Introduction
Gastric cancer is one of the most common cancers in
Eastern Asia, including China, Japan and South Korea, and Eastern
Europe (1). The incidence and
mortality of gastric cancer have declined markedly over the past
half-century in the majority of developed countries, but it remains
the second most common cause of cancer-associated mortality in the
world. An estimated 28,000 incident cases (17,750 in males and
10,250 in females) of gastric cancer will be diagnosed in 2017, and
10,960 mortalities (6,720 in males and 4,240 in females) are
estimated to occur from the disease (2). In China, approximately two-thirds of
patients develop advanced or metastatic disease, and >50% have
recurrent disease following curative surgery (3). Systematic chemotherapy plays a critical
role in the treatment of gastric cancer. Cisplatin (DDP) has been
commonly used in the treatment of gastric cancer (4). Despite an initial response to surgical
debulking and front-line platinum chemotherapy, the majority of
tumors eventually develop a drug resistant relapse selected during
the course of therapy. The reasons for drug resistance are
complicated and several previous studies have aimed to explore the
question (1). The development of
multidrug resistance (MDR) to cancer chemotherapy is a major
obstacle to the effective treatment of advanced gastric cancer
(5). Additionally, the mechanism of
MDR remains obscure. Mechanisms including increased expression of
P-glycoprotein (P-gp) and MDR-associated protein (MRP), cell cycle
arrest, increased DNA damage repair and resistance of tumor cells
to apoptosis may account for MDR (6).
Restoring DDP sensitivity by reversing MDR would be an effective
method of treatment.
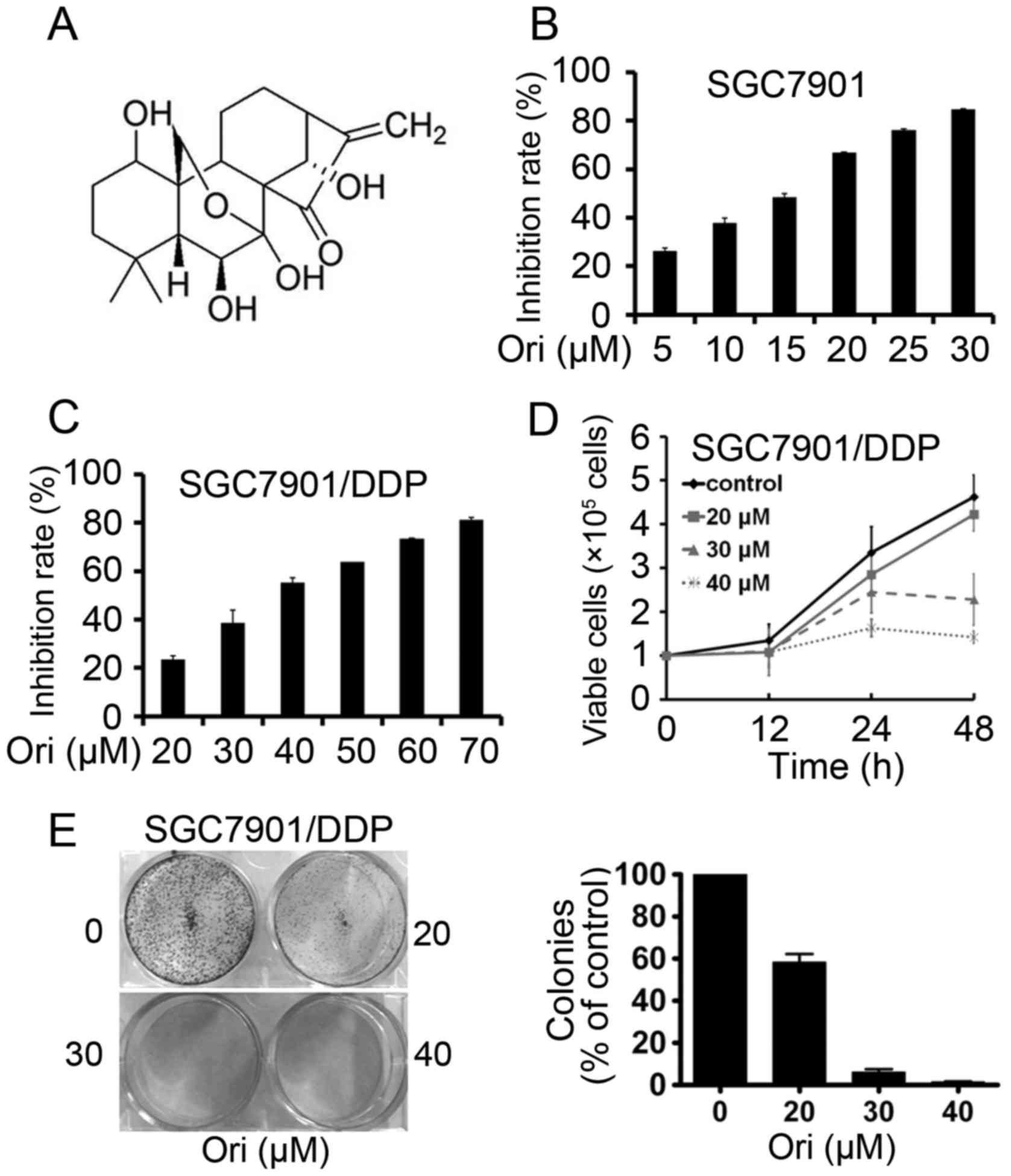
Oridonin (Ori; Fig.
1A), a bitter tetracyclin diterpenoid compound, may be isolated
from Rabdosia rubescens, Isodon japonicus Hara and
Isodon trichocarpus, which are widely distributed in China,
Japan and Korea (7). Studies show
that Ori exhibits potent anticancer activity against a wide range
of cancer cell types, including those from prostate, breast,
pancreatic and non-small cell lung cancer, leukemia, glioblastoma
multiforme and human melanoma cells (8–13).
