Introduction
Glioblastoma multiforme (GBM), the most malignant
and aggressive type of primary brain tumor in adults, accounts for
55% of all incidences of glioma (1).
Due to the diffuse infiltration of tumor cells into normal brain
tissue, local therapies such as surgery and/or radiation therapy
are not effective. Subsequent to diagnosis, the median survival
time of GBM is 12–15 months (2,3). As a
result, preventing the invasion of cancer cells may improve the
prognosis of glioma patients. Tumor invasion is considered to be a
complicated process including the adherence of tumor cells to
normal brain elements and the proteolysis of extracellular matrix
(ECM) components (4). The ECM
proteins that make up the specialized basement membrane (BM) serve
as a barrier for cell invasion (5,6).
Furthermore, the degradation of the BM results in the release or
activation of various growth factors required for angiogenesis,
tumor growth and metastasis (3).
Matrix metalloproteinases (MMPs), a family of
zinc-dependent endopeptidases, are known to serve important roles
in the degradation of the BM and are associated with the invasion
ability of glioma (4,5,7). The MMPs
MMP-2 and MMP-9 perform key roles in glioma progression and
aggression (8,9). Higher levels of MMP-2 and MMP-9 were
observed in high-grade gliomas compared with non-invasive low-grade
astrocytoma and normal brain tissue (4,10). In the
1990s, following the identification of the important role of MMPs
in cancer angiogenesis, growth and metastasis, MMPs became a
therapeutic target for cancer treatment, which determined interest
in the design and evaluation of matrix metalloproteinases
inhibitors (MMPIs) as anticancer agents (11,12). MMPs
have been revealed to be important in regulating cellular
activities and are involved in physiological and pathological
processes (13,14). The first generation of MMPIs, which
had broad-spectrum MMP inhibition activity, caused unexpected side
effects, including musculoskeletal pain and inflammation (12,15,16).
Caffeic acid (CA) is a natural component of numerous
plant-based foods including fruits, wine and coffee (17). In previous studies, CA was confirmed
as a selective MMP-9 inhibitor with an anticancer effect (18–20). A
series of CA derivatives have been synthesized, including CA
phenethyl ester, which can selectively inhibit the activity of
MMP-2/−9, but not MMP-1/−3/−7 (21).
In 2013, Shi et al (22)
synthesized a series of CA amide derivatives as MMPIs, including
3d. The present study synthesized 3d and termed it PT93 (Fig. 1A). The present study demonstrated that
PT93 suppresses the proliferation and migration of T98G and U251
cells, and revealed that PT93 inhibits MMP-2/−9 expression in T98G
cells, which may contribute to the anticancer effect of the CA
