Introduction
Multiple myeloma (MM) accounts for about 10% of the
hematopoietic system tumors. The incidence rate of multiple myeloma
in China is 1/10 million, which is lower than that of western
countries of 4/10 million. However, in recent years the incidence
rate of multiple myeloma is on the rise, and the age of onset is
also increasingly falling (1). In
previous years, effective rate of chemotherapy was 40–60% and the
complete remission rate was <5%. The median survival period was
not more than 3 years. At present, multiple myeloma remains a
malignant disease of which the therapeutic effect is unsatisfactory
(2,3).
In recent years, with the intervention of some new drugs, the
treatment for myeloma has made some progress, but the complete
remission rate is ~30% (4).
Therefore, targeting new markers to further explore the
pathogenesis of multiple myeloma and for searching new therapeutics
has become important.
It is now considered that the occurrence and
development of multiple myeloma is closely related to
microenvironment of bone marrow. Many cytokines can promote the
growth and survival of tumor cells, such as IL-6 paracrine by bone
marrow stromal cells can promote the proliferation of myeloma
cells, resisting the cytotoxicity induced by drugs (5,6). In
addition, insulin-like growth factor 1 (IGF-1) and hepatocyte
growth factor (HGF) are also stimulating signal to multiple myeloma
growth, promoting the proliferation and survival of myeloma cells
(7,8).
The proliferation of B-cell activating factor (BAFF) and the
proliferation-inducing ligand (APRIL), new members of tumor
necrosis factor (TNF) family, have been identified in recent years.
They are the main cytokines that promote the proliferation and
survival of B cells and also have similar effects on plasma cells
(2,9).
Our study focuses on the gene and protein expression
of BAFF and APRIL in bone marrow mononuclear cells (BMMCs) and
plasma of patients with multiple myeloma and correlation analysis
between multiple myeloma clinical indicators and the gene and
protein expression of BAFF and APRIL were performed.
Materials and methods
Subjects
Multiple myeloma cell line KM3 was purchased from
Kangwei Century Biotechnology Co. Ltd. (Beijing, China).
We regarded 60 patients with multiple myeloma
patients as the research objects from February 2011 to May 2013 in
the Department of Hematology in the First Affiliated Hospital of
Jinzhou Medical University. Thirty-three male cases and 27 female
cases were included. The initial treatment was 18 cases. Remission
was 25 patients. Non-remission was 17 patients. Twenty-eight cases
were immunoglobulin G (IgG) type. Eighteen cases were IgA type. One
hundred forty-four cases were light chain type. Sixteen cases were
in stage I. Twenty-one cases were in stage II. Twenty-three cases
were in stage III. The patients were aged from 30 to 75 years and
the median age was 53 years. All the patients conformed to the
diagnostic standard of WHO multiple myeloma.
The initial treatment patients were first diagnosed
without any treatment. After diagnosed with multiple myeloma, the
patients were administered with VAD chemotherapy combined with
thalidomide.
We selected 20 patients with mild to moderate iron
deficiency anemia in the same period in the Department of
Hematology in the First Affiliated Hospital of Jinzhou Medical
University as the control group. Eleven patients were male and 9
were female. They were aged from 30 to 65 years. All patients
agreed to the use of their samples. This study was approved by the
Ethics Committee of The First Affiliated Hospital of Jinzhou
Medical University. Signed written informed consents were obtained
from the patients and/or guardians.
All patients with multiple myeloma and the control
group were excluded from rheumatoid arthritis, systemic lupus
erythematosus, central nervous system diseases, infectious diseases
and diabetes. The renal function in MM patients was normal or
mildly impaired, Cr <176.8 µmol/l.
Real-time fluorescent PCR primer synthesis:
According to the principle of PCR primer design, the primers of
BAFF, APRIL and GAPDH were designed with reference to the
literature. Primer was synthesized by Schreck Biological Technology
Co., Ltd. (Shanghai, China) (7).
BAFF upstream primer was 5′-ACAAACCAGTGAAAACTAT-3′.
BAFF downstream primer was 5′-ATCCTTCCACTACAAAG-3′. Anticipated
product was 282 bp.
APRIL upstream primer was
5′-CCTACGCATTCCTCAACGAA-3′. APRIL downstream primer was
5′-TAAACTACCTGATCCCAGCA-3′. Anticipated product was 214 bp.
