Introduction
Derived from neural crest cells, malignant melanoma
(MM) accounts for ~1.5% of all tumors and is the cutaneous
malignancy with the highest mortality rate (1). There are >10 million new cases of MM
diagnosed every year (1). In total,
~21% of MM patients present with focal skin metastasis and 50% with
regional lymph node metastasis (1).
MM patients with distant metastasis (stage IV) usually have poor
prognoses, with a median survival time of 5–8 months, and the
≥5-year survival rate is <2% (2).
With social and economic development, the incidence of MM is
increasing each year (3).
The poor prognosis of MM patients is reflective of
the lack of an effective therapy. Systemic chemotherapy, including
mono- and poly-chemotherapies, immunomodulatory therapies and
vaccination therapy with dendritic cells (DCs) or genetically
modified tumor cells, remains controversial (2). Although there is evidence showing that
the combined use of interleukin (IL)-2, interferon (IFN) and
chemotherapeutics may achieve a response rate as high as 64%
(4,5),
the efficacy of systemic therapy remains disappointing; systemic
monotherapy usually achieves a clinical response rate of <15%
(6–10). However, prospective, randomized
clinical trials have shown no evidence that other drugs are
superior to dacarbazine (DTIC) for MM patients (10). Thus, it is imperative to identify an
alternative therapeutic strategy to improve the prognosis of
metastatic MM patients. Although MM is highly malignant and
insensitive to chemotherapy and radiotherapy, it maintains high
immunogenicity (11). Thus, immune
therapy may serve as a strategy for advanced MM. However, a
hapten-modified cellular vaccine for melanoma, MVAX®,
has stalled in clinical development due to manufacturing and
regulatory problems (11).
Dinitrophenyl (DNP) is a classic hapten used to
induce contact-related delayed-type hypersensitivity (DTH) due to
its potent antigenicity and high absorption in healthy skin. DTH is
an allergen-induced, T cell-mediated immune response that can
evaluate the cellular immunity in humans (12). However, clinical studies (11,13) have
shown the limited success of immune therapy, which is partially
ascribed to the limited availability of polypeptides that restrict
major histocompatibility complex (MHC) class II presentation and
subsequent induction of tumor-specific cluster of differentiation
(CD)4+ T-cells. In addition to suppression of antigen
presentation as well as secretion of immunosuppressive molecules by
tumor cells, regulatory T cells (Tregs) in the tumor may also
inhibit the antitumor immune response (14,15),
representing a major barrier in antitumor immune therapy (16). Tregs may inhibit the proliferation of
effector T cells, including CD4+CD25− T cells
and CD8+ cytotoxic T lymphocytes, suppress the
maturation and antigen presentation of dendritic cells, and alter
cytokine levels (17,18). Certain studies have identified
increased peripheral Treg levels in patients with metastatic MM
(19–23); therefore, therapies that decrease Treg
levels may be beneficial for these patients.
The energy emitted by a laser may be absorbed by
tissues and transformed into heat, resulting in the release and
presentation of tumor antigens, and a subsequent antitumor immune
response (11). Therefore, the
present study tested the hypothesis that MM patients receiving
localized immunotherapy to induce DTH via DNP in combination with
laser therapy would have improved disease-related outcomes as
compared with patients treated with DNP alone. In addition to
chemotherapy, MM patients received DTH alone or with concomitant
laser therapy, and the extent of DTH, as well as the overall
survival (OS) times of the patients, were determined. In addition,
the levels of peripheral CD4+CD25+ Tregs,
IFN-γ-producing CD8+ T cells and CD4+ cells,
IL-10, and tumor growth factor (TGF)-β were detected. The
combination of immunotherapy with laser therapy may represent a
novel therapeutic strategy for patients with unresectable, advanced
MM.
Materials and methods
Patients
A total of 72 patients with stage III (b or c) or IV
(unresectable) MM, according to the American Joint Committee on
Cancer staging system (24), were
recruited from the First Affiliated Hospital of the Chinese
People's Liberation Army (PLA) General Hospital (Beijing, China)
between February 2008 and March 2012 (Table I). The following inclusion criteria
were employed: i) A pathological diagnosis of MM; ii) normal liver
and kidney function, as well as normal results from a routine blood
test; iii) a Karnofsky score of ≥60 (25); iv) an estimated survival time of >3
months; and v) the presence of unresectable MM (cutaneous MM with
local or distant metastasis). Therapeutic efficacy was evaluated
with the Response Evaluation Criteria in Solid Tumors (26). The present study conforms to the
provisions of the Declaration of Helsinki (2000 revision). Informed
consent was obtained from each patient, and the study was approved
by the Ethics Committee of the First Affiliated Hospital of the
Chinese PLA General Hospital. The clinicaltrial.gov identifier number of the study is
NCT02372708.
 | Table I.Descriptive statistics of demographic
and clinical characteristics in 72 patients with malignant melanoma
of the skin treated with monotherapy (n=36) or combination therapy
(n=36). |
Table I.
Descriptive statistics of demographic
and clinical characteristics in 72 patients with malignant melanoma
of the skin treated with monotherapy (n=36) or combination therapy
(n=36).
| Characteristic | DNP, n (%) | DNP+laser therapy,
n (%) | P-value |
|---|
| Age, years |
|
| 0.448 |
|
≤70 | 26 (72.2) | 23 (63.9) |
|
|
>70 | 10 (27.8) | 13 (36.1) |
|
| Gender |
|
| 0.098 |
|
Male | 23 (63.9) | 16 (44.4) |
|
|
Female | 13 (36.1) | 20 (55.6) |
|
| Tumor
stagea |
|
| 0.409 |
|
IIIb | 11 (30.6) | 9
(25.0) |
|
|
IIIc | 7
(19.4) | 12 (33.3) |
|
| IV | 18 (50.0) | 15 (41.7) |
|
| Location of primary
lesion |
|
| 0.633 |
|
Skin | 20 (55.6) | 22 (61.1) |
|
|
Viscera | 16 (44.4) | 14 (38.9) |
|
Prior to immunotherapy, the medical history of each
patient was completely reviewed, a physical examination was
performed, ultrasonography was undertaken to examine the lymph
nodes of the groin, armpits and neck, and cranial magnetic
resonance imaging and chest/abdominal computed tomography were
performed. The patients were re-examined at 3 months post-therapy,
and subsequent evaluations were performed once every 3 months
thereafter. Adverse events were graded according to the criteria
described in the Common Terminology Criteria for Adverse Events,
version 4.0 (27).