However, to the best of our knowledge, no study has shown the
effect and the potential mechanism of Ori reversing MDR of human
gastric cancer. Therefore, in the present study, the antitumor
effects and possible mechanisms of Ori on the DDP-resistant human
gastric cancer SGC7901/DDP cell line were investigated.
Materials and methods
Reagents
Ori with a purity of up to 98% was purchased from
Shanghai YuanYe BioTechnology Co., Ltd. (Shanghai, China). Ori was
dissolved in dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA,
Darmstadt, Germany) at a stock solution of 100 mM and stored at
−20°C. DDP was purchased from Sigma-Aldrich (Merck KGaA).
Cell culture
Human cisplatin-resistant gastric cancer cell line
SGC7901/DDP and human gastric cancer cell line SGC7901 were
purchased from American Type Culture Collection (Manassas, VA,
USA). SGC7901/DDP cells were grown in RPMI-1640 (Gibco; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) with fetal bovine serum
(FBS; Hyclone; GE Healthcare, Logan, UT, USA), 500 ng/ml DDP,
penicillin (50 U/ml), and streptomycin (50 U/ml), and incubated in
a humidified atmosphere with CO2 at 37°C. SGC7901 cells
were grown in RPMI-1640 with FBS and penicillin (50 U/ml), and
streptomycin (50 U/ml) and incubated in a humidified atmosphere
with CO2 at 37°C.
Cytotoxic assay and cell
viability
Cells were seeded onto a 96-well plate and
pre-cultured for 24 h at 37°C, then treated with Ori (5–70 µM) for
24 h. Cell cytotoxicity was determined by MTT assay (Sigma-Aldrich;
Merck KGaA). The absorbance was measured at 490 nm using the
Automated microplate reader (BioTek Instruments, Inc., Winooski,
VT, USA), and the inhibition rate was calculated as follows:
Inhibition rate (%) = (average A490 of the control
group-average A490 of the experimental group)/(average
A490 of the control group-average A490 of the
blank group) ×100. Cell viability was estimated by Trypan blue dye
exclusion as previously described (14). The reversion fold calculation formula
was used to find the IC50 of DDP on SGC7901/DDP
cells/IC50 of DDP (with CuB) on SGC7901/DDP cells. The
resistance index (RI) calculation formula was calculated as
follows: RI=IC50 of resistant cells/IC50 of
sensitive cells.
Soft-agar colony formation assay
Cells were suspended in 1 ml of RPMI-1640 containing
0.3% low-melting-point agarose (Amresco, Solon, OH, USA) and 10%
FBS, and plated on a bottom layer containing 0.6% agarose and 10%
FBS in 6-well plate in triplicate. After 2 weeks, the plates were
stained with 0.2% gentian violet (Sigma-Aldrich; Merck KGaA) and
the colonies were counted under light microscopy (IX70; Olympus
Corporation, Tokyo, Japan) (15).
Apoptosis determination by DAPI
staining
Approximately 2×105 cells/well were added
to a 12-well plate and treated with Ori (0, 20, 30, 40 µM) for 24
h. Cells in each treatment and control were then stained by DAPI,
examined under fluorescence microscopy and images were captured, as
previously described (16).
Western blot analysis
Cell pellets were lysed in radioimmunoprecipitation
assay buffer containing 50 mM Tris (pH 8.0), 150 mM NaCl, 0.1% SDS,
0.5% deoxycholate, 1% NP-40, 1 mM DTT, 1 mM NaF, 1 mM sodium
vanadate and 1 mM PMSF (Sigma-Aldrich; Merck KGaA), and 1% protease
inhibitor cocktail (Merck KGaA). Protein extracts were quantitated
and loaded onto 8–12% sodium dodecyl sulfate polyacrylamide gel,
electrophoresed, and transferred to a polyvinylidene difluoride
membrane (Merck KGaA). The membrane was incubated overnight at 4°C
with primary antibody, washed, and then incubated with goat
anti-rabbit or anti-mouse immunoglobulin G (H&L) horseradish
peroxidase (HRP)-conjugated washed, and incubated with
HRP-conjugated secondary antibody (1:10,000 dilution; catalog no.
E030120-01 and E030110-01; EarthOx, LLC, San Francisco, CA, USA)
for 1.5 h at room temperature for 1.5 h. Detection was performed by
using a SuperSignal® West Pico Trial kit (catalog no. QA210131;
Pierce Biotechnology, Inc., Rockford, IL, USA). The antibodies used
were as follows: Anti-MRP1 (1:500 dilution; catalog no. sc-13960),
anti-cyclin D1 (1:500 dilution; catalog no. sc-2044), anti-CIP2A
(1:500 dilution; catalog no. sc-80662), anti-Akt (1:500 dilution;
catalog no. sc-8312) and anti-phosphorylated (p) Akt (Ser473; 1:500
dilution; catalog no. sc-7985; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA); anti-caspase-3 (casp-3; 1:1,000 dilution; catalog
no. 9662), anti-PARP (1:1,000 dilution; catalog no. 9542),
anti-catalytic subunit of PP2A (PP2Ac; 1:1,000 dilution; catalog
no. 2038), anti-extracellular signal-regulated kinase (ERK) 1/2
(1:1,000 dilution; catalog no. 9102) and anti-pERK1/2
(Thr202/Tyr204; 1:1,000 dilution; catalog no. 9101) (Cell Signaling
Technology, Inc.); anti-P-gp (1:2,000 dilution; catalog no.
ab170904) (Abcam, Cambridge, UK); and anti-GAPDH (1:5,000 dilution;
catalog no. M20006; Abmart, Shanghai, China).