GAPDH upstream primer was 5′-AATCCACCTCTCAACTACC-3′.
GAPDH downstream primer was 5′-CTCCCCACGCAAGCTTAC-3′. Anticipated
product was 359 bp.
KM3 cell culture
Under sterile conditions, KM3 cells were inoculated
into the RPMI-1640 medium containing 10% fetal bovine serum. Then
it was placed in the incubator with 5% carbon dioxide, 37°C
saturated humidity to be cultured. Then renewed every 2–3 days.
BMMCs collection
Under sterile conditions, 5 ml bone marrow from
patients with multiple myeloma and control group was extracted and
added with heparin (1:10). The fresh bone marrow specimens were
carefully added on the lymphocyte separation medium. Then it was
centrifuged for 10 min, at 1,500 × g. The mononuclear cells in the
middle layer were collected. After centrifugation for 10 min at 700
× g, the supernatant was absorbed and then precipitated by 1X PBS
(Beckman Coulter, Brea, CA, USA) and washed 3 times to obtain
cells.
BMMCs RNA extraction
BMMCs (2×106) were extracted and were
carefully prepared in an environment which was absent of enzyme.
Cells were precipitated in 1 ml TRIzol solution (Invitrogen,
Carlsbad, CA, USA), fully blown uniformly and placed at room
temperature for 5 min. Chloroform solution of 0.3 ml was added,
mixed, placed at room temperature for 5 min, 4°C 8,000 × g
centrifuged for 15 min and supernatant was taken. In the EP tube
(Beckman Coulter) with supernatant, an equal volume of cold
isopropanol solution was added, mixed, placed at room temperature
for 30 min and 4°C 8,000 × g centrifuged for 10 min. Supernatant
was discarded carefully. The gelatinous precipitate on the tube
wall was RNA. Seventy-five percent 0.5 ml ethanol solution (Beckman
Coulter) was added, shaking and washing the precipitate.
Centrifuged at 3,000 × g at 4°C for 5 min, discarding supernatant,
dried at room temperature for 30 min. DEPC water (10 ml; Beckman
Coulter) was added, dissolving the precipitation and mixed. The
mixture was the RNA extract.
Qualitative and quantitative
determination of RNA
One microliter RNA extract was taken and 49 µl DEPC
water was added. With UV visible spectrophotometer (Auxi Scientific
Instrument Co. Ltd., Shanghai, China), we measured OD280 and OD260
value in each tube to calculate the content of RNA in each sample,
and obtain the ratio.
mRNA reverse transcription to
cDNA
With reverse transcription kit, mRNA was reverse
transcribed to cDNA. Steps are as follows: i) 5 µg total RNA was
taken, and 1 µl random primers was added. DEPC water was added to a
total volume of 12 µl. Degeneration at 70°C for 5 min; ii) 5X
buffer 4 µl, 2 µl 10 mmol/l dNTP and ribonuclease inhibitor µl
mixtures were added after reverse transcriptase 1 µl was added, and
placed at room temperature for 5 min; iii) 42°C for 1 h, 99°C for 5
min; and iv) after the reaction was over, it was placed on ice to
cool for 2 min. The synthesized cDNA template was preserved at
−20°C.
Fluorescence quantitative PCR
SYBR-Green I fluorescent was used to perform
quantitative PCR reaction. The reaction system: 2 µl template,
primer 1 µl, and was mixed with 10 µl dye mixture. Ultrapure water
was added to 20 µl. After mixed evenly, it was placed into the PCR
thermal cycler for reaction. After each cycle of degeneration, the
program can automatically record the average fluorescence values of
the last cycle of the last 10% of time, which indicates the amount
of PCR products when last cycle ends. After the reaction was
completed, recorded curves of all samples were obtained.
Plasma samples collection
When BMMCs were collected, plasma was collected at
the same time. The plasma was packed in aseptic EP tubes of 1 ml
and was preserved at −20°C.
