Introduction
Small-cell lung cancer (SCLC) is the most aggressive
type of lung cancer and has a poor prognosis (1). The standard therapy for extensive SCLC
is chemotherapy with a platinum compound (carboplatin or cisplatin)
administered in combination with etoposide, a topoisomerase II
inhibitor (2). A Japanese phase III
study [Japan Clinical Oncology Group (JCOG) 9511] investigated the
clinical outcomes patients who were administered cisplatin and
etoposide, compared with those who received cisplatin and
irinotecan, a topoisomerase I inhibitor (3). Irrespective of the regimen selected, the
majority of patients experience relapse or disease progression
following an initial response to chemotherapy, and second-line
therapy is subsequently required (4,5).
Amrubicin is a synthetic 9-aminoanthracycline that
is converted to its active metabolite, amrubicinol, through the
reduction of its C-13 ketone group to a hydroxy group (6). Amrubicin and amrubicinol are
topoisomerase II inhibitors, which have been demonstrated to exert
antitumor activities in various human tumor xenograft models
(7). The drug has been evaluated in a
number of Japanese studies and reported to yield a response rate of
36–52% and a median survival time of 7–12 months when administered
as a second-line treatment (8–13). The
results of previous studies have indicated that amrubicin is useful
for the treatment of relapsed SCLC (8–13).
Few previous studies have evaluated the efficacy of
amrubicin as a second-line treatment in patients with SCLC with
consideration of the previous chemotherapy regimen. The present
study aimed to evaluate whether there is a significant difference
in the efficacy of amrubicin in patients with SCLC when treated
previously with a platinum agent combined with either the
topoisomerase II inhibitor etoposide or the topoisomerase I
inhibitor irinotecan.
Materials and methods
Patient selection
A retrospective study was conducted using the data
of a cohort of 48 consecutive Japanese patients with SCLC that had
relapsed following treatment with a platinum-based regimen combined
with etoposide or irinotecan, and subsequently received amrubicin
monotherapy at Kitasato University Hospital (Tokyo, Japan) between
January 2009 and November 2014. The study reviewed the medical
records of the patients and excluded those who did not have at
least one measurable lesion, according to the Response Evaluation
Criteria in Solid Tumors (RECIST, version 1.1) (14). The patient characteristics were
identified by a retrospective chart review, including age at
diagnosis, gender, Eastern Cooperative Oncology Group (ECOG)
performance status (PS) at the time of amrubicin treatment
initiation, smoking status, brain metastasis status, type of
relapse (sensitive or refractory) following prior therapy and the
previously administered chemotherapy regimen (platinum agent plus
etoposide, or cisplatin plus irinotecan). The platinum agent was
cisplatin or carboplatin. With regard to their smoking status, the
patients were classified as current smokers, former light smokers
(history of smoking a total of ≤10 pack-year plus smoking cessation
≥15 years previously), and non-smokers (a lifetime history of
smoking <100 cigarettes). Refractory relapse was defined as the
absence of response to a previous chemotherapy regimen, disease
progression during chemotherapy or disease progression within 90
days of completing chemotherapy following the initial confirmation
of an objective response. Sensitive relapse was defined as the
absence of response to a previous chemotherapy regimen, disease
progression during chemotherapy or disease progression ≥90 days
after completing chemotherapy following the initial confirmation of
an objective response.
Treatment
The patients received infusion of amrubicin at 40
mg/m2/day for 3 consecutive days every 21 days; the
treatment was repeated until the appearance of disease progression,
intolerable toxicity or the patient's refusal to continue the
treatment. Prior to the start of treatment, patients were required
to have an absolute neutrophil count of ≥1,500/mm3, a
platelet count of ≥100,000/mm3, serum aspartate
aminotransferase and alanine aminotransferase levels <3-times
the maximum normal value, and serum total bilirubin and creatinine
levels of <1.5-times the maximum normal values. The doses of
amrubicin were modified as required on the basis of hematological
and non-hematological toxicities.