Treatment. All the patients received DTIC-based
chemotherapy. Once all the physical and laboratory examinations
were performed, treatment with DTIC (200 mg/m2; Nanjing
Pharmaceutical Factory Co., Ltd., Nanjing, China) was initiated and
performed once every 3 weeks for a total of 5 courses. When disease
progression became evident, the therapy was discontinued. When
stable disease or disease improvement was observed, the
chemotherapy was continued. Chemotherapy was administered over a
median of 5 courses (range, 2–36 courses).
In the combination therapy group, 36 patients
received DNP (Sinopharm Chemical Reagent Co., Ltd., Shanghai,
China) with focal laser therapy. For the combination therapy group,
diluted DNP-vaseline (2% DNP in 0.1 ml vaseline) was applied on the
primary or metastatic lesions on the first day after chemotherapy
in each course, and the lesions were concurrently irradiated with a
laser for 10 min at 1 W/cm2 and then dressed. After 2
days, the presence of contact dermatitis was confirmed. If the
lymph nodes were resected, sensitization was performed at the
occipital region (2×2-cm area) once weekly (28). In the control group, 36 patients
received DNP alone. Monotherapy consisted of the application of
DNP-vaseline on the lesions; the remaining treatments were similar
to the combination therapy group.
Prior to chemotherapy and at 2, 5, 10 and 20 days
after in situ immunization, fasting venous blood was
collected (10 ml) from all the patients and divided into two parts.
One part was centrifuged (600 × g; 5 min; 4°C), and the
resultant serum was collected and stored at −20°C for the detection
of cytokines; the other part was anti-coagulated with heparin, and
peripheral blood mononuclear cells (PBMCs) were separated by
density gradient separation with lymphocyte separation medium
(Sinopharm Chemical Reagent Co., Ltd.). The PBMCs were counted, and
cell density was adjusted to 1–2×106 cells/ml and
analyzed by flow cytometry.
Flow cytometry
Using PBMCs, CD8+ and CD4+ T
cells were screened separately with 10 µl/107 cells of
anti-CD8 monoclonal antibody magnetic Dynabeads (Life Technologies;
Thermo Fisher Scientific, Inc., Waltham, MA, USA) and anti-CD4
monoclonal antibody magnetic MicroBeads (Miltenyi Biotec GmbH,
Bergisch Gladbach, Germany). Once the beads were removed, the
CD8+ and CD4+ T cells were blocked using
blocking reagents (eBioscience, Inc., San Diego, CA, USA) for 30
min at 4°C, and independently labeled with 10 µl of each antibody
against CD4-fluorescein isothiocyanate (FITC) (mouse monoclonal;
catalogue no. 11-0047-42), CD8-FITC (mouse monoclonal; catalogue
no. 11-0088-42) and IFN-γ-phycoerythrin (PE) (mouse monoclonal;
catalogue no. 12-7319-42) (eBioscience, Inc.) in 1 ml of staining
buffer (1:100 dilution; eBioscience, Inc.). Labeled cells were
detected and analyzed by a FACSCanto analyzer (BD Biosciences,
Franklin Lakes, NJ, USA).
For the analysis of Tregs, the PBMCs (100 µl) were
also mixed with 10 µl of FITC-conjugated mouse anti-human CD4
(eBioscience, Inc.) or PE-conjugated CD25 (eBioscience, Inc.)
antibodies, followed by incubation in the dark for 30 min at 4°C
Upon washing in PBS twice, 500 µl of PBS was added, and
CD4+CD25+ Tregs were detected by flow
cytometry.
ELISA
ELISA was performed to detect the serum levels of
IL-10 (IL-10 ELISA kit; R&D Systems, Inc., Minneapolis, MN,
USA), TGF-β1 and TGF-β2 (TGF-β1/β2 ELISA kit; R&D Systems,
Inc.) according to the manufacturer's protocol.
Antibody titration assay
Blood samples (5 ml) were collected prior to
immunization to establish background antibody levels, and
thereafter they were collected every 2 weeks. After the blood
samples were allowed to clot at room temperature for ≤1 h, they
were then centrifuged at 12,000 × g for 5 min. Serum
fractions were collected and diluted 10-fold in PBS. Anti-DNP
immunoglobulin G titers were determined by ELISA, as previously
described (18). Absorbance was
measured at 492 nm using a 3550 microplate reader (Bio-Rad
Laboratories, Inc, Hercules, CA, USA). Best-fit sigmoidal curves
were obtained from plotting the absorbance vs. the logarithmic
dilution factors using GraphPad Prism 4 (GraphPad Software, Inc.,
La Jolla, CA, USA). Titer levels were obtained as half maximal
effective concentration values from the midpoint of each sigmoidal
curve.
DTH scoring
DTH was assessed as previously described (29). After DNP application, swelling and
even blistering was observed 12–24 h later, but it resolved within
2–5 days. When swelling was present again 1–2 weeks later, an
allergy was suggested, and the swelling was scored as ++++
(diameter, ≥20 mm) or +++ (diameter, ≥15-<20 mm). In the event
that there was no response within 1–2 weeks, 50 µg DNP was
reapplied to the lesion, and the response was observed 24 h later.
Swelling was scored as ++ (diameter, ≥10- <15 mm), + (diameter,
<10 mm) or negative (in the absence of swelling).
Statistical analysis
Baseline demographic and clinical characteristics
are presented as a number and percentage. χ2 tests were
used to examine the associations between baseline characteristics
and treatment groups. As blood samples were collected at baseline
(day 0) and on days 2, 5, 10 and 20, a mixed-effects model was used
to evaluate group and time effects on changes in the levels of T
cells and inhibitory cytokines by taking into account the repeated
measures design. Post-hoc multiple comparisons were made between
groups and time points with Bonferroni correction.