Statistical analysis
All experiments were repeated at least three times
and the data were presented as the mean ± standard deviation unless
stated otherwise. Differences between data groups were evaluated
for significance using Student's t-test for unpaired data or
one-way analysis of variance and Bonferroni post hoc test.
P<0.05 was considered to indicate a statistically significant
difference.
Results
Effects of Ori on SGC7901 and
SGC7901/DDP gastric cancer cells
SGC7901 and SGC7901/DDP cells were seeded onto
96-well plates for 24 h and then treated with different
concentrations of Ori (Fig. 1B and
C). After 24 h, the cell viability was evaluated by the MTT
assay and absorbance at 490 nm was measured. It was found that Ori
had moderate cytotoxicity to SGC7901 and SGC7901/DDP cells with a
half-maximal inhibitory concentration (IC50) of 13.84
and 36.35 µM (Table I). By Trypan
blue exclusion assay, it was found that Ori rapidly reduced the
growth of SGC7901/DDP cells (Fig.
1D). The effect of Ori on cell colony formation activity was
investigated, and the results showed that Ori significantly
inhibited the clonogenic ability of SGC7901/DDP (Fig. 1E). These results suggested that Ori
inhibited the anchorage-dependent (cell proliferation) and
anchorage-independent (colony formation) growth of SGC7901/DDP
cells.
 | Table I.IC50 of Ori in gastric
cancer cell lines. |
Table I.
IC50 of Ori in gastric
cancer cell lines.
| Cell line | IC50,
µM |
|---|
| SGC7901 | 13.84±2.85 |
| SGC7901/DDP | 36.35±5.29 |
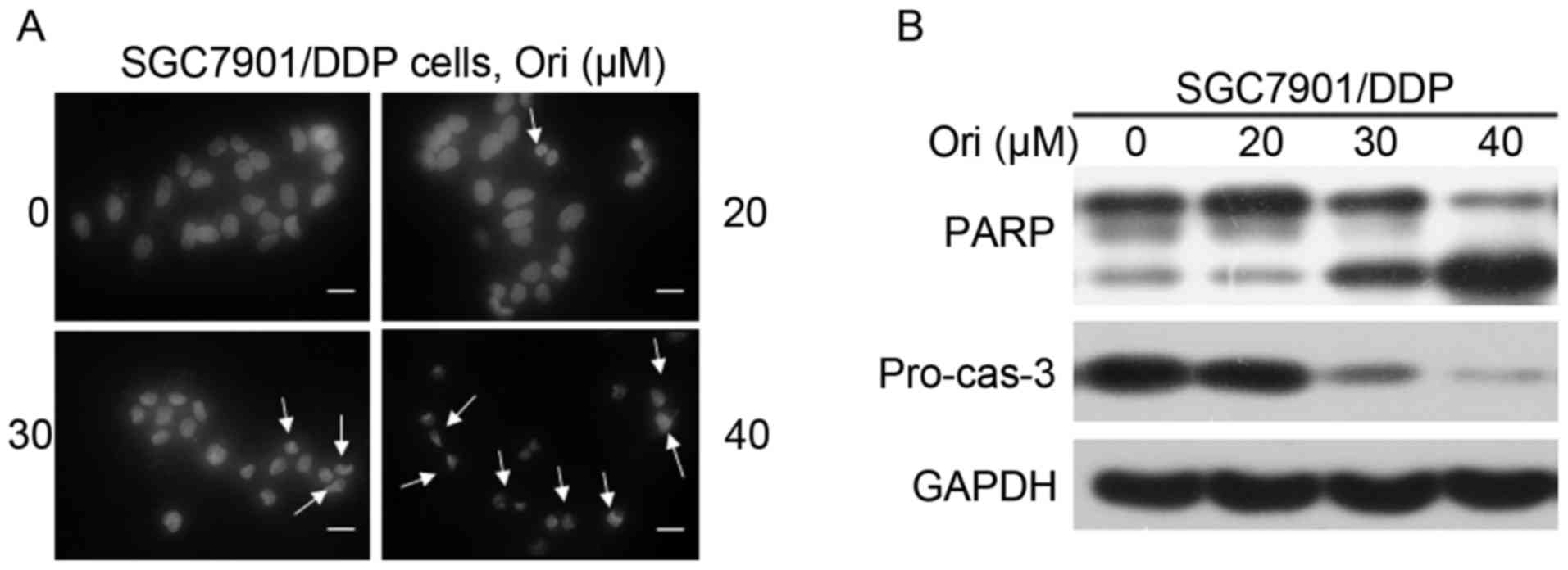
Ori induces apoptosis in SGC7901/DDP
cells
As aforementioned, the mechanism underlying the
inhibition effect of Ori on SGC7901/DDP cells was investigated. The
cell death is reminiscent of the phenomena induced by apoptosis.
Subsequently, whether Ori induces apoptosis of the SGC7901/DDP
cells was assessed. Firstly, the morphological changes of the
nucleus were investigated by DAPI staining. As shown in Fig. 2A, nuclear condensation and
fragmentation was identified following Ori treatment, which are
typical changes in cell apoptosis. Furthermore, western blot
analysis was used to detect the activation of the casp-3 effector
caspase and its substrate, PARP (Fig.
2B). Ori was demonstrated to induce a significant
dose-dependent decrease in pro-casp-3 and the cleavage of PARP, in
SGC7901/DDP cells, indicating that Ori induced caspase-dependent
apoptosis.