Evaluation of response and
toxicities
Tumor response to treatment was classified according
to the RECIST (version 1.1). Patients were evaluated for
progression or regression of the disease by a physical examination
and complete medical history, chest radiography, computed
tomography of the chest and abdomen, magnetic resonance imaging of
the head and positron emission tomography. Patient medical records
were reviewed to identify toxicities, which were graded according
to the National Cancer Institute Common Toxicity Criteria (version
4) grading system (http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm#ctc_40).
Statistical analysis
The distributions of the categorical characteristics
between patient groups, divided according to the previous
chemotherapy regimen, were analyzed using the χ2 test.
Progression-free survival (PFS) time was measured as the duration
from the start of amrubicin therapy to the determination of
treatment failure (mortality or documentation of disease
progression) or the date of censoring at the final follow-up
examination. Overall survival (OS) time was defined as the duration
from the start of amrubicin therapy to patient mortality, or the
date of censoring at the final follow-up examination. The survival
curves were generated using the Kaplan-Meier method and variations
in survival were analyzed by the log-rank test. The variables of
age, gender, PS, prior chemotherapy regimen, status of brain
metastasis and type of relapse were fitted into a Cox
proportional-hazards model to predict the hazard ratios for the PFS
and OS. All statistical analyses were performed using SPSS
software, version 17.0 (SPSS, Inc., Chicago, IL, USA) for Windows.
The data are presented as the median and range, unless otherwise
stated. P<0.05 was considered to indicate a statistically
significant result.
Results
Patient characteristics
The clinical characteristics of the patients are
presented in Table I. A total of 48
patients treated with amrubicin were included in the final
analysis. The median age of the patients was 67 years; 36 patients
(75%) had a PS of 0 or 1 (a PS of 1 or 2 was considered to be good)
and 8 patients (17%) had brain metastasis. Of the 48 patients, 37
(77%) had been previously treated with platinum, including
cisplatin or carboplatin, and etoposide, and 11 patients (23%) had
been treated with cisplatin and irinotecan. A total of 33 patients
(69%) presented with sensitive relapse and 15 patients (31%) with
refractory relapse. There were no significant differences observed
in the clinical characteristics between those patients who had
received platinum and etoposide, compared with patients who had
received cisplatin and irinotecan.
 | Table I.Characteristics of the patients
involved in the present study. |
Table I.
Characteristics of the patients
involved in the present study.
| Category | Total (n=48) | EP (n=37) | IP (n=11) | P-valuea |
|---|
| Age, median
(range) | 67 (34–82) | 67 (34–82) | 67 (56–75) |
|
| Gender, n |
|
|
| 0.52 |
| Male | 42 | 33 | 9 |
|
|
Female | 6 | 4 | 2 |
|
| Smoking status,
n |
|
|
|
|
|
Current | 48 | 37 | 11 |
|
| Non or
former light | 0 | 0 | 0 |
|
| ECOG performance
status, n |
|
|
| 0.84 |
| 0–1 | 36 | 28 | 8 |
|
| 2–3 | 12 | 9 | 3 |
|
| Clinical stage prior
to receiving the previous therapy, n |
|
|
| 0.15 |
| LD | 6 | 6 | 0 |
|
| ED | 42 | 31 | 11 |
|
| Brain metastasis,
n |
|
|
| 0.05 |
|
Positive | 8 | 4 | 4 |
|
|
Negative | 40 | 33 | 7 |
|
| Type of relapse,
n |
|
|
| 0.68 |
|
Sensitive | 33 | 26 | 7 |
|
|
Refractory | 15 | 11 | 4 |
|
Tumor response
Of a total of 48 patients, an objective response (as
determined using RECIST version 1.1) to a first-line platinum
doublet chemotherapy regimen had been identified in 35 patients,
corresponding to an overall response rate of 72.9%. The response
rates following treatment with platinum plus etoposide or cisplatin
plus irinotecan were 73.0 and 72.7%, respectively (P=0.99). An
objective response to the subsequent amrubicin therapy was
identified in 15 patients, equating to a response rate of 31.3%.