The determination of BAFF and APEIL
with ELISA
The enzyme label plate was taken out. One hundred
microliters standard substance was added respectively in the blank
pores and the pores were labeled. Fifty microliters enzyme-marked
solution was added to standard sample holes and sample holes and
incubated for 60 min at 37°C. Concentrated washing liquid and
medical distilled water were diluted at 1:20. Microtiter plate was
washed 5 times repeatedly, each time for 30 sec. Fifty microliters
substrate A, B was added to each hole, incubated for 15 min at
37°C. Fifty microliters of the termination liquid was added to each
hole to terminate reaction. OD value was obtained in the
enzyme-linked immunosorbent assay (ELISA) (Bio-Rad, Philadelphia,
PA, USA) (10).
Statistical analysis
SPSS 15.0 (IBM, Chicago, IL, USA) was used for the
statistical analysis. The homogeneity of variance for the sample
data was tested. According to the homogeneity of variance, one-way
analysis of variance and rank test were used. The results are
expressed as mean ± standard deviation (mean ± SD), P<0.05 was
considered to indicate a statistically significant difference.
Results
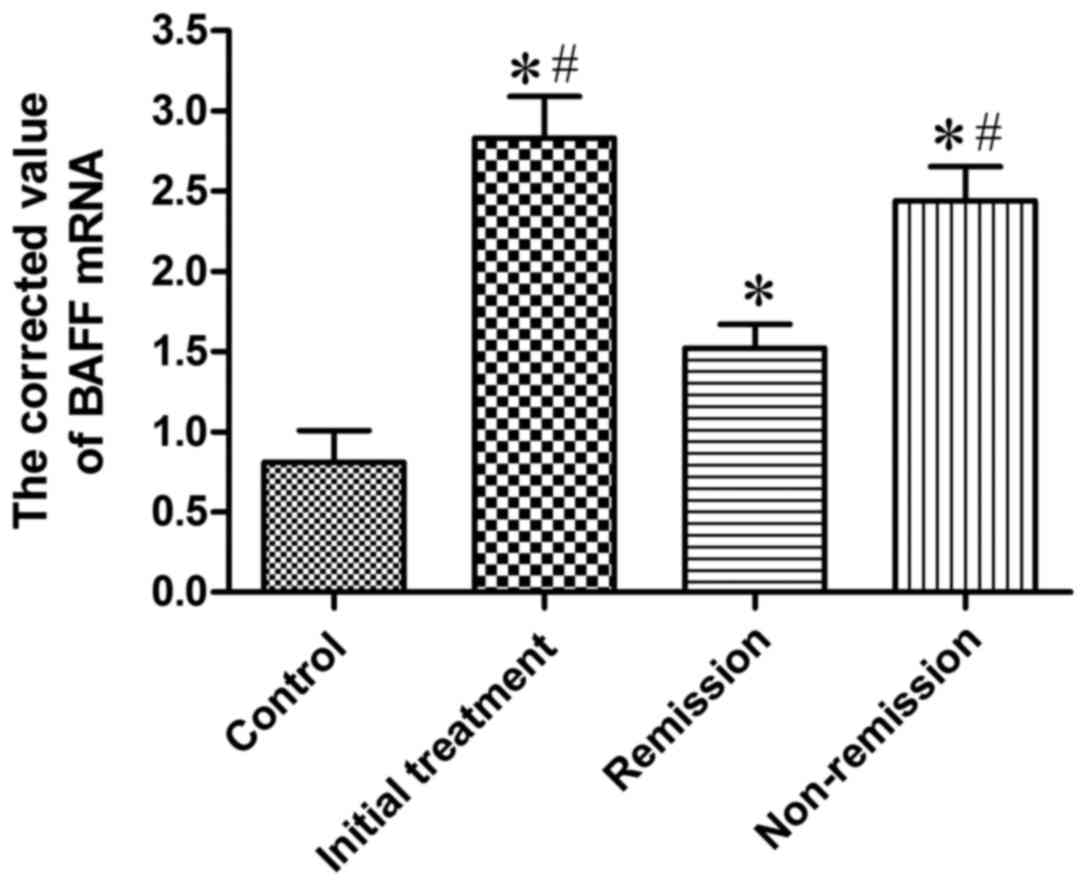
Expression of BAFF mRNA in BMMCs of
the four groups of patients
Real-time fluorescent quantitative RT-PCR was used
to determine BAFF mRNA expression in mononuclear cells in bone
marrow of 20 cases of the control group, 20 cases of initial
treatment group, 8 cases of remission group and 6 cases from
non-remission group. BAFF mRNA was positively expressed in patients
with MM and 20 cases of control group, and the corrected value is
shown in Table I and Fig. 1. The results suggested that the
quantitative PCR corrected value of initial treatment group was
markedly higher than that of control group, which was statistically
different (P<0.05); the quantitative PCR corrected value in
remission group were significantly higher than that of the control
group, and the difference was significant (P<0.05); the
quantitative PCR corrected value in non-remission group was
obviously higher than that in the control group, and the difference
was significant (P<0.05); the quantitative PCR corrected value
in initial treatment group was significantly higher (P<0.05)
than that in remission group; the quantitative PCR corrected value
in non-remission group was significantly higher (P<0.05) than
that in remission group; the quantitative PCR corrected value in
initial treatment group and in non-remission group showed no
significant difference.