derivative.
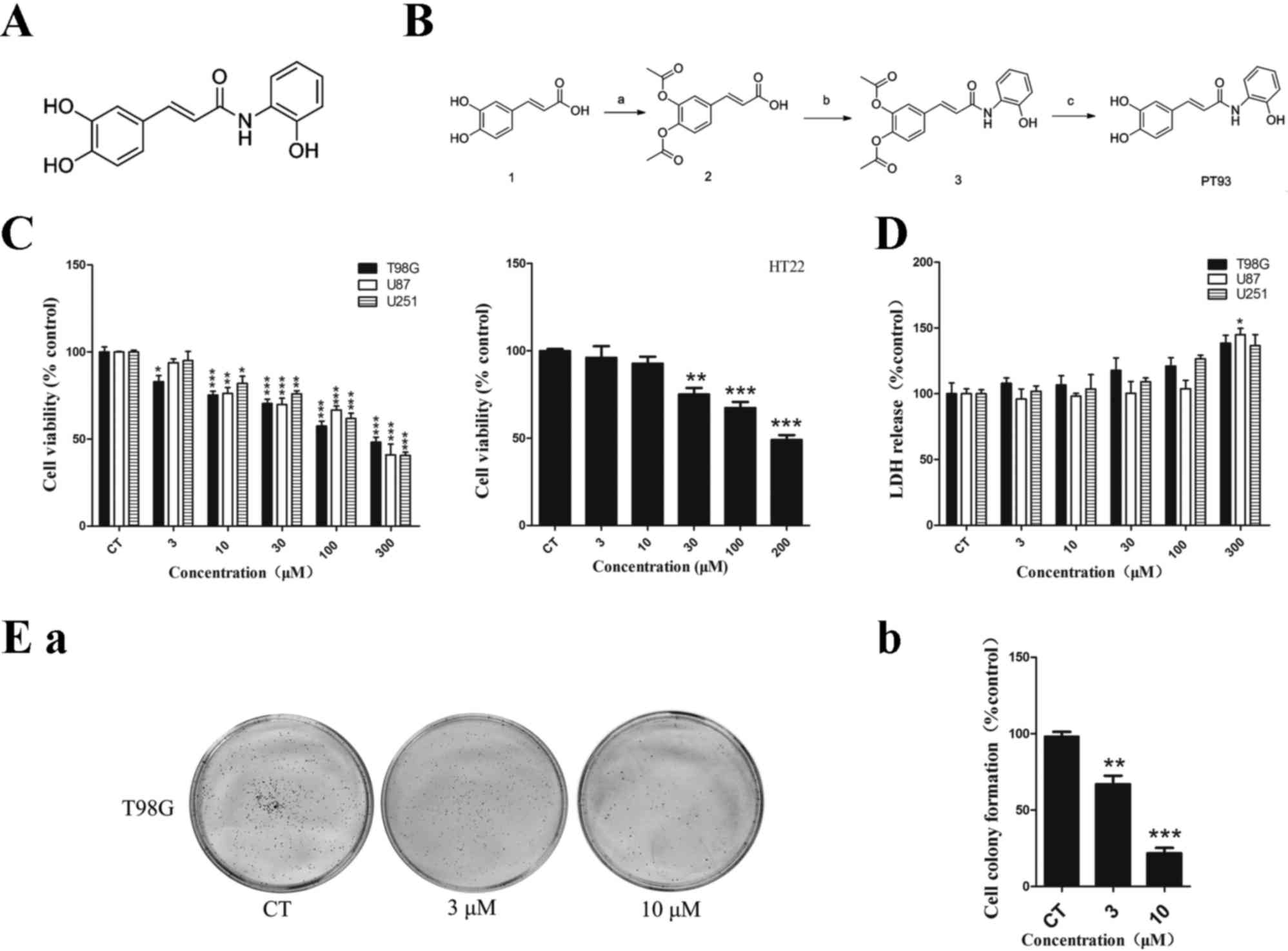 | Figure 1.Chemical structure, synthetic routes,
cytotoxicity and cell proliferative inhibition of PT93. (A)
Chemical structure of PT93. (B) Synthetic routes of PT93. Reagents
and conditions: (a) Acetic anhydride, 4-dimethylaminopyridine,
pyridine, 0°C followed by room temperature; (b)
1-Ethyl-3-(3-dimethylaminopropyl) carbodiimide, triethylamine, dry
dichloromethane, reacted at room temperature; (c) sodium carbonate,
methonal/water, room temperature. (C) Cell viability (%) of human
GBM cell lines T98G, U87, U251 and normal neuron cell line HT22
treated with PT93 was assessed by a MTT assay. Subsequent to
treatment with PT93, cell viability decreased in a dose-dependent
manner and the concentration of inhibitor where inhibition is
reduced by half (IC50) was 300, 272, 272 and 200 µM,
respectively. (D) Although LDH release (%) of GBM cells increased
as concentration of PT93 increased, a significant difference was
only observed at 300 µM in U87 cells compared with the control
group. (E) Proliferation of T98G cells was tested using a cell
colony formation assay. T98G cells were treated with 0, 3 and 10 µM
PT93 for 7 days. (a) The image is representative of 3 biological
replicates. The colony formations were counted subsequent to 7
days. (b) The results show that PT93 can suppress T98G cell
proliferation in a dose-dependent manner. *P<0.05, **P<0.01
and ***P<0.001 compared with the control. GBM, glioblastoma
multiforme; LDH, lactate dehydrogenase. |
Materials and methods
Materials, reagents and
antibodies
Dimethyl sulfoxide (DMSO) and MTT were purchased
from Sigma-Aldrich (Merck Millipore, Darmstadt, Germany).
Dulbecco's modified Eagle's medium (DMEM) and fetal bovine serum
(FBS) were obtained from Gibco (Thermo Fisher Scientific, Inc.
Waltham, MA, USA). PT93 was synthesized in International Joint
Laboratory (SYSU-PolyU HK) of Novel Anti-Dementia Drugs of
Guangdong (Guangzhou, China) and dissolved in DMSO then stored at
−20°C. Enhanced chemiluminescence (ECL) reagents were purchased
from Landbiology (Guangzhou, China). A lactate dehydrogenase (LDH)
assay kit was obtained from Nanjing Jiancheng Bioengineering
Institute (Nanjing, China). MMP-2/9 rabbit primary antibodies
(MMP-9 cat. no., BS1241; dilution, 1:1,000; MMP-2 cat. no., BS1236;
dilution, 1:1,000) were purchased from Bioworld Technology, Inc.
(St. Louis Park, MN, USA). β-actin primary antibody (cat. no.,
ACTN05 (C4); dilution, 1:5,000) was purchased from Thermo Fisher
Scientific, Inc. Rabbit (cat. no., 32460; dilution, 1:1,000) and
mouse (cat. no., 31430; dilution, 1:10,000) were goat derived IgG
coupled to horseradish peroxidase and purchased from Thermo Fisher
Scientific, Inc.
