Introduction
Pancreatic neuroendocrine tumors (PNETs) are a rare
type of malignancy with an annual incidence of 1/100,000 people,
and account for ~1–2% of all pancreatic tumors (1). PNETs are divided into functional tumors,
which cause specific hormonal syndromes such as hypoglycemic
attack, and non-functional tumors (2). The prognosis of PNETs is relatively
favorable compared with that for pancreatic adenocarcinoma;
however, certain patients with PNETs exhibit distant metastasis and
have a less favorable prognosis (3).
In 2010, the World Health Organization (WHO) categorized PNETs into
G1 and G2 PNETs and neuroendocrine carcinoma (NEC), based on
mitotic counts and the Ki-67 index (4). These classifications are useful for
predicting prognosis and postoperative recurrence (5,6). However,
certain patients with low-risk pathologic features may unexpectedly
experience distant metastasis and postsurgical recurrence (5). Little is currently known about the
underlying molecular mechanisms by which distant metastasis is
promoted in patients with PNETs.
Invasion into the surrounding normal tissue is a
critical step for primary tumors to metastasize to distant areas
(7). Recent studies have reported
that the epithelial-mesenchymal transition (EMT) serves an
important role in tumor progression and metastasis (8). Through EMT, cancer cells acquire the
ability to migrate and infiltrate through the extracellular matrix,
which leads to distant metastasis (7). At the molecular level, the
transcriptional reprogramming of EMT is triggered by the
transcription factors Snail, Slug and Twist, leading to subsequent
suppression of specific adhesion molecules, including E-cadherin
(9). E-cadherin is expressed in
epithelial cells and performs an essential role in cell-cell
contact (10). The expression of
E-cadherin is decreased during EMT in embryonic development, tissue
fibrosis and cancer (10). Snail was
initially identified in Drosophila as the zinc-finger
transcriptional repressor that binds to the E-boxes of the
E-cadherin promoter (11,12). Snail is able to effectively induce EMT
via suppressing the transcription of E-cadherin during tumor
progression (13). The loss of
E-cadherin expression promotes Wnt signaling, and is associated
with high levels of Snail in the nucleus (14). It was reported that high expression
levels of Snail and low expression levels of E-cadherin are
inversely correlated with the prognosis of patients with breast
cancer (14). With the aim of gaining
insight into the underlying molecular alterations in metastatic
PNETs, the present study focused on EMT by evaluating Snail and
E-cadherin expression. The significance of Snail and E-cadherin
expression patterns in distant metastasis and prognosis was
investigated in patients who had received surgery for PNETs.
Materials and methods
Patients and tumor samples
Formalin-fixed paraffin-embedded blocks were
obtained, containing tissue samples from 40 patients with
histologically proven PNETs and who underwent surgical resection at
Kagoshima University Hospital (Kagoshima, Japan) between January
1995 and December 2015. All resected tissue specimens were
histologically examined using hematoxylin and eosin staining
according to the tumor-node-metastasis classification and WHO
classification 2010 systems (4,15). The
present study was approved by the Institutional Ethics Review Board
of Kagoshima University Hospital, and written informed consent was
obtained from all patients.
Antibodies
The rabbit anti-human polyclonal antibody directed
against Snail (catalogue no. ab85936; Abcam, Cambridge, UK) was
diluted at 1:500. The mouse anti-human monoclonal antibody against
E-cadherin (catalogue no. M361229-2; Dako; Agilent Technologies,
Inc., Santa Clara, CA, USA) was diluted at 1:500.