OS time was defined as the time elapsed from the
date of surgery to the date of mortality. Disease-free survival
(DFS) time was defined as the time elapsed from the date of surgery
to the date of the first swelling/blistering. To evaluate
differences in OS time and DFS time between treatment and DTH
response groups, Kaplan-Meier survival analyses were performed.
Log-rank tests were conducted to examine differences in OS time and
DFS time between the groups. All statistical analyses were
performed using SPSS statistical software version 22 for Windows
(IBM SPSS, Armonk, NY, USA). A two-tailed P-value of <0.05 was
considered to indicate a statistically significant difference.
Results
Baseline demographics and clinical
characteristics
There were 36 patients in the treatment group (23
male cases and 13 female cases) and 36 cases in the control group
(16 male cases and 20 female cases). The mean age of the patients
was 60.0 years (range, 29–85 years). As shown in Table I, the majority of the patients were
<70 years old (72.2 and 63.9% for the DNP monotherapy and
combination therapy groups, respectively), and ~1/2 of the patients
were diagnosed with stage IV melanoma. All demographic and clinical
characteristics were comparable between the monotherapy control and
combination treatment groups (all P>0.05; Table I).
Effects of immunotherapy combined with
laser therapy on peripheral Treg levels
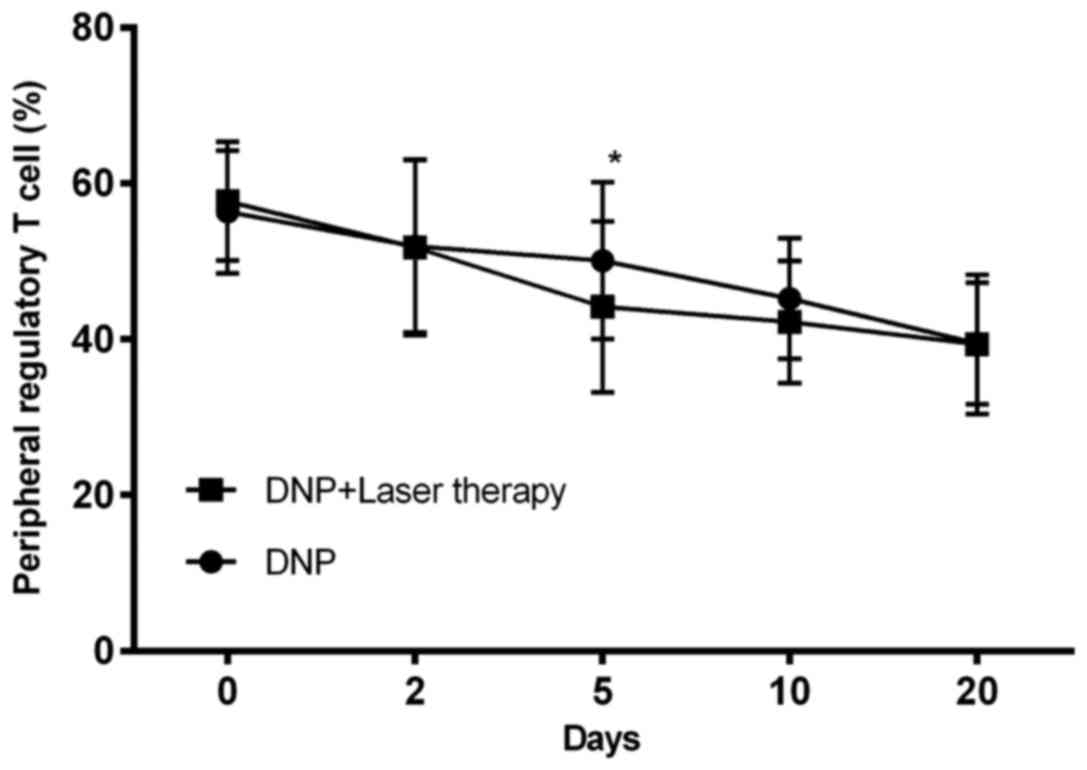
As shown in Fig. 1,
the levels of peripheral Tregs significantly decreased over time in
each group (P<0.001). However, there was no significant
difference between the combination therapy groups after considering
repeated measurements across time (P=0.098).