Chemistry
Acetic anhydride (850 mg, 8.33 mmol) was added to a
chilled solution of CA (500 mg, 2.78 mmol) and
4-dimethylaminopyridine (16.95 mg, 138.77 µmol) in pyridine (4 ml).
The mixture was stirred at room temperature for 1 h and poured over
crushed ice. The solution was acidified using 1 M HCl (pH <2),
extracted with ethyl acetate (30 ml × 2 times), dried over
anhydride sodium sulfate and filtered. The filtrate was
concentrated by rotary evaporator yielding compound 1 as a white
powder (655 mg, 87%), which was used directly in the next step
without additional purification.
A total of 217.65 mg (1.14 mmol)
1-ethyl-3-(3-dimethylaminopropyl) carbodiimide hydrochloride was
added, at room temperature, to a mixture of compound 1 (200 mg,
756.91 µmol), 2-aminophenol (165.2 mg, 1.51 mmol) and
trimethylamine (314.76 µl, 2.27 mmol) in anhydrous dichloromethane
(6 ml). The mixture was stirred at room temperature for 4 h. The
reaction was quenched by the addition of water (10 ml) and
extracted thrice with dichloromethane. The combined organic layers
were washed with saline (10 ml), dried over sodium sulfate,
filtered and concentrated to remove the solvent. The resulting
residue was purified by flash chromatography on silica gel
(methanol/dichloromethane 1/50) yielding compound 3 as brown/yellow
solid (150 mg, 73%).
To a solution of compound 3 (100 mg, 281.42 µmol) in
4 ml methanol, a solution of sodium carbonate (59.65 mg, 562.84
µmol) in 3 ml H2O was added, and the mixture was stirred
at room temperature for 1 h. The solution was extracted with
dichloromethane (20 ml at 3 time points) and the combined organic
layers were washed with saline (10 ml), dried over
Na2SO4, filtered and concentrated to remove
the solvent. The resulting residue was purified by flash
chromatography on silica gel (methanol/dichloromethane 1/50-1/20)
yielding PT93 as a yellow solid (51 mg, 66.8%). Proton nuclear
magnetic resonance (400 MHz, DMSO) δ 9.48 (s, 4H), 7.83 (d,
J=7.8 Hz, 1H), 7.40 (d, J=15.5 Hz, 1H), 7.07–6.72 (m,
7H). Single peak at 254 nm and 215 nm in analytical high
performance liquid chromatography (Fig.
1A and B).
Cell lines and cell culture
The human malignant GBM T98G, U87 and U251 cell
lines and normal mouse neuron HT22 cells were purchased from
Shanghai Institute of Biochemistry and Cell Biology (Shanghai,
China). The cell lines were cultured and maintained in DMEM
supplemented with 10% (volume of solute/volume of solution) ×100
(volume percent) FBS and incubated at 37°C with 5% CO2
humidified atmosphere. The cells were passaged subsequent to 80%
fusion.
MTT assay and LDH release assay
Cell viability was determined using MTT and LDH
assays. The release of LDH in the culture medium was determined
using a commercial kit. Following treatment with 0, 3, 10, 30, 100
and 300 µm PT93 for 24 h, the supernatant was drew from the media
of per well and centrifuged at 400 × g (RCF) for 5 min at
4°C, then 20 µl supernatant was transferred into another 96-well
microplate to determine LDH levels prior to adding MTT, according
to the manufacturers protocol. The optical density was measured
using a microplate reader (Omega Bio-Tek, Inc., Norcross, GA, USA)
at 450 nm. For the MTT assay, MTT (5 mg/ml) was added to each well
and the mixture was incubated for 2 h at 37°C. The MTT reagent was
then replaced with DMSO (100 µl per well) to dissolve the formazan
crystals. Subsequent to the mixture being agitated at room
temperature for 10 min, absorbance was determined at 570 nm using a
microplate reader (Omega Bio-Tek, Inc.). The samples without any
drug were the control group. The results were expressed as the
percentage absorbance of the control cells, which was set as 100%.
All experiments were performed in triplicate, n=3 per group.
Cell colony formation assay
T98G cells were seeded onto a 6-well plate at a
density of 50–60 cells per well. The cells were maintained in DMEM
containing 10% FBS and 0, 3 and 10 µm PT93, and incubated at 37°C
with 5% CO2 humidified atmosphere for 1–2 weeks. The
samples not administered any drug were the control group. After
treated with PT93 for 1–2 weeks (w), cells was washed with PBS,
then fixed with 5 ml methanol for 20 min and stained with Giemsa
staining solution for 30 min. The stained cells were washed with
PBS and air-dried, then observed using a low power lens. Colonies
with >50 cells were counted. The results were expressed as the
percentage of control group, which was set as 100%.