The response rate was 36.4% for patients previously treated with
cisplatin and irinotecan, and 30.0% for patients previously treated
with a platinum agent and etoposide; no significant differences
were observed between the two groups (P=0.68). The tumor responses
are presented in Table II.
 | Table II.Clinical response in patients treated
previously with cisplatin and irinotecan, and patients treated
previously with a platinum agent and etoposide. |
Table II.
Clinical response in patients treated
previously with cisplatin and irinotecan, and patients treated
previously with a platinum agent and etoposide.
| Groups | Total, n | Number of
responders | RR, % | P-value |
|---|
| Platinum
agent+etoposide | 37 | 11 | 30.0 | 0.68a |
|
Cisplatin+irinotecan | 11 | 4 | 36.4 |
|
| Total | 48 | 15 | 31.3 |
|
Toxicities of amrubicin
A comparison of toxicities between the patients who
had received platinum and etoposide and patients who had received
cisplatin and irinotecan is presented in Table III. There were no significant
differences identified in the frequencies of each type of toxicity
between the two groups.
 | Table III.Toxicities of amrubicin in patients
who had received platinum and etoposide and patients who had
received cisplatin and irinotecan. |
Table III.
Toxicities of amrubicin in patients
who had received platinum and etoposide and patients who had
received cisplatin and irinotecan.