Ori reverses the resistance of
SGC7901/DDP cells to DDP
The MTT assay revealed a significant difference
between the growth-inhibiting effect of DDP on normal SGC7901 cells
and on DDP resistant SGC7901/DDP cells (data not shown). The
IC50 for the SGC7901 cells was 14.30 µM, compared with
the IC50 of 34.71 µM for the SGC7901/DDP cells. However,
the DDP IC50 for SGC7901/DDP cells was 27.87 µM
subsequent to treatment with 10 µM Ori treatment and 14.29 µM
subsequent to treatment with 20 µM Ori (Table II). The resistance index (RI) was
2.43 in the SGC7901/DDP parent group, 1.95 in the SGC7901/DDP Ori
10 µM group and 1.00 in the SGC7901/DDP Ori 20 µM group. The RI
calculation formula was calculated as follows: RI=IC50
of resistant cells/IC50 of sensitive cells. Following
the treatment with 10 and 20 µM Ori, the IC50 of DDP in
the SGC7901/DDP cells was reduced from 34.71 µM to 27.87 and 14.29
µM, by 1.25-fold and 2.43-fold, respectively.
 | Table II.Reversing effect of Ori on SGC7901/DDP
cells. |
Table II.
Reversing effect of Ori on SGC7901/DDP
cells.
| Treatments | Inhibition rate,
% | IC50,
µM | Resistance index | Reversion fold |
|---|
| 0 µM Ori |
| 34.71 | 2.43 | 1 |
| 20 µM
DDP | 29.35±2.15 |
|
|
|
| 30 µM
DDP | 43.92±3.80 |
|
|
|
| 40 µM
DDP | 62.89±1.53 |
|
|
|
| 50 µM
DDP | 62.89±2.69 |
|
|
|
| 60 µM
DDP | 67.40±5.27 |
|
|
|
| 70 µM
DDP | 68.88±4.04 |
|
|
|
| 10 µM Ori |
| 27.87 | 1.95 | 1.25 |
| 20 µM
DDP | 32.55±4.79 |
|
|
|
| 30 µM
DDP | 65.83±2.15 |
|
|
|
| 40 µM
DDP | 68.83±4.36 |
|
|
|
| 50 µM
DDP | 70.69±5.01 |
|
|
|
| 60 µM
DDP | 78.05±3.27 |
|
|
|
| 70 µM
DDP | 85.49±4.62 |
|
|
|
| 20 µM Ori |
| 14.29 | 1 | 2.43 |
| 20 µM
DDP | 67.50±2.51 |
|
|
|
| 30 µM
DDP | 68.67±4.01 |
|
|
|
| 40 µM
DDP | 72.81±1.92 |
|
|
|
| 50 µM
DDP | 77.89±3.37 |
|
|
|
| 60 µM
DDP | 88.17±2.95 |
|
|
|
| 70 µM
DDP | 95.28±3.52 |
|
|
|
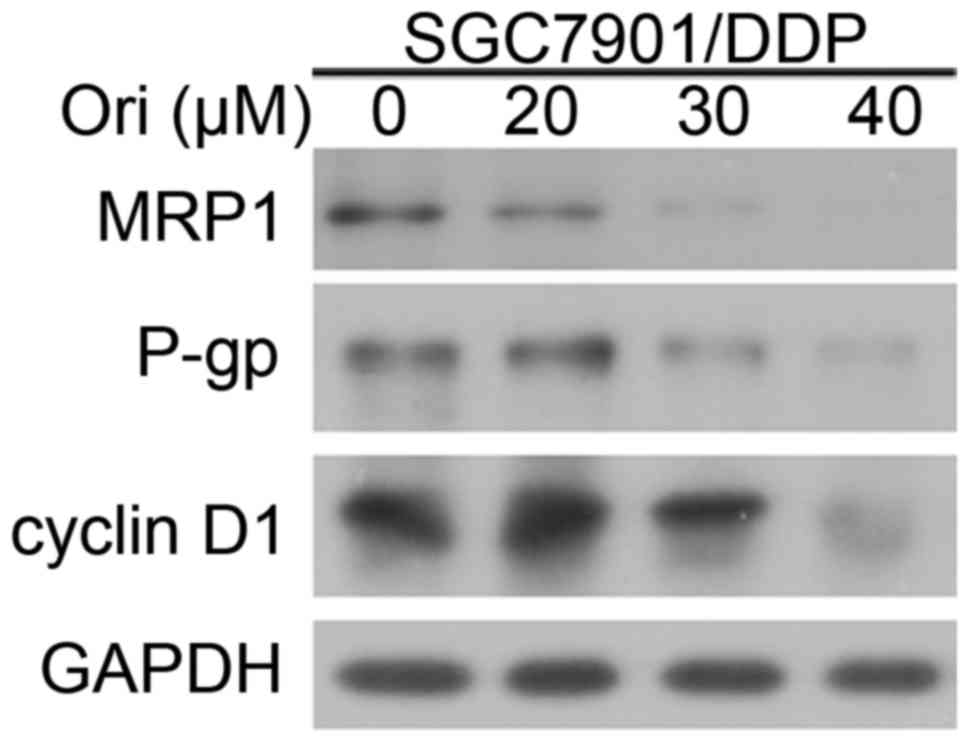
Ori affected the expression of P-gp,
MRP1 and cyclin D1
To investigate the mechanism by which Ori induces
reversing MDR of SGC7901/DDP cells, the expression levels of P-gp,
MRP1 and cell cycle protein cyclin D1 were detected by western blot
analysis (Fig. 3). The results
indicated that P-gp, MRP1 and cyclin D1 expression of SGC7901/DDP
cells were downregulated by treatment with increasing
concentrations of Ori. The decreased expression of P-gp and MRP1 in
SGC7901/DDP cells may contribute to the reversal of
chemoresistance.