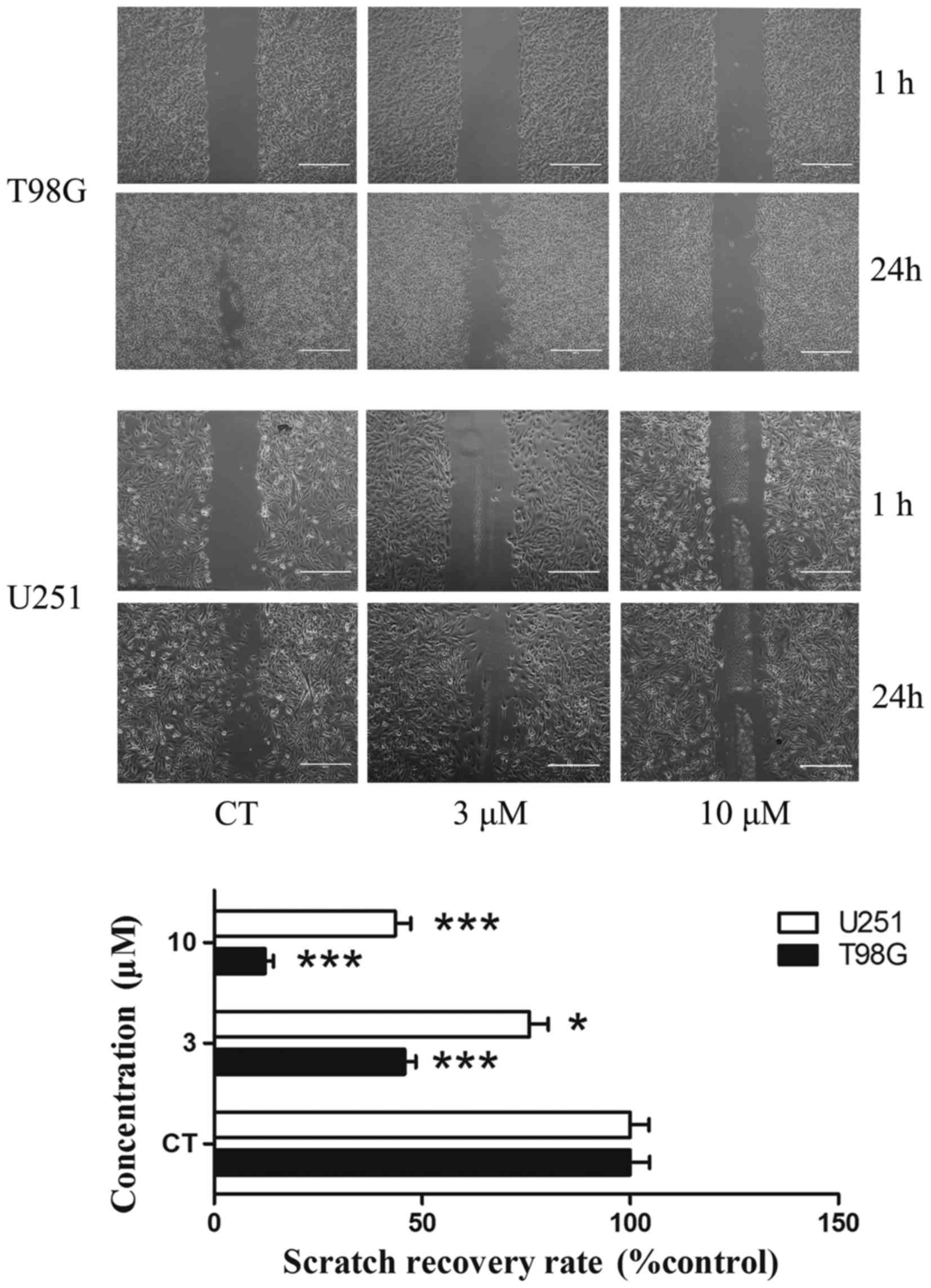
Scratch test
The cell migration ability was assessed by a scratch
test. T98G and U251 cells were seeded onto a 6-well plate at a
density of 2×105 cells/ml, and cultured at 37°C with 5%
CO2 humidified atmosphere for 24 h. The linear scratch
was made with a 200 µl sterile pipette tip, and the cells were
subjected to 0, 3 and 10 µm PT93 treatment. The samples not
administered any drug were the control group. Subsequent to 24 h,
scratch wound healing was observed under an inverted microscope.
The recovery distances of the scratches were compared with the
control group.