|
| All toxicity grades,
n (%) | Toxicity grade ≥3, n
(%) |
|---|
|
|
|
|
|---|
| Toxicity | IP | EP | aP-value | IP | EP | aP-value |
|---|
| Nausea | 2 (18.2) | 5 (13.5) | NS | 0 (0) | 0 (0) | – |
| Fatigue | 3 (27.3) | 5 (13.5) | NS | 0 (0) | 0 (0) | – |
| Anorexia | 3 (27.3) | 8 (21.6) | NS | 0 (0) | 1 (2.7) | NS |
| Constipation | 3 (27.3) | 3 (8.1) | NS | 0 (0) | 0 (0) | NS |
| Anemia | 3 (27.3) | 9 (24.3) | NS | 0 (0) | 3 (8.1) | NS |
| Thrombocytopenia | 7 (63.6) | 17 (45.9) | NS | 1 (9.1) | 5 (13.5) | NS |
| Leukopenia | 10 (90.9) | 33 (89.2) | NS | 3 (27.3) | 16 (43.2) | NS |
| Neutropenia | 10 (90.9) | 32 (86.5) | NS | 3 (27.3) | 16 (43.2) | NS |
| Neutropenic
fever | 0 (0) | 4 (10.8) | NS | 0 (0) | 4 (10.8) | NS |
| AST | 1 (9.1) | 4 (10.8) | NS | 1 (9.1) | 1 (2.7) | NS |
| ALT | 1 (9.1) | 4 (10.8) | NS | 0 (0) | 0 (0) | – |
| Creatinine
increased | 2 (18.2) | 1 (2.7) | NS | 1 (9.1) | 1 (2.7) | NS |
| Pneumonitis | 0 (0) | 1 (2.7) | NS | 0 (0) | 1 (2.7) | NS |
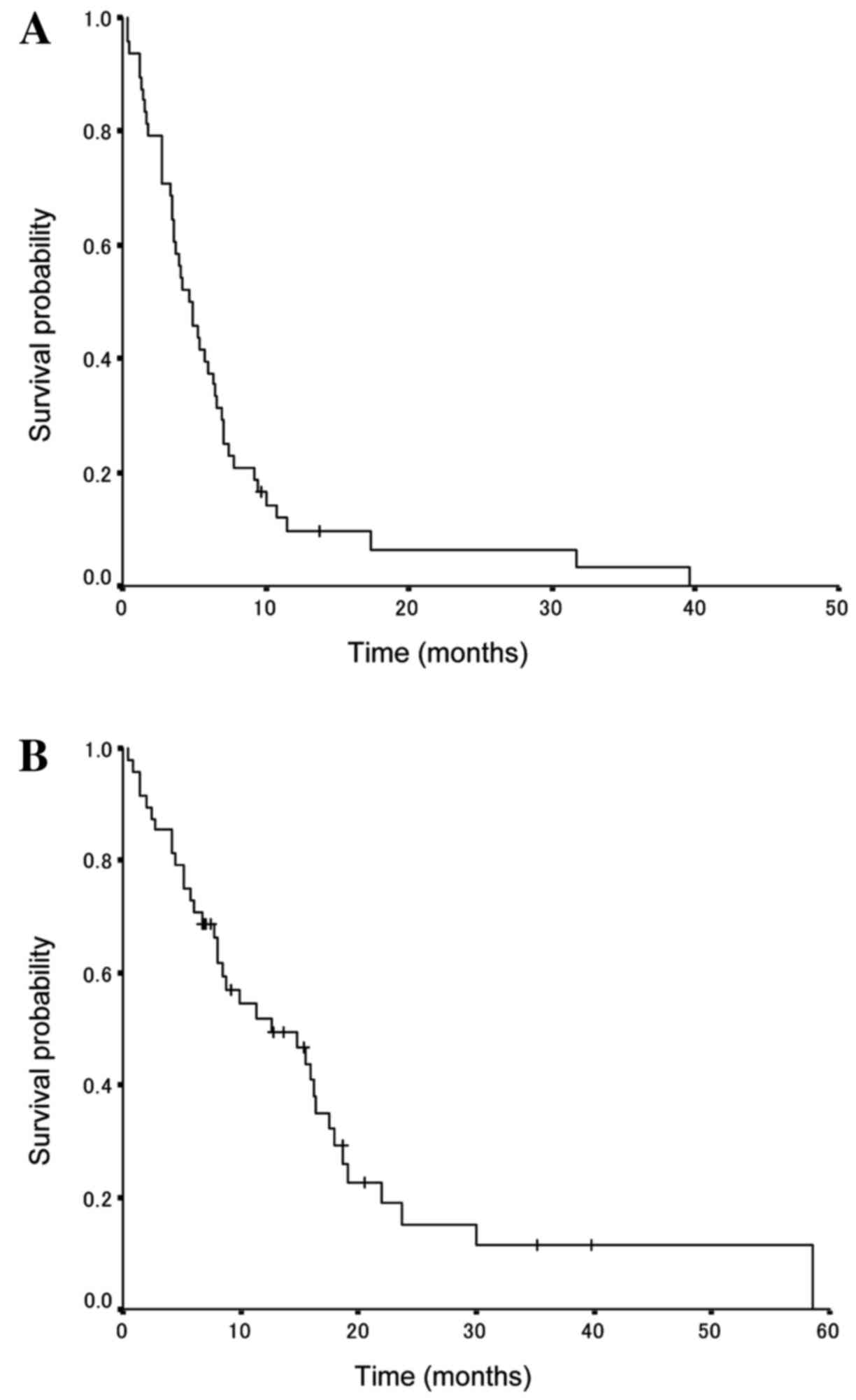
PFS and OS
The survival data update was completed by January
2015 and the median follow-up period was determined to be 12.7
months. The amrubicin treatment results for all patients indicated
that the median PFS and OS times were 7.1 months [95% confidence
interval (CI), 4.6–9.5 months; Fig.
1A] and 17.0 months (95% CI, 11.5–22.5 months; Fig. 1B), respectively.