Immunohistochemical staining
All specimens were fixed in 10% formalin at room
temperature and processed routinely. Sections (3 µm-thick) were cut
from the paraffin blocks of primary tumors. Following
deparaffinization in xylene and rehydration in graded solutions of
ethanol, endogenous peroxidase activity was blocked by immersing
the slides in absolute methanol solution containing 3% hydrogen
peroxide for 30 min at room temperature. The sections were then
treated with 1% goat serum albumin for Snail (catalogue no. S-1000)
or 1% horse serum albumin for E-cadherin (catalogue no. S-2000)
(both Vector Laboratories, Inc., Burlingame, CA, USA) for 30 min at
room temperature to block nonspecific reactions. Heat-induced
antigen retrieval via autoclave pretreatment (120°C for 5 min) in
citrate buffer solution (pH 6.0) was performed. The sections were
rinsed with PBS and incubated with the Snail antibody (dilution,
1:500) for 180 min and the E-cadherin antibody (dilution, 1:500)
for 60 min at room temperature. Following incubation, the specimens
were visualized using an Vectastain Elite ABC IgG Rabbit (catalogue
no. PK-6101) or Mouse (catalogue no. PK-6102) kits (both Vector
Laboratories, Inc.) and the Liquid DAB+ Substrate Chromogen system
(catalogue no. K3468; Dako; Agilent Technologies, Inc.), according
to the manufacturer's protocol. The slides were counterstained with
hematoxylin prior to mounting. All reactions were performed with
positive controls (mouse heart tissue for Snail and normal
pancreatic tissue for E-cadherin). For the negative control, the
primary antibody was replaced with PBS. No significant staining was
observed in the negative-control sections.
Evaluation of
immunohistochemistry
All tissue sections were simultaneously assessed by
two investigators who were blinded to the patient
clinicopathological data. Each slice was observed under an Olympus
CX31 optical microscope (Olympus Corporation, Tokyo, Japan)
(magnification, ×400). Stained cells were assessed and quantified
in five randomly selected fields. The immunohistochemical staining
of Snail was classified as high expression if ≥10% of neoplastic
cells exhibited nuclear staining, or as low expression if <10%
of neoplastic cells were stained. The expression of E-cadherin was
compared between tumor cells and normal islet cells located
adjacent to the tumor. Tumor cells that exhibited equivocal
staining to normal islet cells were considered to have preserved
expression of E-cadherin, whereas those that exhibited less intense