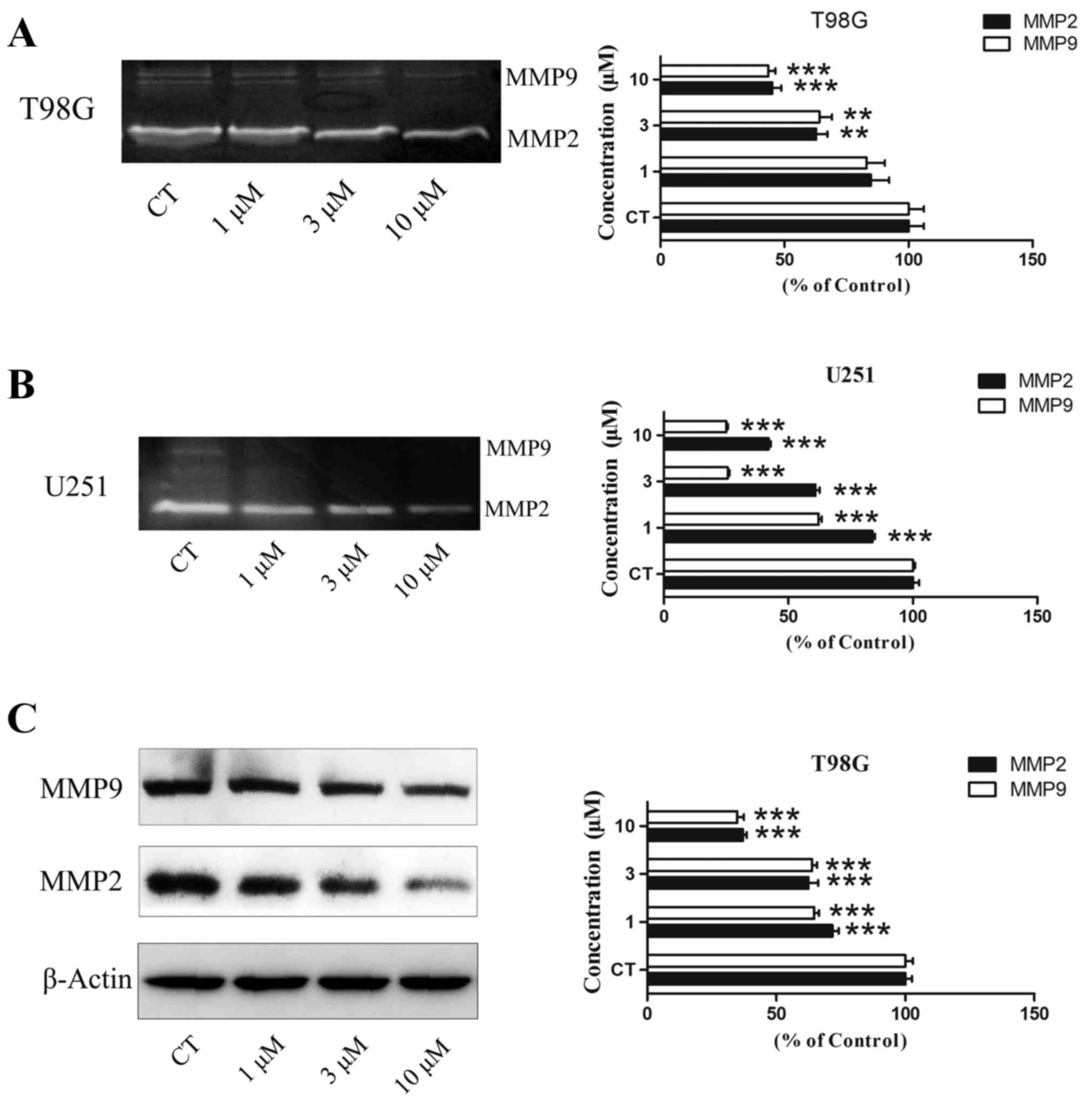
Gelatin zymography analysis
The activity levels of MMP-2 and MMP-9 were
estimated by gelatin zymography analysis. Cells were incubated with
0, 1, 3 and 10 µm PT93 in serum-free DMEM at 37°C for 24 h. Then
culture supernatants were collected and centrifuged at 356 ×
g (RCF) for 10 min at 4°C, and the supernatants were
collected again. The cells not administered any drug were the
control groups. MMP activity in the supernatant was measured using
the MMP Zymography assay kit (Chemicon, Merck Millipore), according
to the protocol of the manufacturer. The samples were separated by
SDS-PAGE, under non-reducing conditions, using gels containing 10%
gelatin and at 4°C. Subsequent to electrophoresis, the gels were
washed twice for 30 min each with solution A, then incubated with
solution B for 10 h. Finally, the gel was stained with Coomassie
Blue R-250 (Bio-Rad Laboratories, Inc., Hercules, CA, USA). Clear
bands indicating digested substrate were detected against a dark
blue background, representing the areas of MMP activity. Bands were
quantified using image quantitative analysis software (ImageJ 1.45,
USA).