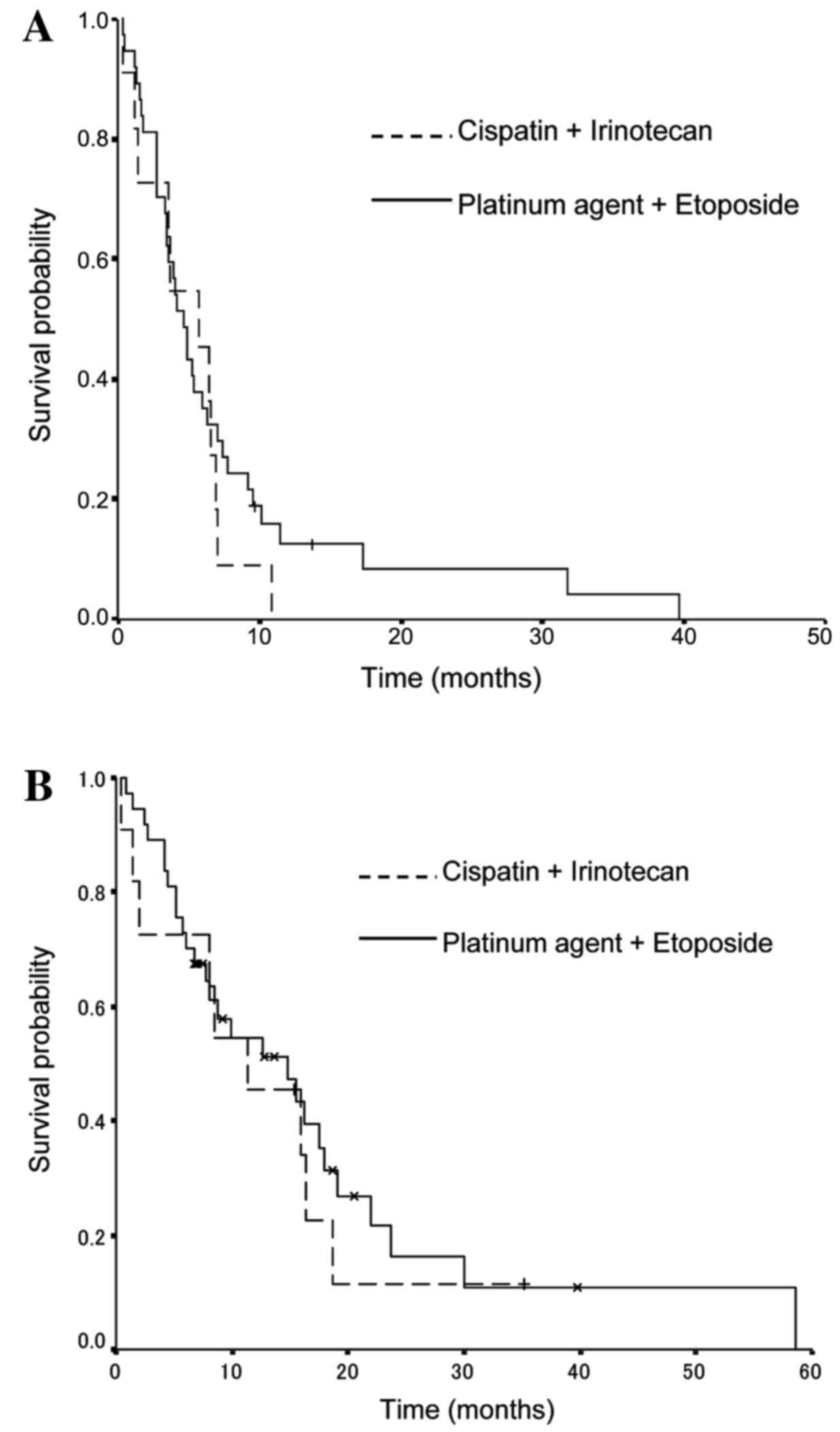
With regard to the previous chemotherapy regimen,
the median PFS times were 5.7 months (95% CI, 3.5–5.9 months) in
the cisplatin and irinotecan group, and 4.7 months (95% CI, 2.7–8.7
months) in the platinum and etoposide group (P=0.43; Fig. 2A). The median OS times were 11.4
months (95% CI, 3.4–19.4 months) in the cisplatin and irinotecan
group, and 14.8 months (95% CI, 6.9–22.7 months) in the platinum
agent and etoposide group (P=0.23; Fig.