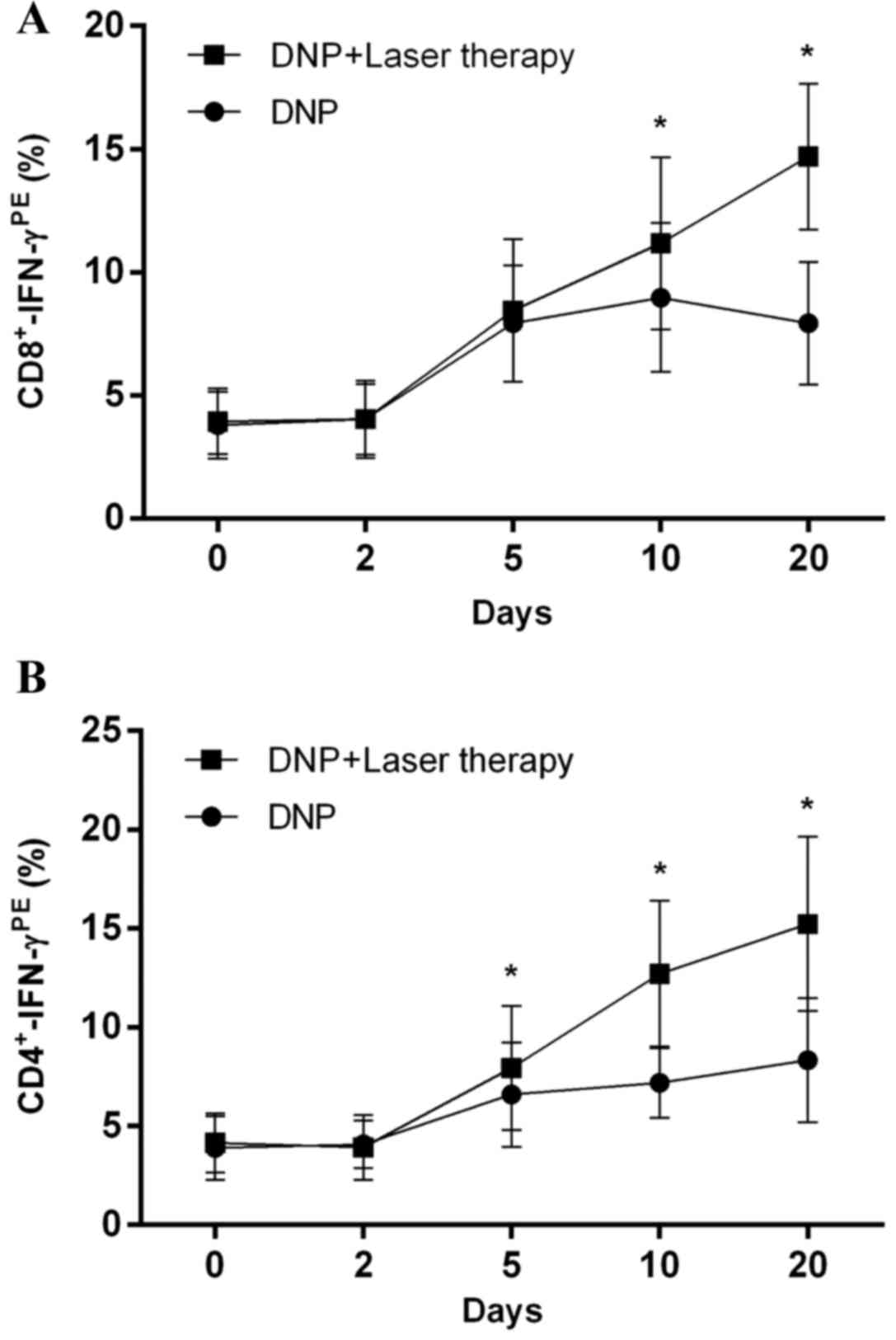
Combination therapy increases IFN-γ
secretion by T cells and significantly reduces inhibitory cytokine
levels
As shown in Fig. 2,
patients receiving combination treatment exhibited significantly
higher IFN-γ production by CD8+ T cells [F (1,70)=56.00;
P<0.001] and CD4+ T cells [F (1,70)=86.79,
P<0.001] than patients receiving DNP monotherapy. Post-hoc
multiple comparisons revealed that IFN-γ secretion by
CD8+ T cells significantly increased in the combination
therapy group at days 10 and 20, compared with control group (both
P<0.001; Fig. 2A). Similar results
were observed with CD4+ T cells at days 5, 10 and 20
(P=0.036, P<0.001 and P<0.001, respectively; Fig. 2B).
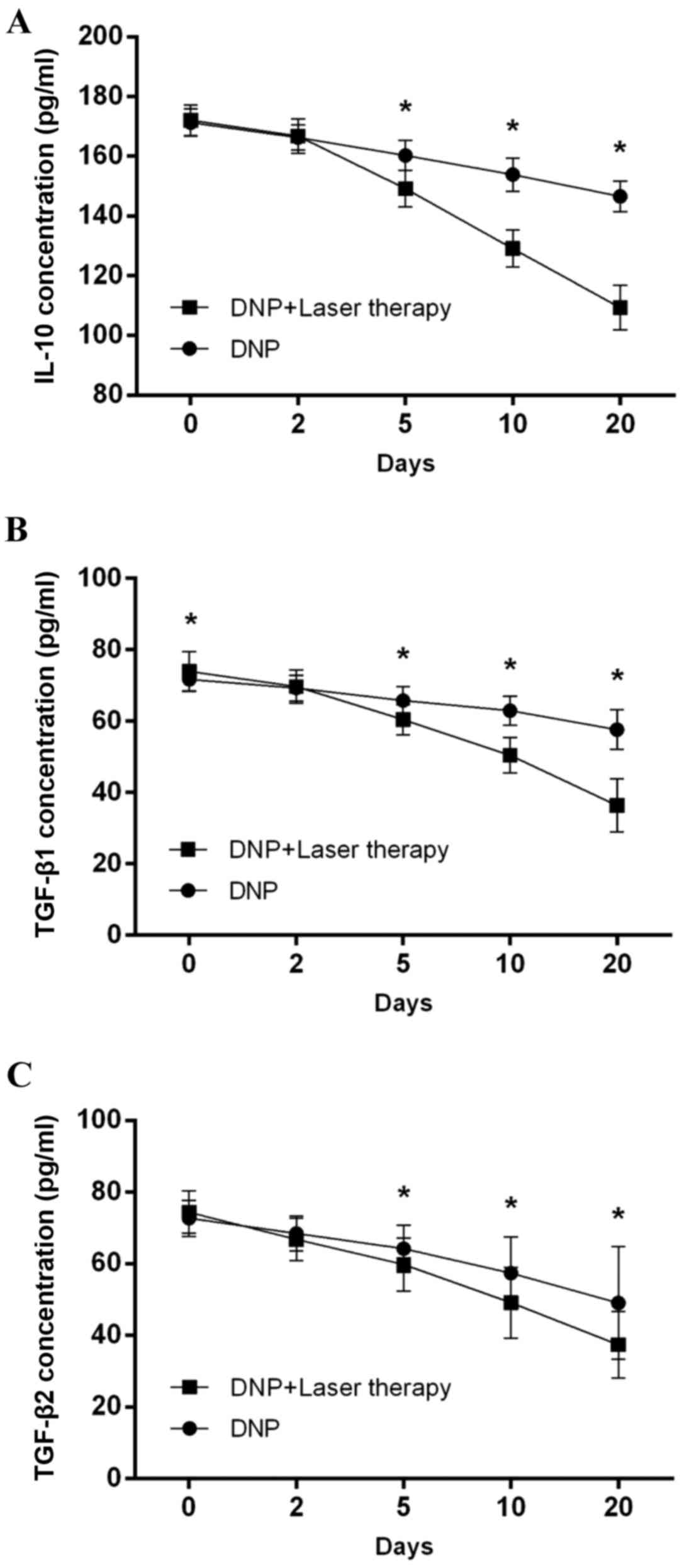
Compared with patients receiving DNP monotherapy,
those receiving combination therapy exhibited a significant
reduction in the levels of IL-10 [(F(1,70)=341.87, P<0.001;
Fig. 3A], TGF-β1 [F(1,70)=75.33,
P<0.001; Fig. 3B] and TGF-β2 [F
(1,70)=7.65, P=0.007; Fig. 3C], as
indicated in Fig. 3.