Cell extraction and western
blotting
Cells were washed twice with ice-cold PBS and
suspended in 80 µl of lysis buffer (20 mM Tris-hydrochloride, 150
mM sodium chloride, 1 mM EDTA, 1% Triton X-100, protease inhibitor
cocktail, 2 mM sodium orthovanadate and 10 mM sodium fluoride, pH
7.5). The protein concentration was determined using a
bicinchoninic acid assay kit (Pierce, Thermo Fisher Scientific
Inc.). A total of 20 µg protein was loaded in each lane. The
proteins were separated using SDS-PAGE and electrically transferred
to a polyvinylidene fluoride membrane (EMD Millipore). Subsequent
to blocking the membrane with 5% skim milk, the target proteins
were immunodetected using primary antibodies aforementioned in the
reagents section. Following incubation with the secondary
antibodies, the bands were detected by the enhanced
chemiluminescence technique (Landbiology, Guangzhou, China).
Statistical analysis
All experiments described in the present study were
repeated at ≥3 time points. The data were presented as the mean ±
standard deviation. Statistical analyses between 2 groups were
performed by unpaired Student's t-test. Differences among groups
were tested by one-way analysis of variance following by a Tukey's
test. P<0.05 was considered to indicate a statistically
significant difference.
Results
PT93 showed no significant
cytotoxicity on GBM and normal neuron cell lines at low
concentration
The structure and synthesis process of PT93 are
demontrated in Fig. 1A and B. The
inhibitory effect of PT93 on cell viability in human GBM T98G,
U251, U87 cells and normal mouse neuron HT22 cells was measured
using an MTT assay subsequent to 24 h exposure. A
concentration-dependent inhibitive effect on cell viability was
observed in the aforementioned 4 types of cell lines. As shown in
Fig. 1C, the concentration of
inhibitor where inhibition is reduced by half (IC50) in
T98G, U87, U251 and HT22 cells were 300, 272, 272 and 200 µM,
respectively.
Furthermore, the present study tested the
cytotoxicity effects of PT93 in T98G, U87, U251 cells using an LDH
assay. No significant cytotoxicity was observed at low
concentrations (<30 µM) in T98G, U251 and U87 cells. As shown in
Fig. 1C, although the level of LDH
release of T98G, U251 and U87 cells increased at concentrations of
100 and 300 µM, a significant difference (P=0.0252) was only
observed at 300 µM in U87 cells.
PT93 inhibited the proliferation of
T98G cells
The proliferative ability of the T98G cells was
tested with a cell colony formation assay. The cells were treated
with 0, 3 or 10 µM PT93 for 1 week and the results of cell colonies
are shown in Fig. 1Ea and Eb. PT93
significantly (P=0.0051 and 0.0007) inhibited cell colony formation
in a dose-dependent manner in T98G cells. The colony numbers
decreased between 251±45 and 200±26 and 103±15 when the cells were
treated with 3 and 10 µM PT93, respectively.
Scratch migration assay
A scratch migration assay was performed to confirm
the influence of PT93 in the migration of T98G and U251 cells. T98G
cells were treated with 0, 3 and 10 µM PT93 subsequent to a wound
being made in the cell layer. In the scratch migration assay, PT93
inhibited the migration of T98G and U251 cells in a dose-dependent
manner. PT93 significantly (P<0.05 or P<0.001) limited the
migration of the cell lines at 3 and 10 µM (Fig. 2).
Gelatin zymography and western
blotting assay
Gelatin zymography was used to test the level of
MMP-2/−9 activity. Subsequent to the T98G and U251 cells being
treated with PT93 at 0, 1, 3 and 10 µM for 24 h, the supernatant
was extracted to detect the activity of MMP-2 and MMP-9. The
results revealed that PT93 significantly (P<0.01 or P<0.001)
decreased the level of MMP-2 and MMP-9 activity in a
concentration-dependent manner (Fig. 3A
and B). Western blot analysis was used to measure the level of
MMP-2/−9 expression. The results demonstrated that PT93 suppresses
MMP-2/−9 expression in a dose-dependent manner (Fig. 3C).
Discussion
The conventional treatments of GBM include surgery
and radiotherapy, however the prognosis is extremely poor (23). Subsequent to the approval of
temozolomide (TMZ) by the Food and Drug Administration in 1999,
post-operative radiotherapy (RT) combined with TMZ chemotherapy has
been developed as the standard therapy for newly diagnosed GBM
(24). The median survival length
subsequent to treatment with RT plus TMZ was 14.6 months compared
with 12.1 months for RT alone (10,23,25).
Therefore, the exploration of novel agents that could be used alone
or combined with TMZ to improve the outcome of GBM is required.