2B).
Multivariate analysis identified the PS, status of
brain metastasis and type of relapse following the previous regimen
as significant predictors of PFS. The PS and type of relapse
following previous chemotherapy were also determined to be
significant predictors of OS (Table
IV).
 | Table IV.Progression-free survival and overall
survival analysis by the Cox regression model. |
Table IV.
Progression-free survival and overall
survival analysis by the Cox regression model.
|
| Univariate
analysis | Multivariate
analysis |
|---|
|
|
|
|
|---|
| Variable | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| PFS |
|
|
|
|
| Age
(<75 vs. >75) | 0.99
(0.44–2.24) | 0.98 | 0.97
(0.34–2.54) | 0.96 |
|
Gender | 0.73
(0.30–1.75) | 0.48 | 0.61
(0.24–1.58) | 0.31 |
|
Performance status (0–1 vs.
2–3) | 3.27
(1.63–6.55) | 0.0009 | 4.21
(1.86–9.57) | 0.001 |
|
Clinical stage (LD vs.
ED) | 0.77
(0.32–1.82) | 0.55 | 0.91
(0.32–2.54) | 0.85 |
|
Previous regimen (EP vs.
IP) | 1.32
(0.66–2.64) | 0.44 | 1.43
(0.63–3.24) | 0.40 |
| Brain
metastasis | 0.72
(0.32–1.62) | 0.43 | 0.35
(0.13–0.95) | 0.04 |
| Type of
relapse (sensitive vs. refractory) | 2.59
(1.33–5.07) | 0.005 | 3.19
(1.54–6.61) | 0.002 |
| OS |
|
|
|
|
| Age
(<75 vs. >75) | 0.86
(0.33–2.23) | 0.76 | 1.09
(0.35–3.39) | 0.88 |
|
Gender | 0.52
(0.20–1.38) | 0.19 | 0.43
(0.15–1.21) | 0.11 |
|
Performance status (0–1 vs.
2–3) | 4.10
(1.93–8.73) | 0.0003 | 4.49
(1.88–10.73) | 0.001 |
|
Clinical stage (LD vs.
ED) | 0.73
(0.26–2.06) | 0.55 | 0.97
(0.28–3.40) | 0.97 |
|
Previous regimen (EP vs.
IP) | 1.21
(0.56–2.58) | 0.63 | 0.90
(0.35–2.30) | 0.83 |
| Brain
metastasis | 1.35
(0.55–3.32) | 0.51 | 1.14
(0.37–3.47) | 0.82 |
| Type of
relapse (sensitive vs. refractory) | 3.55
(1.58–8.00) | 0.002 | 4.00
(1.66–9.60) | 0.002 |
Discussion
Few previous studies have evaluated the efficacy of
amrubicin monotherapy in patients with SCLC with regard to the
previously administered chemotherapy regimen. Following the
evaluation of the objective response, PFS and OS, the present study
revealed no significant differences in the clinical efficacy of
amrubicin monotherapy in patients treated with cisplatin and
irinotecan (a topoisomerase I inhibitor) compared with patients
treated with a platinum agent and etoposide (a topoisomerase II
inhibitor).
However, preclinical studies (15–18) have
indicated that treatment with topoisomerase I inhibitors induces
downregulation of topoisomerase I and upregulation of topoisomerase
II, increasing cell sensitivity to topoisomerase II inhibitors.