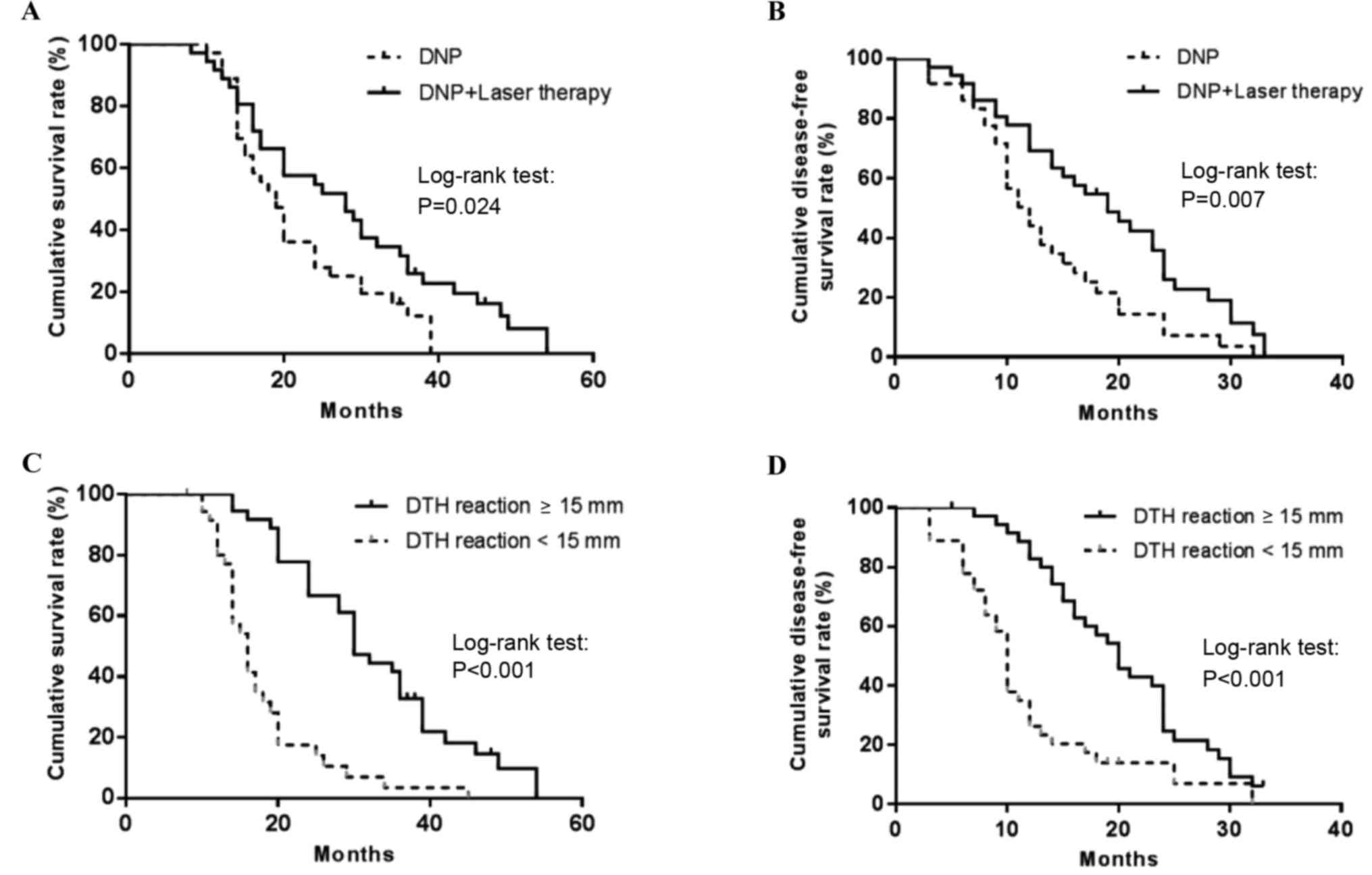
Comparisons of survival rates by
treatment and DTH response
The 3-year OS rate was 12.2% in patients receiving
DNP and 25.9% in those receiving combination therapy, with a median
survival time of 19.0 and 28.0 months, respectively. Survival time
is considered the equivalent of follow-up time. Kaplan-Meier
analysis of survival rates revealed that the patients in the
combination treatment group experienced significantly longer OS
times than those in the monotherapy group (P=0.024; Fig. 4A). Furthermore, the 1-year DFS rate
was 44.0 and 69.1% in the monotherapy and combination therapy
treatment groups, respectively. Log-rank analysis revealed that the
patients in the combination therapy group experienced a longer DFS
time than those in the monotherapy group (19.0 vs. 12.0 months,
respectively; P=0.007; Fig. 4B).
Patients with a DTH response of ≥15 mm experienced a
longer OS time than patients with a DTH response of <15 mm (30.0
vs. 16.0 months, respectively; P<0.001; Fig. 4C). In addition, log-rank analysis
revealed that patients with a DTH response of <15 mm experienced
a longer DFS time than those with a DTH response of <15 mm (20.0
vs. 10.0 months, respectively; P=0.001; Fig. 4D).