The present study investigated the effects of PT93
in GBM cell lines, examining cell viability, cytotoxicity,
proliferation, migration and MMP inhibition. Firstly, the present
study tested the inhibitory effect of PT93 on the cell viability of
the GBM T98G, U87 and U251 cell lines, and the normal mouse neuron
HT22 cell line, using an MTT assay. The MTT assay results showed
that PT93 reduced cell viability in the 4 types of cell line in a
dose-dependent manner (Fig. 1C). The
IC50 in T98G, U87, U251 and HT22 was 300, 272, 272 and
200 µM, respectively. Furthermore, the present study tested the
cytotoxicity of PT93 in T98G, U87 and U251 cells using an LDH
assay. The result of the LDH assay demonstrated that although the
release of LDH increased as the concentration of PT93 increased, a
statistically significant difference was only observed at 300 µM in
the U87 cell line (P<0.05). The results of the MTT and LDH
assays suggested that cell viability reduced by PT93 may be
associated with the inhibition of cell proliferation.
The present study tested the anti-proliferation
effect of PT93 using a cell colony formation assay. PT93 exhibited
potent inhibition of the colony formation of T98G cells. In
contrast to the control group, PT93 significantly suppressed the
proliferation of the T98G cells at concentrations of 3 and 10 µM
(Fig. 1D). The present then study
investigated the effect of PT93 on the migration of T98G and U251
cells using a scratch test. Under low-serum conditions, PT93
significantly suppressed the migration of the aforementioned cells
at concentrations of 3 and 10 µM compared with the control group (0
µM). Cell motility and MMPs are involved in the invasion ability of
tumors. PT93 was reported as a selective MMP inhibitor, inhibiting
MMP-2 and MMP-9 (22). A number of
studies demonstrated that MMPs are associated with the ability of
tumors to migrate and invade (12,26,27). First
generation MMPIs with broad-spectrum MMP inhibitory ability cause
unexpected side effects, including musculoskeletal pain and
inflammation (12,15,16). Thus,
MMPIs which selectively inhibit MMP-2/9 may suppress the migration
of cancer cells and reduce the number of side effects. The present
study hypothesized that the anticancer effect of PT93 in GBM cell
lines may be associated with MMP inhibition, and confirmed this by
treating the T98G cells with increasing concentrations of PT93 and
testing the MMP activity using gelatin zymography. In the
aforementioned analysis, the activity of MMP-2 and MMP-9, which
were secreted by T98G and U251 cells, decreased as drug
concentration increased. At 10 µm, PT93 significantly suppressed
extracellular MMP-2 and MMP-9 activity compared with the control
group (Fig. 3A and B). Furthermore,
the present study used western blotting to measure the expression
of MMP-2/−9 in T98G. The results of the western blot analysis
revealed that PT93 suppresses intracellular MMP-2 and MMP-9
expression. The authors infer that PT93 may also reduce the level
of MMP-2/−9 secretion, potentially explaining why the CA derivative
affects extracellular MMP-2 and MMP-9 activity.
The present study confirmed that the anticancer
effect of PT93 was associated with the inhibition of the migration
and proliferation of GBM cells, and the level of MMP-2/−9
expression. In contrast with first generation MMPIs, PT93 may
exhibit fewer side effects. TMZ has been reported to inhibit the
migration of U251 cells at a concentration of 100 µM (24). Therefore, the effect of PT93 may
combine with the effect of TMZ to enhance the anti-migration
ability and reduce the invasiveness of GMB. PT93 exhibits potential
in the treatment of GBM as a new generation MMPI.
Acknowledgements
The present study was supported by the Fundamental
Research Funds for Guangdong Provincial Project of Science and
Technology (grant nos. 2014A020212091, 2014A020212096 and
2016A020215061).
References
|
1
|
Shi J, Sun B, Shi W, Zuo H, Cui D, Ni L
and Chen J: Decreasing GSH and increasing ROS in chemosensitivity
gliomas with IDH1 mutation. Tumour Biol. 36:655–662. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Baldock AL, Ahn S, Rockne R, Johnston S,
Neal M, Corwin D, Clark-Swanson K, Sterin G, Trister AD, Malone H,
et al: Patient-specific metrics of invasiveness reveal significant
prognostic benefit of resection in a predictable subset of gliomas.
PLoS One. 9:e990572014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Bellail AC, Hunter SB, Brat DJ, Tan C and
Van Meir EG: Microregional extracellular matrix heterogeneity in
brain modulates glioma cell invasion. Int J Biochem Cell Biol.
36:1046–1069. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Wang M, Yoshida D, Liu S and Teramoto A:
Inhibition of cell invasion by indomethacin on glioma cell lines:
In vitro study. J Neurooncol. 72:1–9. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Jacob A and Prekeris R: The regulation of
MMP targeting to invadopodia during cancer metastasis. Front Cell
Dev Biol. 3:42015. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Rao JS: Molecular mechanisms of glioma
invasiveness: The role of proteases. Nat Rev Cancer. 3:489–501.