Similarly, treatment with topoisomerase II inhibitors has been
reported to induce the downregulation of topoisomerase II and
upregulation of topoisomerase I. A phase II study conducted by
Murakami et al (19) reported
that amrubicin monotherapy is effective against refractory SCLC; a
subset analysis of this study revealed that the response to
amrubicin was less pronounced and the survival rate was lower in
patients who were previously treated with etoposide, a
topoisomerase II inhibitor, compared with patients previously
treated with irinotecan. By contrast, the results of a phase II
study that evaluated the efficacy of amrubicin monotherapy for
relapsed SCLC suggested that the absence of any significant
difference in the PFS following amrubicin therapy was dependent on
the previous platinum and topoisomerase inhibitor-based therapy
(11). Furthermore, two retrospective
studies also demonstrated an equivalent amrubicin efficacy against
relapsed SCLC, irrespective of the prior platinum and topoisomerase
inhibitor therapy (20,21).
To the best of our knowledge, this study is the
first to evaluate the effectiveness of amrubicin in terms of the
response rate, PFS and OS with respect to the type of chemotherapy
previously administered, and to subsequently demonstrate the
equivalent efficacy of the drug, regardless of the first-line
chemotherapy regimen used. There were a number of limitations in
the current study; as it was retrospective the results cannot be
regarded as definitive. Additionally, the small sample size may not
have been sufficient to be fully representative, and no
pharmacokinetic validation of the efficacy of amrubicin was
conducted.
In conclusion, amrubicin may be a valid choice as a
second-line chemotherapeutic agent for patients with SCLC,
irrespective of the type of platinum agent and topoisomerase
inhibitor-based chemotherapy regimen previously administered.
References
|
1
|
Jackman DM and Johnson BE: Small-cell lung
cancer. Lancet. 366:1385–1396. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Davies AM, Lara PN, Lau DH and Gandara DR:
Treatment of extensive small cell lung cancer. Hematol Oncol Clin
North Am. 18:373–385. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Noda K, Nishiwaki Y, Kawahara M, Negoro S,
Sugiura T, Yokoyama A, Fukuoka M, Mori K, Watanabe K, Tamura T, et
al: Irinotecan plus cisplatin compared with etoposide plus
cisplatin for extensive small-cell lung cancer. N Engl J Med.
346:85–91. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Schiller JH, Adak S, Cella D, DeVore RF
III and Johnson DH: Topotecan versus observation after cisplatin
plus etoposide in extensive stage small cell lung cancer: E7593-A
phase III trial of the Eastern Cooperative Oncology Group. J Clin
Oncol. 19:2114–2122. 2001.PubMed/NCBI
|
|
5
|
Stupp R, Monnerat C, Turrisi AT III, Perry
MC and Leyvraz S: Small cell lung cancer: State of the art and
future perspectives. Lung Cancer. 45:105–117. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ishizumi K, Ohashi N and Tanno N:
Stereospecific total synthesis of 9-aminoanthracyclines:
(+)-9-amino-9-deoxydaunomycin and related compounds. J Org Chem.
52:4477–4485. 1987. View Article : Google Scholar
|
|
7
|
Noda T, Watanabe T, Kohda A, Hosokawa S
and Suzuki T: Chronic effects of a novel synthetic anthracycline
derivative (SM-5887) on normal heart and doxorubicin-induced
cardiomyopathy in beagle dogs. Invest New Drugs. 16:121–128. 1998.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Nogami N, Kiura K, Takigawa N, Harita S,
Chikamori K, Shibayama T, Tabata M, Hotta K, Shinkai T and Tanimoto
M: A phase II trial of combination chemotherapy with topotecan and
amrubicin in small cell lung cancer (SCLC). J Clin Oncol.
28:(Suppl). 70542010.
|
|
9
|
Inoue A, Sugawara S, Yamazaki K, Maemondo
M, Suzuki T, Gomi K, Takanashi S, Inoue C, Inage M, Yokouchi H, et
al: Randomized phase II trial comparing amrubicin with topotecan in
patients with previously treated small-cell lung cancer: North
Japan Lung Cancer Study Group Trial 0402. J Clin Oncol.