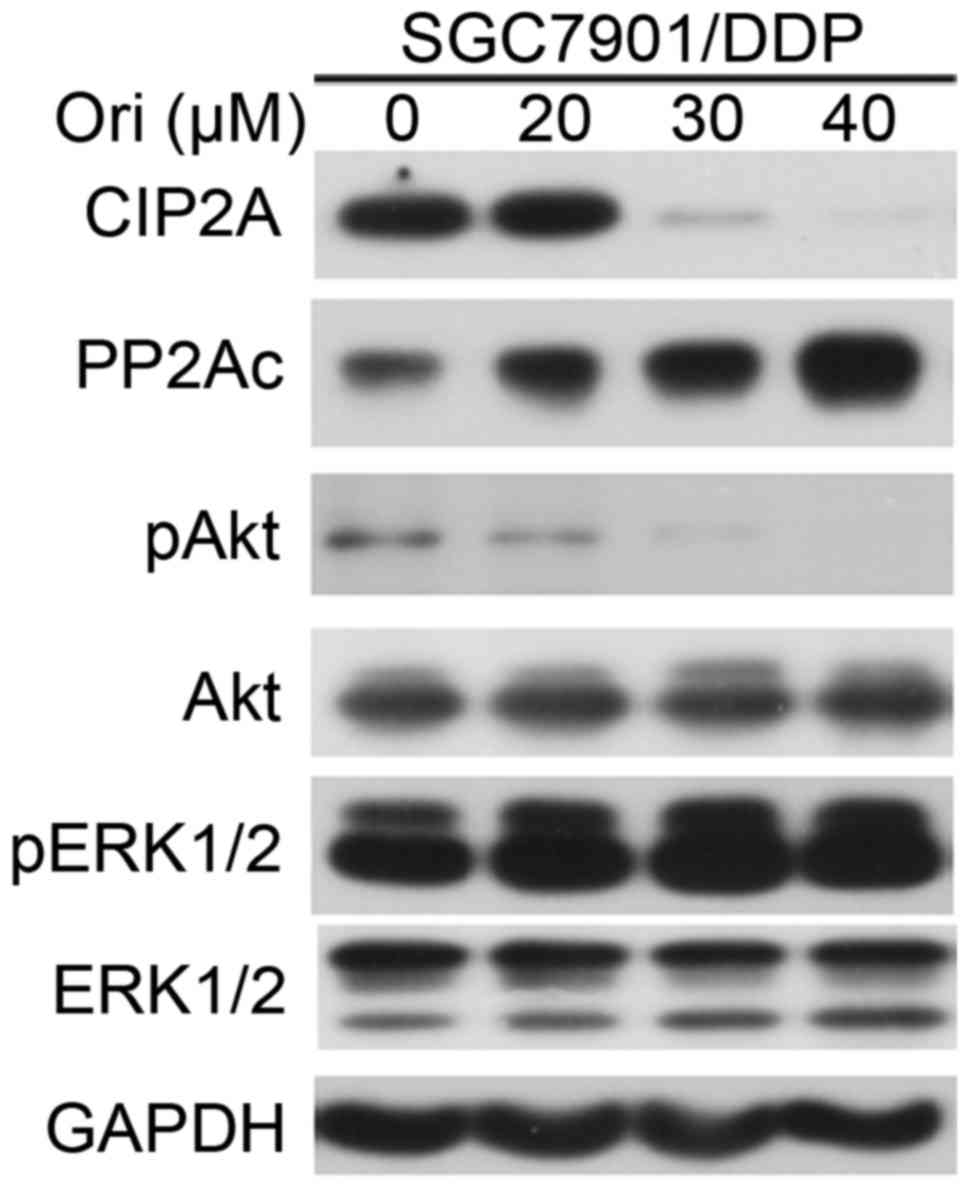
Ori inhibited Akt signaling
pathway-associated molecules
MDR occurs due to the positive feedback signaling
loops generating cancer extreme robustness. Akt is a cancer MDR
locus. Targeting that locus by oxidant/antioxidant balance
modulation, positive feedback loops are converted into negative
feedback loops, leading to disappearance of MDR (17). The present study examined the effect
of Ori on phosphorylated (p)Akt in SGC7901/DDP cells. It was shown
that treatment with Ori resulted in downregulation of pAkt, but not
phosphorylated (p)ERK1/2 (Fig. 4).
PP2A, one of the main serine-threonine phosphatases, has a critical
role in the regulation of cell-cycle progression, survival and
differentiation by negatively regulating the PI3K/Akt pathway, and
dephosphorylating and inactivating mitogen-activated protein kinase
kinase 1 and ERK family kinases (18). The present study tested the effects of
Ori on PP2Ac (catalytic subunit), and found that Ori upregulated
PP2Ac and downregulated cancerous inhibitor of protein phosphatase
2A (CIP2A), a critical oncoprotein in several types of cancer
(19), at protein level (Fig. 4). Thus, the present study concluded
that the inhibitory effect of Ori on the MDR of SGC7901/DDP cells
may be partially due to the regulation of the CIP2A/PP2A/Akt signal
cascade.
Ori exerts synergistic effect
combining with DDP in SGC7901/DDP cells
The low toxicity (10 and 20 µM) doses were selected
for additional experiments to detect whether Ori can reverse the
resistance of SGC7901/DDP cells to low doses of DDP (10 and 20 µM)
(Fig. 5A). Ori in combination with
DDP had a greater effect on the SGC-7901/DDP cells compared with
DDP or Ori alone (P<0.05). The combination index (CI) value was
also analyzed using CalcuSyn software (version 2.1; Biosoft,
Cambridge, UK) and found that the CI values were <1 (Table III), which indicated that Ori and
DDP played a synergistic effect in SGC7901/DDP cells. Western blot
analysis further confirmed the synergistic effect and found that
Ori and DDP combination resulted in elevated levels of casp-3
activation, cleavage of PARP, and decreased P-gp, MRP1, cyclin D1
and CIP2A expression (Fig. 5B).