2003. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Alam S and Kelleher SL: Cellular
mechanisms of zinc dysregulation: A perspective on zinc homeostasis
as an etiological factor in the development and progression of
breast cancer. Nutrients. 4:875–903. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Tian L, Zhang Y, Chen Y, Cai M, Dong H and
Xiong L: EMMPRIN is an independent negative prognostic factor for
patients with astrocytic glioma. PLoS One. 8:e580692013. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lee EJ, Kim SY, Hyun JW, Min SW, Kim DH
and Kim HS: Glycitein inhibits glioma cell invasion through
down-regulation of MMP-3 and MMP-9 gene expression. Chem Biol
Interact. 185:18–24. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Levicar N, Nuttall RK and Lah TT:
Proteases in brain tumour progression. Acta Neurochir (Wien).
145:825–838. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Alcantara MB and Dass CR: Pigment
epithelium-derived factor as a natural matrix metalloproteinase
inhibitor: A comparison with classical matrix metalloproteinase
inhibitors used for cancer treatment. J Pharm Pharmacol.
66:895–902. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Hadler-Olsen E, Winberg JO and
Uhlin-Hansen L: Matrix metalloproteinases in cancer: Their value as
diagnostic and prognostic markers and therapeutic targets. Tumour
Biol. 34:2041–2051. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Singh D, Srivastava SK, Chaudhuri TK and
Upadhyay G: Multifaceted role of matrix metalloproteinases (MMPs).
Front Mol Biosci. 2:192015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Yoshizaki T, Sato H and Furukawa M: Recent
advances in the regulation of matrix metalloproteinase 2
activation: From basic research to clinical implication (Review).
Oncol Rep. 9:607–611. 2002.PubMed/NCBI
|
|
15
|
Dufour A and Overall CM: Missing the
target: Matrix metalloproteinase antitargets in inflammation and
cancer. Trends Pharmacol Sci. 34:233–242. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Vandenbroucke RE and Libert C: Is there
new hope for therapeutic matrix metalloproteinase inhibition? Nat
Rev Drug Discov. 13:904–927. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
17
|
Liu RH: Health-promoting components of
fruits and vegetables in the diet. Adv Nutr. 4:384S–392S. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Park WH, Kim SH and Kim CH: A new matrix
metalloproteinase-9 inhibitor 3,4-dihydroxycinnamic acid (caffeic
acid) from methanol extract of Euonymus alatus: Isolation and
structure determination. Toxicology. 207:383–390. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Chiang EP, Tsai SY, Kuo YH, Pai MH, Chiu
HL, Rodriguez RL and Tang FY: Caffeic acid derivatives inhibit the
growth of colon cancer: Involvement of the PI3-K/Akt and AMPK
signaling pathways. PLoS One. 9:e996312014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Ozturk G, Ginis Z, Akyol S, Erden G, Gurel
A and Akyol O: The anticancer mechanism of caffeic acid phenethyl
ester (CAPE): Review of melanomas, lung and prostate cancers. Eur
Rev Med Pharmacol Sci. 16:2064–2068. 2012.PubMed/NCBI
|
|
21
|
Chung TW, Moon SK, Chang YC, Ko JH, Lee
YC, Cho G, Kim SH, Kim JG and Kim CH: Novel and therapeutic effect
of caffeic acid and caffeic acid phenyl ester on hepatocarcinoma
cells: Complete regression of hepatoma growth and metastasis by
dual mechanism. FASEB J. 18:1670–1681. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shi ZH, Li NG, Shi QP, Tang H, Tang YP, Li
W, Yin L, Yang JP and Duan JA: Synthesis and structure-activity
relationship analysis of caffeic acid amides as selective matrix
metalloproteinase inhibitors. Bioorg Med Chem Lett. 23:1206–1211.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Stupp R, Mason WP, van den Bent MJ, Weller
M, Fisher B, Taphoorn MJ, Belanger K, Brandes AA, Marosi C, Bogdahn
U, et al: Radiotherapy plus concomitant and adjuvant temozolomide
for glioblastoma. N Engl J Med. 352:987–996. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Villano JL, Seery TE and Bressler LR:
Temozolomide in malignant gliomas: Current use and future targets.
Cancer Chemother Pharmacol. 64:647–655. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wait SD, Prabhu RS, Burri SH, Atkins TG
and Asher AL: Polymeric drug delivery for the treatment of
glioblastoma. Neuro Oncol. 17:(Suppl 2). ii9–ii23. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Davies KJ: The complex Interaction of
matrix metalloproteinases in the migration of cancer cells through
breast tissue stroma. Int J Breast Cancer. 2014:8390942014.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Könnecke H and Bechmann I: The role of
microglia and matrix metalloproteinases involvement in
neuroinflammation and gliomas. Clin Dev Immunol. 2013:9141042013.
View Article : Google Scholar : PubMed/NCBI
|