26:5401–5406. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kaira K, Sunaga N, Tomizawa Y, Yanagitani
N, Shimizu K, Imai H, Utsugi M, Iwasaki Y, Iijima H, Tsurumaki H,
et al: A phase II study of amrubicin, a synthetic
9-aminoanthracycline, in patients with previously treated lung
cancer. Lung Cancer. 69:99–104. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Onoda S, Masuda N, Seto T, Eguchi K,
Takiguchi Y, Isobe H, Okamoto H, Ogura T, Yokoyama A, Seki N, et
al: Phase II trial of amrubicin for treatment of refractory or
relapsed small-cell lung cancer: Thoracic Oncology Research Group
Study 0301. J Clin Oncol. 24:5448–5453. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hasegawa Y, Takeda K and Kashii T:
Clinical experience of amrubicin hydrochloride (Calsed) monotherapy
in previously treated patients with small-cell lung cancer. Jpn J
Lung Cancer. 45:811–815. 2005. View Article : Google Scholar
|
|
13
|
Hirose T, Shirai T, Kusukoto S, Sugiyama
T, Yamaoka T, Okuda K, Ohmori T, Ohnishi T and Adachi M: Showa
University School of Medicine, Tokyo, Japan: Phase II study of
amrubicin and carboplatin in patients with the refractory or
relapsed small cell lung cancer (SCLC). J Clin Oncol.
28:S152010.
|
|
14
|
Eisenhauer EA, Therasse P, Bogaerts J,
Schwartz LH, Sargent D, Ford R, Dancey J, Arbuck S, Gwyther S,
Mooney M, et al: New response evaluation criteria in solid tumours:
Revised RECIST guideline (version 1.1). Eur J Cancer. 45:228–247.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Gupta RS, Gupta R, Eng B, Lock RB, Ross
WE, Hertzberg RP, Caranfa MJ and Johnson RK: Camptothecin-resistant
mutants of Chinese hamster ovary cells containing a resistant form
of topoisomerase I. Cancer Res. 48:6404–6410. 1988.PubMed/NCBI
|
|
16
|
Sugimoto Y, Tsukahara S, Oh-hara T, Isoe T
and Tsuruo T: Decreased expression of DNA topoisomerase I in
camptothecin-resistant tumor cell lines as determined by a
monoclonal antibody. Cancer Res. 50:6925–6930. 1990.PubMed/NCBI
|
|
17
|
Sugimoto Y, Tsukahara S, Oh-hara T, Liu LF
and Tsuruo T: Elevated expression of DNA topoisomerase II in
camptothecin-resistant human tumor cell lines. Cancer Res.
50:7962–7965. 1990.PubMed/NCBI
|
|
18
|
Tan KB, Mattern MR, Eng WK, McCabe FL and
Johnson RK: Nonproductive rearrangement of DNA topoisomerase I and
II genes: Correlation with resistance to topoisomerase inhibitors.
J Natl Cancer Inst. 81:1732–1735. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Murakami H, Yamamoto N, Shibata T, Takeda
K, Ichinose Y, Ohe Y, Yamamoto N, Takeda Y, Kudoh S, Atagi S, et
al: A single-arm confirmatory study of amrubicin therapy in
patients with refractory small-cell lung cancer: Japan Clinical
Oncology Group Study (JCOG0901). Lung Cancer. 84:67–72. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Hirukawa I, Yokoyama T, Hirata A, Takata
S, Ishii H and Takizawa H: A retrospective study of amrubicin
treatment in patients with relapsed small-cell lung cancer. Jpn J
Lung Cancer. 5:3812014.
|
|
21
|
Sudo J, Tsuzuki H, Takahashi S, Yamane Y,
Kurimoto F and Sakai H: Second-line chemotherapy for relapsed
small-cell lung cancer. Nihon Kokyuki Gakkai Zasshi. 3:2332014.
|