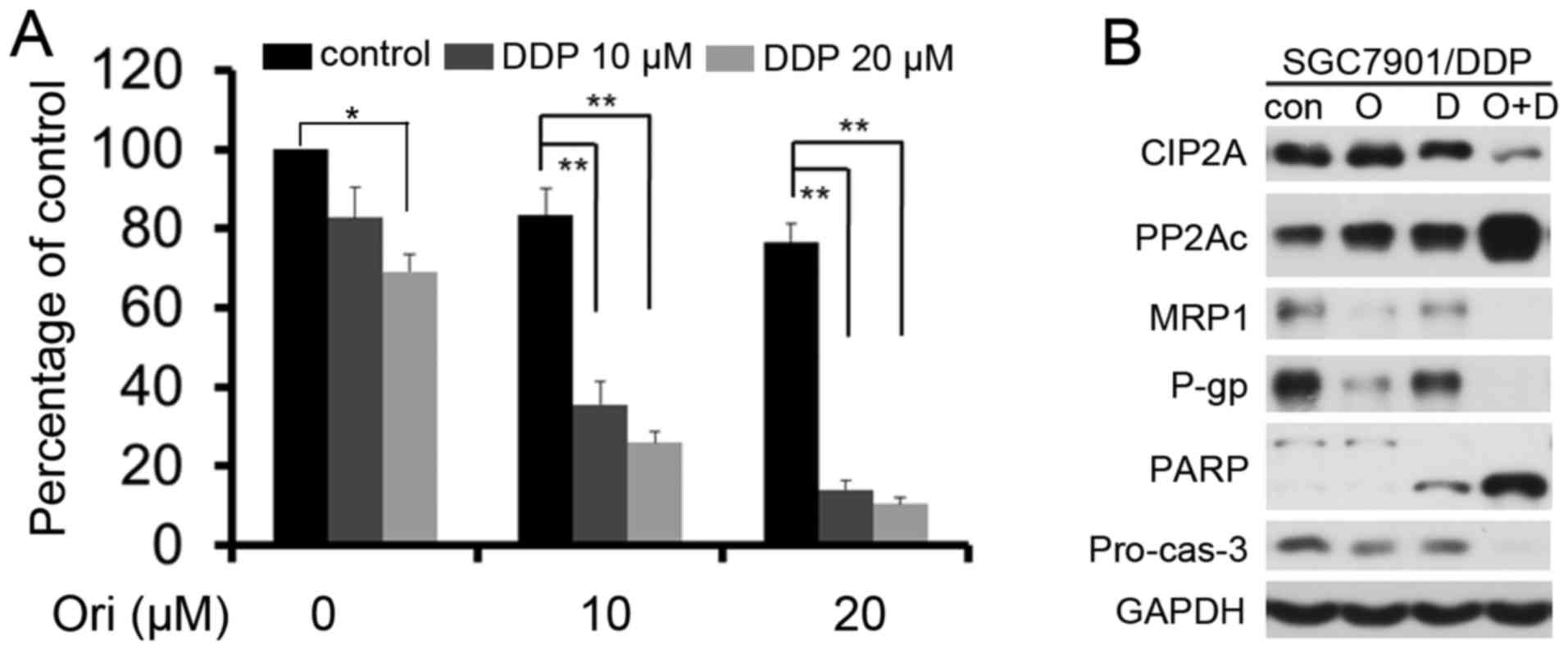 | Figure 5.Ori exerts synergistic effects in
combination with DDP. (A) SGC7901/DDP cells were treated for 24 h
with Ori and/or DDP, and then assessed by MTT assay. *P<0.05;
**P<0.01. (B) SGC7901/DDP cells were treated with Ori (O; 20
µM), DDP (D; 20 µM) alone or together (O+D) for 24 h. The treated
cells were collected, lysed and assessed by western blot analysis
using the indicated antibodies. DDP, cisplatin; Ori oridonin;
CIP2A, cancerous inhibitor of protein phosphatase 2A; PP2Ac,
catalytic subunit of protein phosphatase 2A; MRP1, multi-drug
resistance-associated protein; P-gp, P-glycoprotein; PARP, poly
(ADP-ribose) polymerase; Pro-cas-3, pro-caspase-3. |
 | Table III.Ori and DDP CI values. |
Table III.
Ori and DDP CI values.
| Treatment | CI |
|---|
| 10 µM Ori + 10 µM
DDP | 0.17 |
| 10 µM Ori +20 µM
DDP | 0.20 |
| 20 µM Ori + 10 µM
DDP | 0.05 |
| 20 µM Ori + 20 µM
DDP | 0.07 |
Discussion
Gastric cancer is a major type of cancer worldwide
and its incidence rate is among the three most frequent neoplasms
in China (1). Gastric cancer is also
the third most frequent cause of cancer-associated mortality
following lung and liver cancer in males, and following breast and
lung cancer in females (1,2). To improve the outcome of gastric cancer,
different treatment strategies have been evaluated, including
chemotherapy, radiotherapy and extended resection (20). Chemotherapy is the most common
treatment of choice for gastric cancer, but its application is
limited due to drug resistance. DDP is a platinum chemotherapeutic
agent that is widely used in several malignancies and is beneficial
in certain patients, but treatment with DDP may cause DDP
resistance (21). Therefore,
identification and development of novel drugs that can overcome the
DDP resistance is required to prolong the overall survival time of
gastric cancer patients. To overcome such resistance, exploring
novel compounds is urgent. Compounds from natural sources
constitute an indispensable candidate drug library for
pharmacotherapy. In the present study, the efficacy of the
combination of Ori, a bitter tetracyclin diterpenoid compound
isolated from traditional medicinal herbs (8), and DDP on the gastric cancer SGC7901/DDP
cell line was investigated.
Inhibition of cell proliferation is an efficient
strategy in cancer therapy. In this present study, Ori was shown to
inhibit the proliferation (Fig. 1C),
cell viability (Fig. 1D) and
soft-agar colony formation (Fig. 1E)
of the gastric cancer SGC7901/DDP cell line.
