene. 22:3243–3251. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bentires-Alj M, Barbu V, Fillet M, Chariot
A, Relic B, Jacobs N, Gielen J, Merville MP and Bours V: NF-κB
transcription factor induces drug resistance through MDR1
expression in cancer cells. Oncogene. 22:90–97. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Pham CG, Bubici C, Zazzeroni F, Knabb JR,
Papa S, Kuntzen C and Franzoso G: Upregulation of Twist-1 by NF-κB
blocks cytotoxicity induced by chemotherapeutic drugs. Mol Cell
Biol. 27:3920–3935. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Sun H, Wu Y, Fu D, Liu Y and Huang C:
SIRT6 regulates osteogenic differentiation of rat bone marrow
mesenchymal stem cells partially via suppressing the nuclear
factor-κB signaling pathway. Stem Cells. 32:1943–1955. 2014.
View Article : Google Scholar : PubMed/NCBI
|