staining patterns compared with the normal islet cells, or those
that did not stain at all, were considered to have reduced
expression of E-cadherin.
Statistical analysis
Associations between different categorical variables
were assessed using Fisher's exact test and the χ2 test.
The Kaplan-Meier method was used for survival analysis, and
variations in survival rates were estimated using the log-rank
test. All statistical analysis was performed using SPSS version
22.0 (IBM SPSS, Armonk, NY, USA). All tests were two-sided.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Patient characteristics
Table I lists the
clinicopathological features of the patients enrolled in the
present study. The study group comprised 17 men and 23 women with
an age range between 18 and 79 years (mean, 56.1 years). Lymph node
metastasis was observed in 7 patients (17.5%). Distant metastasis
was observed in 9 patients (22.5%), and the site of metastasis was
the liver. Postoperative recurrence or an increase in the residual
tumor size was observed in 10 (25.0%) patients.
 | Table I.Clinicopathological parameters of
pancreatic neuroendocrine tumors (n=40). |
Table I.
Clinicopathological parameters of
pancreatic neuroendocrine tumors (n=40).
| Characteristics | n (%) |
|---|
| Age, years |
|
|
>60 | 17 (42.5) |
| ≤60 | 23 (57.5) |
| Gender |
|
| Male | 17 (42.5) |
|
Female | 23 (57.5) |
| Tumor size |
|
| >20
mm | 13 (32.5) |
| ≤20
mm | 27 (67.5) |
| Lymph node
metastasis |
|
| No | 33 (82.5) |
| Yes | 7 (17.5) |
| Liver metastasis |
|
| No | 31 (77.5) |
| Yes | 9 (22.5) |
| WHO
classification |
|
| G1 | 22 (55.0) |
| G2 | 15 (37.5) |
| NEC | 3 (7.5) |
| Vascular
invasion |
|
| No | 24 (60.0) |
| Yes | 16 (40.0) |
| Lymphatic
invasion |
|
| No | 28 (70.0) |
| Yes | 12 (30.0) |
| Functionality |
|
|
Functioning | 23 (57.5) |
|
Inslinoma | 15 (37.5) |
|
Glucagonoma | 4 (10.0) |
|
Gastrinoma | 4 (10.0) |
|
Nonfunctioning | 17 (42.5) |
Expression of Snail and E-cadherin in
PNETs
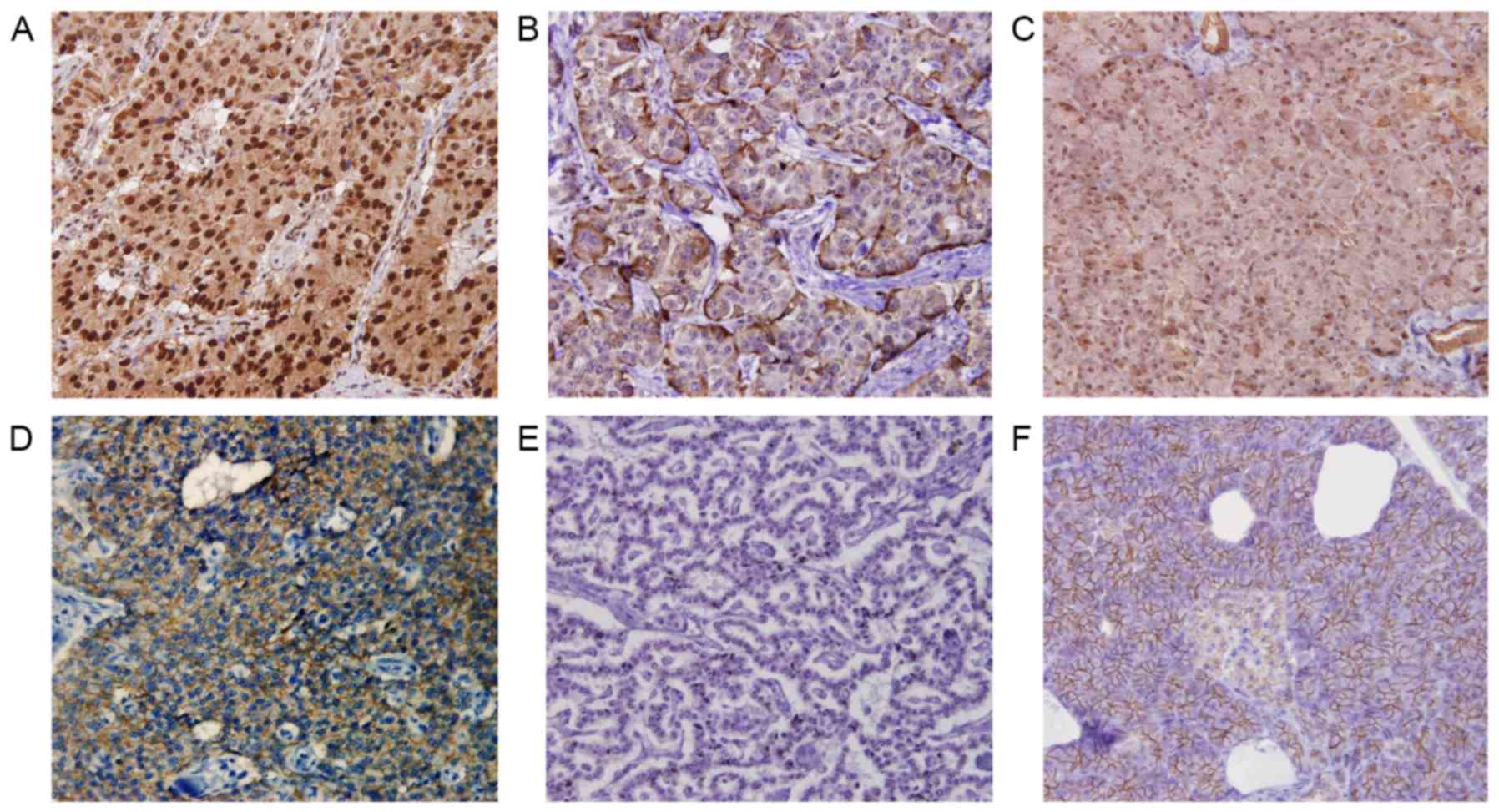
Fig. 1 depicts
representative images of Snail and E-cadherin expression. Snail
expression was identified in the nucleus of neoplastic cells. The
proportion of immunoreactive cells varied, ranging from 0 to
>50% of neoplastic cells. The expression of E-cadherin in
neoplastic cells was heterogeneous, ranging from 0 to ~100% of
neoplastic cells. High and low Snail expression levels were
observed in 11 (27.5%) and 29 (72.5%) patients, respectively.