Evading apoptosis is one of the hallmarks of drug
resistance, and targeting apoptosis has become a cancer therapeutic
strategy (22). The present study
found that the change in cellular morphology and nucleus
condensation, which were typical characters of apoptosis (Fig. 2A). Therefore, Ori may have the ability
to induce cell apoptosis. The extrinsic and intrinsic apoptotic
pathways that ultimately lead to activation of effector caspases
(casp-3, −2 and −7) have been characterized (23,24). The
decrease in pro-casp-3 expression and the proteolysis of PARP
(Fig. 2B) indicate that casp-3 is
activated. Thus, Ori may trigger apoptosis by activating the
casppase-dependent apoptosis pathway.
A number of experimental strategies to overcome DDP
resistance act at the preclinical or clinical levels, including the
introduction of pro-apoptotic genes and the inhibition of
antioxidants that protect against oxidative stress and prevent
damage to cells (25,26). Of particular significance are the
combinations of chemotherapy drug treatments with other drugs,
radiation and the emerging gene therapy regimens. The effect of the
combination of Ori and DDP on the SGC7901/DDP cells was
investigated, and it was identified that Ori in combination with
DDP had an improved effect compared with DDP or Ori alone.
Following treatment with Ori (10 and 20 µM), the IC50 of
DDP to the SGC7901/DDP cells was significantly reduced from 34.71
µM to 27.87 and 14.29 µM by 1.25-fold and 2.43-fold, respectively
(Table II). The RI was 2.43 in the
SGC7901/DDP parent group, 1.95 in the SGC7901/DDP Ori 10 µM group
and 1.00 in the SGC7901/DDP Ori 20 µM group. Furthermore, the
present study explored the mechanisms of Ori in reversing DDP
resistance.
Drug resistance is largely mediated through
overexpression of MDR, MRP, drug resistance protein and proteasome
subunits, increases in antioxidant defenses, and TOP2 activity;
these results have been widely verified (27–29). In
addition, suppression of cyclin D1 levels has been shown to
potentiate the response of human pancreatic cancer cells to DDP
(30). The present study identified
that the treatment with Ori was able to reverse the MDR of the
SGC7901/DDP cells via the downregulation of P-gp, MRP1 and cyclin
D1 (Fig. 3).
To assess whether Akt and CIP2A are the targets of
Ori, their activation and expression in SGC7901/DDP cells treated
with Ori was measured. Akt phosphorylation and CIP2A expression
were inhibited by treatment with Ori (Fig. 4). This suggests that Ori reverses MDR,
at least in part, through suppression of the CIP2A/PP2A/Akt
signaling cascade.
The low toxicity (10 and 20 µM) doses of Ori and DDP
were combined to investigate a possible synergistic effect, and the
results showed that Ori could significantly elevate DDP anti-tumor
efficiency, and the CI was <1, as calculated by CalcuSyn
software (version 2.1). Additionally, the expression of full-length
casp-3 was reduced and PARP was cleaved. Additionally, the MDR
genes P-gp and MRP1, and CIP2A were also significantly inhibited by
combined treatment with Ori and DDP. Cisplatin, which reacts with
DNA and forms DNA adducts, is the main drug for treatment of
gastric cancer (26). Notably, the
two drugs, which have different mechanisms, synergistically
inhibited cell growth. The combination of these drugs may have
clinic potential in cisplatin-resistant human gastric cancer
treatment.
In conclusion, the present results have confirmed
that Ori combination with DDP could overcome chemoresistance of
SGC7901/DDP cells. One possible mechanism is that Ori induced
apoptosis, modulated drug resistance and cell cycle proteins, P-gp,
MRP1 and cyclin D1. The inhibition of the CIP2A/PP2A/Akt signal
cascade pathway is a key role in the process of Ori plays its
function. Ori may be a promising new drug due to its ability to
reverse MDR in gastric cancer.
Acknowledgements
The present study was supported by grants from the
Natural Science Foundation of Hubei Province (grant no.
2016CFB528); the Foundation of Health and Family planning
Commission of Hubei Province (grant no. WJ2017F065); the Foundation
of Hubei University of Medicine (grant no. FDFR201605); the
Foundation for Innovative Research Team of Hubei University of
Medicine (grant no. 2014CXX05) and the Key Discipline Project of
Hubei University of Medicine.
References
|
1
|
Niccolai E, Taddei A, Prisco D and Amedei
A: Gastric cancer and the epoch of immunotherapy approaches. World
J Gastroenterol. 21:5778–5793. 2015.PubMed/NCBI
|
|
2
|
Siegel RL, Miller KD and Jemal A: Cancer
statistics, 2015. CA Cancer J Clin. 65:5–29. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Yang X, Cai H, Liang Y, Chen L, Wang X, Si
R, Qu K, Jiang Z, Ma B, Miao C, et al: Inhibition of c-Myc by
let-7b mimic reverses mutidrug resistance in gastric cancer cells.
Oncol Rep. 33:1723–1730. 2015.PubMed/NCBI
|
|
4
|
Ni P, Xu W, Zhang Y, Chen Q, Li A, Wang S,
Xu S and Zhou J: TXNL1 induces apoptosis in cisplatin resistant
human gastric cancer cell lines. Current Cancer Drug Targets.
14:850–859. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Fan K, Fan D, Cheng LF and Li C:
Expression of multidrug resistance-related markers in gastric
cancer. Anticancer Res. 20:4809–4814. 2000.PubMed/NCBI
|
|
6
|
Hong L, Piao Y, Han Y, Wang J, Zhang X, Du
Y, Cao S, Qiao T, Chen Z and Fan D: Zinc ribbon domain-containing 1
(ZNRD1) mediates multidrug resistance of leukemia cells through
regulation of P-glycoprotein and Bcl-2. Mol Cancer Ther.
4:1936–1942. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Owona BA and Schluesener HJ: Molecular
insight in the multifunctional effects of oridonin. Drugs R D.