Evaluation of safety
No severe adverse events were observed in any of the
72 patients enrolled in the present study. The most common side
effects included a low-grade fever and fatigue (Table II). Thus, in situ
immunotherapy with DNP in combination with laser therapy
demonstrated favorable efficacy and an acceptable safety
profile.
 | Table II.Adverse events in 72 patients with
malignant melanoma treated with dinitrophenyl alone or in
combination with laser therapy. |
Table II.
Adverse events in 72 patients with
malignant melanoma treated with dinitrophenyl alone or in
combination with laser therapy.
|
| Grade, n |
|---|
|
|
|
|---|
| Adverse events | 0 | 1 | 2 | 3 | 4 |
|---|
| Fever | 56 | 10 | 4 | 2 | 0 |
| Fatigue | 49 | 16 | 5 | 2 | 0 |
| Neutropenia | 65 | 4 | 3 | 0 | 0 |
| Nausea,
vomiting | 56 | 12 | 4 | 0 | 0 |
| Diarrhea | 67 | 5 | 0 | 0 | 0 |
| Epistaxis | 70 | 1 | 1 | 0 | 0 |
| Hemoptysis or
hemafecia | 72 | 0 | 0 | 0 | 0 |
| Increase in blood
pressure | 59 | 13 | 0 | 0 | 0 |
| Anemia | 66 | 6 | 0 | 0 | 0 |
Discussion
For the majority of patients with stage IV MM, the
survival time is ≤1 year after diagnosis (29) due in large part to the lack of an
effective therapy. As MM maintains high immunogenicity (11), the present study aimed to compare the
clinical outcomes in MM patients receiving localized immunotherapy
to induce DTH via DNP in combination with laser therapy against
those observed in patients treated with DNP alone. DNP monotherapy
and combination therapy decreased Treg levels over time to a
similar extent. However, patients receiving the combination
treatment exhibited significantly higher IFN-γ production by
CD8+ and CD4+ T cells, and significantly
reduced levels of IL-10, TGF-β1 and TGF-β2 than the control group.
Furthermore, the patients in the combination treatment group
experienced significantly longer OS and DFS times than the control
group. Given that no severe adverse events were observed in either
treatment group, which is consistent with our previous study
(30), DNP treatment combined with
laser immunotherapy may represent a novel therapeutic strategy for
advanced MM. These results are consistent with other previous
studies, which reported that laser immunotherapy in combination
with immune adjuvant therapy could achieve strong immune responses
in MM and breast cancer patients (31–33).
Terheyden et al (34) used dinitrochlorobenzene to induce
contact dermatitis, which in combination with DTIC therapy, was
used to treat metastatic MM. The objective response rate was 62% in
patients with stage III MM (n=39) and 9% in those with stage IV MM
(n=33), and >50% of patients remained progression-free for 1
year regardless of the stage of MM (35). In the present study, DNP hapten was
used to induce DTH through DNP-mediated activation of T cells. MHC
presentation of the hapten by antigen-presenting cells can also
induce the production of tumor antigens, which may cause
cross-antigen presentation (12).
Subsequent hapten-induced presentation of tumor antigens can be
recognized by the immune system (13). Cutaneous DTH could be used as an
indicator to evaluate immune therapy (35), as well as being a predictor of
survival (36). In 27 patients with
stage IV MM receiving a DC vaccine to induce DTH, the median
survival time was 22.9 months (n=19) in DTH-positive patients and
4.8 months in DTH-negative patients (37). Similarly, in the present study, the
extent of the DTH reaction was associated with OS and DFS. By
contrast, in a study of 284 melanoma patients treated with an
autologous tumor cell vaccine in combination with DNP, a positive
DTH response (≥5 mm) was not associated with the number of living
melanoma cells, but with the number of dead tumor cells (36). Thus, further studies will determine if
combination therapy is associated with tumor cell death.
Along with the extent of DTH, combination therapy
was associated with increased OS and DSF times in the present
study. As local radiotherapy is associated with the regression of
metastatic cancer at a distance from the irradiated site, a
phenomenon called the abscopal effect may be observed, which may be
mediated by a systemic immune response (38). Further studies will assess whether the
increased OS and DFS times in patients receiving combination
therapy were associated with reduced metastasis.
The extent of DTH induced by chemoimmunotherapy is
also consistent with an increase in the proportion of
CD8+-IFN-γ+ cells (39,40). In
the present study, increased proportions of IFN-γ-producing
CD8+ and CD4+ T cells were observed over time
with each treatment; however, the proportions were significantly
greater in the patients receiving combination therapy. These
results suggested that the extent to which DTH was induced was
greater with combination therapy than with monotherapy.
Tregs have an essential role in sustaining
self-tolerance and immune homeostasis by suppressing a number of
physiological and pathological immune responses, including those in
the tumor microenvironment (19). In
the present study, the levels of peripheral
CD4+CD25+ Tregs decreased in the two
treatment groups; however, no differences between the groups were
noted over time. The decrease in Treg levels coincided with reduced
serum levels of inhibitory cytokines, including IL-10, TGF-β1 and
TGF-β2. Furthermore, the suppression of these cytokines was
significantly greater in patients receiving combination therapy at
days 5, 10 and 20. These results are consistent with those reported
for patients treated with a vaccine consisting of melanoma cells
with DNP, in which OS time was reduced in patients with high IL-10
levels in the tumor microenvironment (41).
The present study is limited by its relatively small
sample size. Therefore, additional studies with larger numbers of
patients and studies taking place at different institutions are
necessary to confirm the results of the present study. In addition,
the patients in the present study were sensitized to DNP via its
topical application; therefore, further studies assessing the
possible additive effects of using laser therapy with a previously
described DNP-modified melanoma cell vaccine are necessary
(33,40). Finally, the mechanism by which
combination therapy enhances OS and DFS was not examined. It is
possible that the localized treatment impacted the incidence of
metastasis or reduced metastatic lesions through the abscopal
effect, which will be analyzed in further studies (38,42).
Taken together, the present results indicate that
the cutaneous application of DNP may induce a highly specific
systemic immune response that can be enhanced with laser therapy.
This immune response may reduce the incidence of distant
metastases, which may improve the survival time of MM patients.