Preserved and reduced E-cadherin expression was observed in 19
(47.5%) and 21 (52.5) patients, respectively.
Association between Snail and
E-cadherin expression and clinicopathological features
Table II demonstrates
the association between Snail/E-cadherin expression and various
clinicopathological factors. High Snail expression levels were
significantly associated with high incidences of lymphatic and
vascular invasion and liver metastasis (P=0.0078, P<0.0001 and
P=0.0067, respectively; Table II).
Reduced E-cadherin expression levels were also significantly
associated with high incidences of lymphatic and vascular invasion
and liver metastasis (P=0.0015, P=0.0270 and P=0.0214,
respectively; Table II). No
significant difference was observed in Snail and E-cadherin
expression according to gender, tumor size and tumor functionality.
Furthermore, on the basis of the expression profiles of Snail and
E-cadherin, all patients were divided into two groups as follows:
Low Snail and preserved E-cadherin expression, and the ‘other
group’ (patients with high Snail and reduced E-cadherin, high Snail
and preserved E-cadherin, and low Snail and reduced E-cadherin
expression) (Table III). Patients
in the ‘other group’ experienced a markedly increased lymph node
metastasis, liver metastasis, G2 and NEC in the WHO classification,
vascular invasion and lymphatic invasion, as compared with in the
low Snail and preserved E-cadherin expression group. Patients with
low Snail and preserved E-cadherin expression were not observed to
exhibit lymph node metastasis or liver metastasis during the
present study. In total, 1/22 patients with a G1 classified tumor
experience liver metastasis; these particular tumor tissues
exhibited high Snail and reduced E-cadherin expression levels.
 | Table II.Association between the expression of
Snail/E-cadherin and clinicopathological factors in pancreatic
neuroendocrine tumors. |
Table II.
Association between the expression of
Snail/E-cadherin and clinicopathological factors in pancreatic
neuroendocrine tumors.
|
|
| Snail expression,
n |
| E-cadherin
expression, n |
|
|---|
|
|
|
|
|
|
|
|---|
| Characteristics | Total, n | Low | High | P-value | Reduced | Preserved | P-value |
|---|
| Total | 40 | 29 | 11 |
| 21 | 19 |
|
| Age, years (mean ±
SD) |
| 56.0±3.20 | 56.4±4.46 | NS | 59.4±3.62 | 52.5±3.63 | NS |
| Gender |
|
|
| NS |
|
| NS |
| Male | 17 | 11 | 6 |
| 9 | 8 |
|
|
Female | 23 | 18 | 5 |
| 12 | 11 |
|
| Tumor size |
|
|
| NS |
|
| NS |
| >20
mm | 13 | 9 | 4 |
| 9 | 4 |
|
| ≤20
mm | 27 | 20 | 7 |
| 12 | 15 |
|
| Lymph node
metastasis |
|
|
|
|
|
| NS |
| No | 33 | 26 | 7 |
| 15 | 18 |
|
|
Yes | 7 | 3 | 4 | NS | 6 | 1 |
|
| Liver
metastasis |
|
|
| 0.0067 |
|
| 0.0214 |
| No | 31 | 26 | 5 |
| 13 | 18 |
|
|
Yes | 9 | 3 | 6 |
| 8 | 1 |
|
| WHO
classification |
|
|
| NS |
|
| NS |
| G1 | 22 | 18 | 4 |
| 9 | 13 |
|
| G2 +
NEC | 18 | 11 | 7 |
| 12 | 6 |
|
| Vascular
invasion |
|
|
| <0.0001 |
|
| 0.0270 |
| No | 24 | 23 | 1 |
| 9 | 15 |
|
|
Yes | 16 | 6 | 10 |
| 12 | 4 |
|
| Lymphatic
invasion |
|
|
| 0.0078 |
|
| 0.0015 |
| No | 28 | 24 | 4 |
| 10 | 18 |
|
|
Yes | 12 | 5 | 7 |
| 11 | 1 |
|
| Functionality |
|
|
| NS |
|
| NS |
|
Functioning | 23 | 18 | 5 |
| 12 | 11 |
|
|
Nonfunctioning | 17 | 11 | 6 |
| 9 | 8 |
|
 | Table III.Association between the expression of
Snail and E-cadherin and clinicopathological factors in pancreatic
neuroendocrine tumors. |
Table III.