15:233–244. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhou GB, Kang H, Wang L, Gao L, Liu P, Xie
J, Zhang FX, Weng XQ, Shen ZX, Chen J, et al: Oridonin, a
diterpenoid extracted from medicinal herbs, targets AML1-ETO fusion
protein and shows potent antitumor activity with low adverse
effects on t(8;21) leukemia in vitro and in vivo. Blood.
109:3441–3450. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xu B, Shen W, Liu X, Zhang T, Ren J, Fan Y
and Xu J: Oridonin inhibits BxPC-3 cell growth through cell
apoptosis. Acta Biochim Biophys Sin (Shanghai). 47:164–173. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Li Y, Wang Y, Wang S, Gao Y, Zhang X and
Lu C: Oridonin phosphate-induced autophagy effectively enhances
cell apoptosis of human breast cancer cells. Med Oncol. 32:3652015.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Chen RY, Xu B, Chen SF, Chen SS, Zhang T,
Ren J and Xu J: Effect of oridonin-mediated hallmark changes on
inflammatory pathways in human pancreatic cancer (BxPC-3) cells.
World J Gastroenterol. 20:14895–14903. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang YY, Lv YF, Lu L and Cai L: Oridonin
inhibits mTOR signaling and the growth of lung cancer tumors.
Anticancer Drugs. 25:1192–1200. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zhao Z and Chen Y: Oridonin, a promising
antitumor natural product in the chemotherapy of hematological
malignancies. Curr Pharm Biotechnol. 15:1083–1092. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Liu Y, Cao W, Zhang B, Liu YQ, Wang ZY, Wu
YP, Yu XJ, Zhang XD, Ming PH, Zhou GB and Huang L: The natural
compound magnolol inhibits invasion and exhibits potential in human
breast cancer therapy. Sci Rep. 3:30982013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Cao W, Liu Y, Zhang R, Zhang B, Wang T,
Zhu X, Mei L, Chen H, Zhang H, Ming P and Huang L:
Homoharringtonine induces apoptosis and inhibits STAT3 via
IL-6/JAK1/STAT3 signal pathway in Gefitinib-resistant lung cancer
cells. Sci Rep. 5:84772015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Chou CC, Yang JS, Lu HF, Ip SW, Lo C, Wu
CC, Lin JP, Tang NY, Chung JG, Chou MJ, et al: Quercetin-mediated
cell cycle arrest and apoptosis involving activation of a caspase
cascade through the mitochondrial pathway in human breast cancer
MCF-7 cells. Arch Pharm Res. 33:1181–1191. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Radisavljevic Z: AKT as locus of cancer
multidrug resistance and fragility. J Cell Physiol. 228:671–674.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liu H, Gu Y, Wang H, Yin J, Zheng G, Zhang
Z, Lu M, Wang C and He Z: Overexpression of PP2A inhibitor SET
oncoprotein is associated with tumor progression and poor prognosis
in human non-small cell lung cancer. Oncotarget. 6:14913–14925.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Khanna A and Pimanda JE: Clinical
significance of cancerous inhibitor of protein phosphatase 2A in
human cancers. Int J Cancer. 138:525–532. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Zhang JL, Liu XZ, Wang PY, Chen GW, Jiang
Y, Qiao SK, Zhu J, Wang X, Pan YS and Liu YC: Targeting HCCR
expression resensitizes gastric cancer cells to chemotherapy via
down-regulating the activation of STAT3. Sci Rep. 6:241962016.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Simonian PL, Grillot DA and Nuñez G: Bcl-2
and Bcl-XL can differentially block chemotherapy-induced cell
death. Blood. 90:1208–1216. 1997.PubMed/NCBI
|
|
22
|
Hanahan D and Weinberg RA: Hallmarks of
cancer: The next generation. Cell. 144:646–674. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Nicholson DW: Caspase structure,
proteolytic substrates, and function during apoptotic cell death.
Cell Death Differ. 6:1028–1042. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Johnstone RW, Ruefli AA and Lowe SW:
Apoptosis: A link between cancer genetics and chemotherapy. Cell.
108:153–164. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Zhao Z, Wang J, Tang J, Liu X, Zhong Q,
Wang F, Hu W, Yuan Z, Nie C and Wei Y: JNK- and Akt-mediated Puma
expression in the apoptosis of cisplatin-resistant ovarian cancer
cells. Biochem J. 444:291–301. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Hasegawa M, Ishiguro K, Ando T and Goto H:
Geranylgeranylacetone attenuates cisplatin-induced reductions in
cell viability by suppressing the elevation of intracellular p53
content without heat shock protein induction. Nagoya J Med Sci.
74:123–131. 2012.PubMed/NCBI
|
|
27
|
Alcantara LM, Kim J, Moraes CB, Franco CH,
Franzoi KD, Lee S, Freitas-Junior LH and Ayong LS:
Chemosensitization potential of P-glycoprotein inhibitors in
malaria parasites. Exp Parasitol. 134:235–243. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Luo L, Sun YJ, Yang L, Huang S and Wu YJ:
Avermectin induces P-glycoprotein expression in S2 cells via the
calcium/calmodulin/NF-kappaB pathway. Chem Biol Interact.
203:430–439. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Ying L, Zu-An Z, Qing-Hua L, Qing-Yan K,
Lei L, Tao C and Yong-Ping W: RAD001 can reverse drug resistance of
SGC7901/DDP cells. Tumour Biol. 35:9171–9177. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Kornmann M, Arber N and Korc M: Inhibition
of basal and mitogen-stimulated pancreatic cancer cell growth by
cyclin D1 antisense is associated with loss of tumorigenicity and
potentiation of cytotoxicity to cisplatinum. J Clin Invest.
101:344–352. 1998. View
Article : Google Scholar : PubMed/NCBI
|