Thus, given that no severe adverse events were observed in either
treatment group, DNP treatment combined with laser immunotherapy
may represent a novel therapeutic strategy for advanced MM.
Acknowledgements
The present study was supported in part by grants
from the Beijing Municipal Commission of Science and Technology
(no. 2131107002213040), the National Natural Science Foundation of
China (no. 81000994) and the Beijing Natural Science Foundation
(no. 81572953). The authors would also like to thank the Burns
Institute of the First Affiliated Hospital of the Chinese PLA
General Hospital.
References
|
1
|
Meier F, Will S, Ellwanger U,
Schlagenhauff B, Schittek B, Rassner G and Garbe C: Metastatic
pathways and time courses in the orderly progression of cutaneous
melanoma. Br J Dermatol. 147:62–70. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Becker JC, Kämpgen E and Bröcker EB:
Classical chemotherapy for metastatic melanoma. Clin Exp Dermatol.
25:503–508. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Garbe C, Peris K, Hauschild A, Saiag P,
Middleton M, Spatz A, Grob JJ, Malvehy J, NewtonBishop J, Stratigos
A, et al: Diagnosis and treatment of melanoma: European consensus
based interdisciplinary guideline. Eur J Cancer. 46:270–283. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Legha SS, Ring S, Eton O, Bedikian A,
Buzaid AC, Plager C and Papadopoulos N: Development of a
biochemotherapy regimen with concurrent administration of
cisplatin, vinblastine, dacarbazine, interferon alfa, and
interleukin-2 for patients with metastatic melanoma. J Clin Oncol.
16:1752–1759. 1998.PubMed/NCBI
|
|
5
|
O'Day SJ, Gammon G, Boasberg PD, Martin
MA, Kristedja TS, Guo M, Stern S, Edwards S, Fournier P, Weisberg
M, et al: Advantages of concurrent biochemotherapy modified by
decrescendo interleukin-2, granulocyte colony-stimulating factor
and tamoxifen for patients with metastatic melanoma. J Clin Oncol.
17:2752–2761. 1999.PubMed/NCBI
|
|
6
|
Middleton MR, Grob JJ, Aaronson N,
Fierlbeck G, Tilgen W, Seiter S, Gore M, Aamdal S, Cebon J, Coates
A, et al: Randomized phase III study of temozolomide versus
dacarbazine in the treatment of patients with advanced metastatic
malignant melanoma. J Clin Oncol. 18:158–166. 2000.PubMed/NCBI
|
|
7
|
Atkins M: The treatment of metastatic
melanoma with chemotherapy and biologics. Curr Opin Oncol.
9:205–213. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Legha S, Ring S, Papadopoulos N, Raber M
and Benjamin RS: A phase II trial of taxol in metastatic melanoma.
Cancer. 65:2478–2481. 1990. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Bedikian A, Weiss G, Legha S, Burris HA
III, Eckardt JR, Jenkins J, Eton O, Buzaid AC, Smetzer L, Von Hoff
DD, et al: Phase II trial of docetaxel in patients with advanced
cutaneous malignant melanoma previously untreated with
chemotherapy. J Clin Oncol. 13:2895–2899. 1995.PubMed/NCBI
|
|
10
|
Avril M, Aamdal S, Grob J, Hauschild A,
Mohr P, Bonerandi JJ, Weichenthal M, Neuber K, Bieber T, Gilde K,
et al: Fotemustine compared with dacarbazine in patients with
disseminated malignant melanoma: A phase III study. J Clin Oncol.
22:1118–1125. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Berd D: A tale of two pities: Autologous
melanoma vaccines on the brink. Hum Vaccin Immunother. 8:1146–1151.
2012. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Calalona WJ, Taylor PT, Rabson AS and
Chretien PB: A method for dinifrochlorobengene contact
sensitigafron: A clinico pathological study. N Engl J Med.
286:399–402. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Fujisawa Y, Nabekura T, Nakao T, Nakamura
Y, Takahashi T, Kawachi Y, Otsuka F and Onodera M: The induction of
tumor-specific CD4+ T cells via major histocompatibility
complex class II is required to gain optimal anti-tumor immunity
against B16 melanoma cell line in tumor immunotherapy using
dendritic cells. Exp Dermatol. 18:396–403. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Terabe M and Berzofsky JA:
Immunoregulatory T cells in tumor immunity. Curr Opin Immunol.
16:157–162. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Somasundaram R, Jacob L, Swoboda R, Caputo
L, Song H, Basak S, Monos D, Peritt D, Marincola F, Cai D, et al:
Inhibition of cytolytic T lymphocyte proliferation by autologous
CD4+/CD25+ regulatory T cells in a colorectal
carcinoma patient is mediated by transforming growth factor-beta.
Cancer Res. 62:5267–5272. 2002.PubMed/NCBI
|
|
16
|
Jacobs JF, Nierkens S, Figdor CG, de Vries
IJ and Adema GJ: Regulatory T cells in melanoma: The final hurdle
towards effective immunotherapy? Lancet Oncol. 13:e32–e42. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wolf AM, Wolf D, M Gastl G Steurer,
Gunsilius E and Grubeck-Loebenstein B: Increase of regulatory T
cell in the peripheral blood of cancer patients. Clin Cancer Res.
9:606–612. 2003.PubMed/NCBI
|
|
18
|
Read S, Malmström V and Powrie F:
Cytotoxic T lymphocyte-associated antigen 4 plays an essential role
in the function of CD25(+)CD4(+) regulatory cells that control
intestinal inflammation. J Exp Med. 192:295–302. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Correll A, Tuettenberg A, Becker C and
Jonuleit H: Increased regulatory T-cell frequencies in patients
with advanced melanoma correlate with a generally impaired T-cell
responsiveness and are restored after dendritic cell-based
vaccination. Exp Dermatol. 19:e213–e221. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ladányi A, Mohos A, Somlai B, Liszkay G,
Gilde K, Fejos Z, Gaudi I and Tímár J: FOXP3+ cell
density in primary tumor has no prognostic impact in patients with
cutaneous malignant melanoma. Pathol Oncol Res. 16:303–309. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Lagouros E, Salomao D, Thorland E, Hodge
DO, Vile R and Pulido JS: Infiltrative T regulatory cells in
enucleated uveal melanomas. Trans Am Ophthalmol Soc. 107:223–228.