Association between the expression of
Snail and E-cadherin and clinicopathological factors in pancreatic
neuroendocrine tumors.
|
| Snail and
E-cadherin expression, n |
|
|---|
|
|
|
|
|---|
| Characteristic | Total | Snail low
+E-cadherin preserved | Other
groupa | P-value |
|---|
| Total | 40 | 16 | 24 |
|
| Age, years (mean ±
SD) |
| 51.8±4.23 | 59.0±3.22 | NS |
| Gender |
|
|
| NS |
|
Male | 17 | 6 | 11 |
|
|
Female | 23 | 10 | 13 |
|
| Tumor size |
|
|
| NS |
| >20
mm | 13 | 4 | 9 |
|
| ≤20
mm | 27 | 12 | 15 |
|
| Lymph node
metastasis |
|
|
| 0.0295 |
| No | 33 | 16 | 17 |
|
|
Yes | 7 | 0 | 7 |
|
| Liver
metastasis |
|
|
| 0.0060 |
| No | 31 | 16 | 15 |
|
|
Yes | 9 | 0 | 9 |
|
| WHO
classification |
|
|
| 0.0349 |
| G1 | 22 | 10 | 12 |
|
| G2 +
NEC | 18 | 6 | 12 |
|
| Vascular
invasion |
|
|
| 0.0007 |
| No | 24 | 15 | 9 |
|
|
Yes | 16 | 1 | 15 |
|
| Lymphatic
invasion |
|
|
| 0.0009 |
| No | 28 | 16 | 12 |
|
|
Yes | 12 | 0 | 12 |
|
| Functionality |
|
|
| NS |
|
Functioning | 23 | 10 | 13 |
|
|
Nonfunctioning | 17 | 6 | 11 |
|
Prognostic impact of Snail and
E-cadherin expression
The 5-year and 10-year survival rates for the
patient cohort were determined to be 91.7 and 59.5%, respectively.
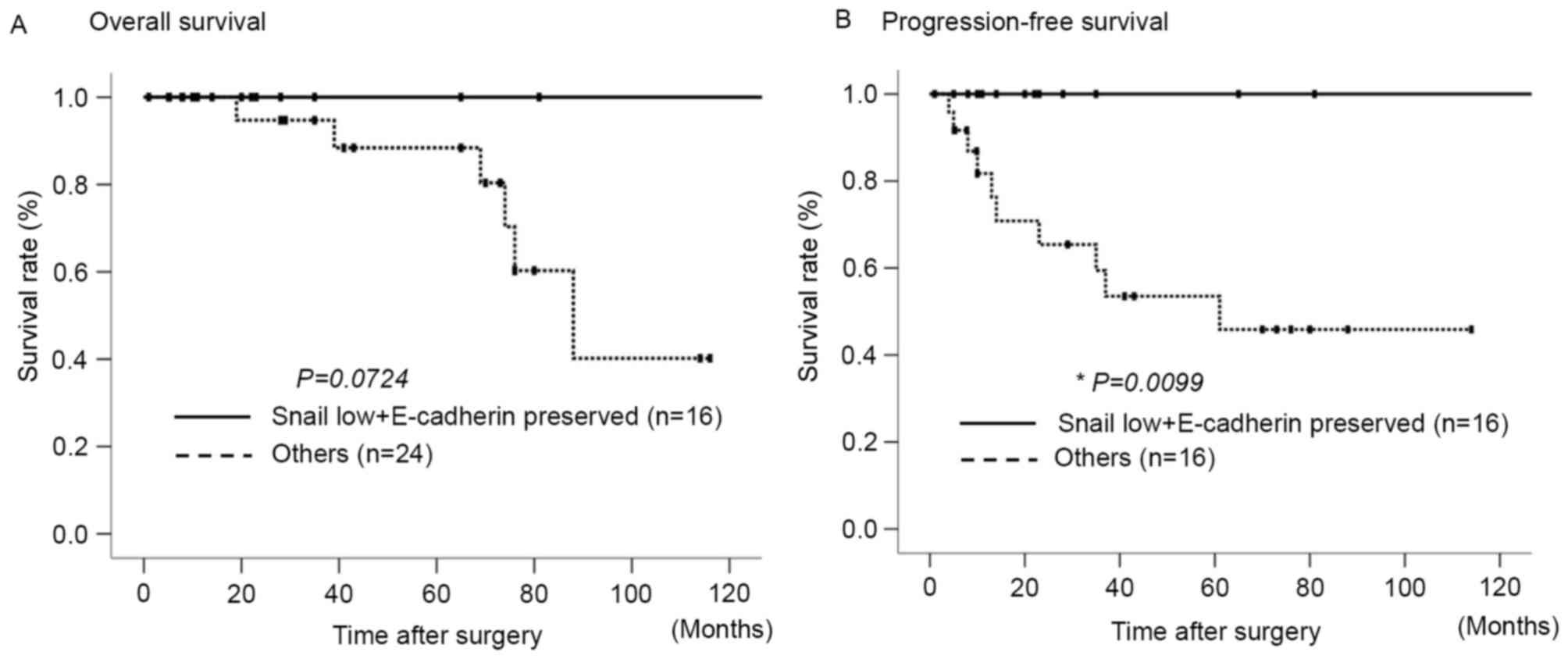
Fig. 2 presents the overall survival
(OS) and progression-free survival (PFS) rates following surgery in
the aforementioned two groups, based on the expression levels of
Snail and E-cadherin. The ‘other group’ exhibited a poorer OS rate,
as compared with the low Snail and preserved E-cadherin expression
group, although these differences were not statistically
significant (Fig. 2A). By contrast, a
significant difference was observed between the two groups with
regard to the rate of PFS (P=0.0099; Fig.
2B).
Discussion
The present study demonstrated that the analysis of
Snail and E-cadherin co-expression may be useful for predicting
vascular invasion, lymphatic invasion and liver metastasis in
patients with PNETs. The PNET tissue samples in the present study
were divided into the following two groups: Tumors with low Snail
and preserved E-cadherin expression, and the other tumors which
consisted of any tumor tissues with high Snail and reduced
E-cadherin, high Snail and preserved E-cadherin, and low Snail and
reduced E-cadherin expression. Tumors in the low Snail and
preserved E-cadherin expression group and in the ‘other group’ were
considered to have epithelial and mesenchymal phenotypes,
respectively. PNETs with a mesenchymal phenotype exhibited a
significantly higher frequency of vascular and lymphatic invasion,
lymph node metastasis and liver metastasis. Even in G2 tumors and
NEC, those with low Snail and preserved E-cadherin expression did
not have lymph node and liver metastasis. In PNETs, liver
metastasis is the most frequent mode of recurrence and a
significant adverse prognostic factor (16,17). Our
study indicated that EMT serves an important role in lymphatic
invasion, vascular invasion, lymph node metastasis and liver
metastasis in PNETs. Immunohistochemical evaluations of the EMT
markers Snail and E-cadherin were useful for predicting
metastasis.
It has been reported that EMT performs a fundamental
role in tumor invasion and metastasis in various types of cancer.
In pancreatic adenocarcinoma, numerous previous studies have
revealed that Snail, zinc finger E-box-binding homeobox (ZEB) 1,
ZEB2 and nectins are associated with EMT and with poor prognosis
(18–20). However, the role of EMT in PNETs is
still unclear. Fendrich et al (21) immunohistochemically evaluated the
expression patterns of the EMT markers E-cadherin, Snail and Twist
in human PNETs, and observed a loss of E-cadherin and the
overexpression of Snail and Twist in the majority of malignant
PNETs. In addition, the formation of islet cell tumors in the
RIP-Tag2 transgenic mouse model was prevented by inhibiting Snail
expression using polyethylene glycol. This result indicated that
EMT serves a key role in the tumorigenesis of PNETs. Galvan et
al (22) also used
immunohistochemistry to assess the expression of EMT markers in 91
cases of gastroenteropancreatic (GEP) NET, including 22 cases of
PNET. It was reported that alteration of the E-cadherin/β-catenin
complex, specifically the high Snail expression and cytoplasmic
E-cadherin pattern, reduced the survival rates of patients with
GEP-NETs. Taken together, the current study and previous reports on
PNETs have demonstrated that tumor cells that have acquired an
invasive phenotype through EMT may easily detach from the primary
tumor, invade surrounding tissues and enter microvessels, therefore
spreading into the circulation (21,22).