2009.PubMed/NCBI
|
|
22
|
Mougiakakos D, Johansson CC, Trocme E,
AllEricsson C, Economou MA, Larsson O, Seregard S and Kiessling R:
Intratumoral forkhead boxP3-positive regulatory T cells predict
poor survival in cyclooxygenase-2-positive uveal melanoma. Cancer.
116:2224–2233. 2010.PubMed/NCBI
|
|
23
|
Mourmouras V, Fimiani M, Rubegni P,
Epistolato MC, Malagnino V, Cardone C, Cosci E, Nisi MC and Miracco
C: Evaluation of tumour-infiltrating
CD4+CD25+FOXP3+ regulatory T cells
in human cutaneous benign and atypical naevi, melanomas and
melanoma metastases. Br J Dermatol. 157:531–539. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Edge SB and Cancer A.J.C.O.: AJCC cancer
staging handbook: From the AJCC cancer staging manual.
Springer-Verlag; New York: 2010
|
|
25
|
Karnofsky DA, Abelmann WH, Craver LF and
Burchenal JH: The use of the nitrogen mustards in the palliative
treatment of carcinoma. With particular reference to bronchogenic
carcinoma. Cancer. 1:634–656. 1948. View Article : Google Scholar
|
|
26
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
U.S. Department of Health and Human
Services, National Institutes of Health, National Cancer Institute,
. Common Terminology Criteria for Adverse Events (CTCAE) Version
4.0. Published 28 May 2009 (v4.03: June 14, 2010); cited 14 January
2014. Available from. http://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-2006-14_QuickReference_5x7.pdf
|
|
28
|
Trcka J, Kämpgen E, Becker JC, Schwaaf A
and Bröcker EB: Immune chemotherapy of malignant melanoma. Epifocal
administration of dinitrochlorobenzene (DNCB) combined with
systemic chemotherapy with dacarbazine (DTIC). Hautarzt. 49:17–22.
1998.(In German). View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Calalona WJ, Taylor PT, Rabson AS and
Chretien PB: A method for dinitrochlorobenzene contact
sensitization. A clinicopathological study. N Engl J Med.
286:399–402. 1972. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Barth A, Wanek LA and Morton DL:
Prognostic factors in 1,521 melanoma patients with distant
metastasis. J Am Coll Surg. 181:193–201. 1995.PubMed/NCBI
|
|
31
|
Li X, Min M, Gu Y and Du N: Laser
immunotherapy: Concept, possible mechanism, clinical applications
and recent experimental results. IEEE J Selected Topics Quantum
Electronics. 18:1434–1438. 2012. View Article : Google Scholar
|
|
32
|
Li X, Naylor MF, Le H, Nordquist RE,
Teague TK, Howard CA, Murray C and Chen WR: Clinical effects of in
situ photoimmunotherapy on late-stage melanoma patients: A
preliminary study. Cancer Biol Ther. 10:1081–1087. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Li X, Le H, Wolf RF, Chen VA, Sarkar A,
Nordquist RE, Ferguson H, Liu H and Chen WR: Long-term effect on
EMT6 tumors in mice induced by combination of laser immunotherapy
and surgery. Integrative Cancer Ther. 10:368–373. 2011. View Article : Google Scholar
|
|
34
|
Terheyden P, Kortüm AK, Schulze HJ, Durani
B, Remling R, Mauch C, Junghans V, Schadendorf D, Beiteke U, Jünger
M, Becker JC and Bröcker EB: Chemoimmunotherapy for cutaneous
melanoma with dacarbazine and epifocal contact sensitizers: Results
of a nationwide survey of the German Dermatologic Co-operative
Oncology Group. J Cancer Res Clin Oncol. 133:437–444. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Quillien V, Lesimple T and Toujas L:
Vaccinal cell therapy in melanoma. Bull Cancer. 90:722–733.
2003.(In French). PubMed/NCBI
|
|
36
|
Berd D, Sato T and Mastrangelo MJ: Effect
of the dose and composition of an autologous hapten-modified
melanoma vaccine on the development of delayed-type
hypersensitivity responses. Cancer Immunol Immunother. 51:320–326.
2002. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Ridolfi L, Petrini M, Fiammenqhi L,
Granato AM, Ancarani V, Pancisi E, Brolli C, Selva M, Scarpi E,
Valmorri L, et al: Dendritic cell-based vaccine in advanced
melanoma: Update of clinical outcome. Melanoma Res. 21:524–529.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Stamell EF, Wolchok JD, Gnjatic S, Lee NY
and Brownell I: The abscopal effect associated with a systemic
anti-melanoma immune response. Int J Radiat Oncol Biol Phys.
85:293–295. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Wack C, Kirst A, Becker JC, Lutz WK,
Bröcker EB and Fischer WH: Chemoimmunotherapy for melanoma with
dacarbazine and 2,4-dinitrochlorobenzene elicits a specific T
cell-dependent immune response. Cancer Immunol Immunother.
51:431–439. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Kumamoto T, Huang EK, Paek HJ, Morita A,
Matsue H, Valentini RF and Takashima A: Induction of tumor-specific
protective immunity by in situ Langerhans cell vaccine. Nat
Biotechnol. 20:64–69. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Mahipal A, Terai M, Berd D, Chervoneva I,
Patel K, Mastrangelo MJ and Sato T: Tumor-derived interleukin-10 as
a prognostic factor in stage III patients undergoing adjuvant
treatment with an autologous melanoma cell vaccine. Cancer Immunol
Immunother. 60:1039–1045. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Postow MA, Callahan MK, Barker CA, Yamada
Y, Yuan J, Kitano S, Mu Z, Rasalan T, Adamow M, Ritter E, et al:
Immunologic correlates of the abscopal effect in a patient with
melanoma. N Engl J Med. 366:925–931. 2012. View Article : Google Scholar : PubMed/NCBI
|