In the present study, the expression of Snail and
E-cadherin was not significantly associated with the rate of OS.
This is partly as the recurrence of PNETs, which most commonly
involves liver metastasis, is usually treatable by transcatheter
arterial chemoembolization or transcatheter arterial infusion, and
then effectively controlled (23–25). The
current study had various limitations; the study design was
retrospective, and the patient cohort was small. However, the
expression patterns of Snail and E-cadherin were identified to be
potent predictors of lymph node and liver metastasis. To the best
of our knowledge, this is the first study to demonstrate that EMT
may perform an important role in the spread of tumor cells into the
lymphatic flow and circulation, and in the subsequent establishment
of metastasis in PNETs. Additional studies, with larger patient
cohorts are required to verify the results of the present
study.
In conclusion, the current study demonstrated that
EMT may serve an important role in vessel invasion and metastasis
in PNETs, and that the immunohistochemical evaluation of Snail and
E-cadherin expression is useful for predicting the invasive and
metastatic phenotype of PNETs.
Acknowledgements
The authors would like to thank Enago (www.enago.jp) for reviewing the English language of
the original manuscript.
References
|
1
|
Oberg K and Eriksson B: Endocrine tumours
of the pancreas. Best Pract Res Clin Gastroenterol. 19:753–781.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Klöppel G, Rindi G, Anlauf M, Perren A and
Komminoth P: Site-specific biology and pathology of
gastroenteropancreatic neuroendocrine tumors. Virchows Arch. 451
Suppl 1:S9–S27. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Metz DC and Jensen RT: Gastrointestinal
neuroendocrine tumors: Pancreatic endocrine tumors.
Gastroenterology. 135:1469–1492. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bosman FT, Carneiro F, Hruban R and Theise
N: WHO Classification of Tumours of the Digestive System. 3. 4th.
IARC Press; 2010
|
|
5
|
Fischer L, Bergmann F, Schimmack S, Hinz
U, Prieß S, Müller-Stich BP, Werner J, Hackert T and Büchler MW:
Outcome of surgery for pancreatic neuroendocrine neoplasms. Br J
Surg. 101:1405–1412. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tsutsumi K, Ohtsuka T, Fujino M, Nakashima
H, Aishima S, Ueda J, Takahata S, Nakamura M, Oda Y and Tanaka M:
Analysis of risk factors for recurrence after curative resection of
well-differentiated pancreatic neuroendocrine tumors based on the
new grading classification. J Hepatobiliary Pancreat Sci.
21:418–425. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
7
|
Jiang JH, Liu C, Cheng H, Lu Y, Qin Y, Xu
YF, Xu J, Long J, Liu L, Ni QX and Yu XJ: Epithelial-mesenchymal
transition in pancreatic cancer: Is it a clinically significant
factor? Biochim Biophys Acta. 1855:43–49. 2015.PubMed/NCBI
|
|
8
|
Kalluri R and Weinberg RA: The basics of
epithelial-mesenchymal transition. J Clin Invest. 119:1420–1428.
2009. View
Article : Google Scholar : PubMed/NCBI
|
|
9
|
Maeda M, Johnson KR and Wheelock MJ:
Cadherin switching: Essential for behavioral but not morphological
changes during an epithelium-to-mesenchyme transition. J Cell Sci.
118:873–887. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Gunji N, Oda T, Todoroki T, Kanazawa N,
Kawamoto T, Yuzawa K, Scarpa A and Fukao K: Pancreatic carcinoma:
Correlation between E-cadherin and alpha-catenin expression status
and liver metastasis. Cancer. 82:1649–1656. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Nieto MA: The snail superfamily of
zinc-finger transcription factors. Nat Rev Mol Cell Biol.
3:155–166. 2002. View
Article : Google Scholar : PubMed/NCBI
|
|
12
|
Thomson S, Buck E, Petti F, Griffin G,
Brown E, Ramnarine N, Iwata KK, Gibson N and Haley JD: Epithelial
to mesenchymal transition is a determinant of sensitivity of
non-small-cell lung carcinoma cell lines and xenografts to
epidermal growth factor receptor inhibition. Cancer Res.
65:9455–9462. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Carver EA, Jiang R, Lan Y, Oram KF and
Gridley T: The mouse snail gene encodes a key regulator of the
epithelial-mesenchymal transition. Mol Cell Biol. 21:8184–8188.
2001. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Blanco MJ, Moreno-Bueno G, Sarrio D,
Locascio A, Cano A, Palacios J and Nieto MA: Correlation of Snail
expression with histological grade and lymph node status in breast
carcinomas. Oncogene. 21:3241–3246. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Rindi G, Klöppel G, Alhman H, Caplin M,
Couvelard A, de Herder WW, Erikssson B, Falchetti A, Falconi M,
Komminoth P, et al: TNM staging of foregut (neuro)endocrine tumors:
A consensus proposal including a grading system. Virchows Arch.
449:395–401. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Birnbaum DJ, Turrini O, Vigano L,
Russolillo N, Autret A, Moutardier V, Capussotti L, Le Treut YP,
Delpero JR and Hardwigsen J: Surgical management of advanced
pancreatic neuroendocrine tumors: Short-term and long-term results
from an international multi-institutional study. Ann Surg Oncol.
22:1000–1007. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Saeed A, Buell JF and Kandil E: Surgical
treatment of liver metastases in patients with neuroendocrine
tumors. Ann Transl Med. 1:62013.PubMed/NCBI
|
|
18
|
Hotz B, Arndt M, Dullat S, Bhargava S,
Buhr HJ and Hotz HG: Epithelial to mesenchymal transition:
Expression of the regulators snail, slug, and twist in pancreatic
cancer. Clin Cancer Res. 13:4769–4776. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Kurahara H, Takao S, Maemura K, Mataki Y,
Kuwahata T, Maeda K, Ding Q, Sakoda M, Iino S, Ishigami S, et al:
Epithelial-mesenchymal transition and mesenchymal-epithelial
transition via regulation of ZEB-1 and ZEB-2 expression in
pancreatic cancer. J Surg Oncol. 105:655–661. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Izumi H, Hirabayashi K, Nakamura N and
Nakagohri T: Nectin expression in pancreatic adenocarcinoma:
Nectin-3 is associated with a poor prognosis. Surg Today.
45:487–494. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Fendrich V, Maschuw K, Waldmann J,
Buchholz M, Rehm J, Gress TM, Bartsch DK and König A:
Epithelial-mesenchymal transition is a critical step in
tumorgenesis of pancreatic neuroendocrine tumors. Cancers (Basel).
4:281–294. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Galvan JA, Astudillo A, Vallina A, Fonseca
PJ, Gómez-Izquierdo L, García-Carbonero R and González MV:
Epithelial-mesenchymal transition markers in the differential
diagnosis of gastroenteropancreatic neuroendocrine tumors. Am J
Clin Pathol. 140:61–72. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Therasse E, Breittmayer F, Roche A, De
Baere T, Indushekar S, Ducreux M, Lasser P, Elias D and Rougier P:
Transcatheter chemoembolization of progressive carcinoid liver
metastasis. Radiology. 189:541–547. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Ruszniewski P, Rougier P, Roche A, Legmann
P, Sibert A, Hochlaf S, Ychou M and Mignon M: Hepatic arterial
chemoembolization in patients with liver metastases of endocrine
tumors. A prospective phase II study in 24 patients. Cancer.
71:2624–2630. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Lee E, Pachter H Leon and Sarpel U:
Hepatic arterial embolization for the treatment of metastatic
neuroendocrine tumors. Int J Hepatol. 2012:4712032012. View Article : Google Scholar : PubMed/NCBI
|