 | Table I.The expression of BAFF mRNA in BMMCs
of the four groups of patients. |
Table I.
The expression of BAFF mRNA in BMMCs
of the four groups of patients.
| Groups | Number | BAFF mRNA corrected
value |
|---|
| Control | 20 |
0.81±0.34 |
| Initial
treatment | 18 |
2.83±0.45a,b |
| Remission | 25 |
1.52±0.26a |
| Non-remission | 17 |
2.44±0.37a,b |
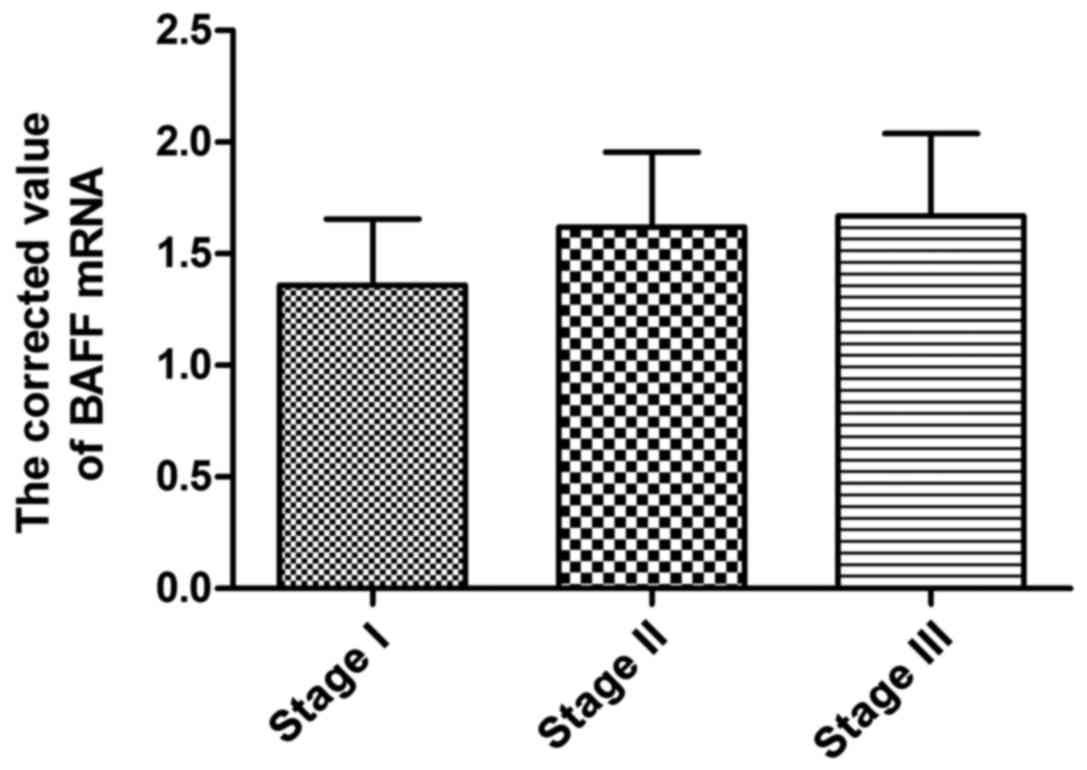
Expression of BAFF mRNA in BMMCs of
patients in different stages
According to ISS stage standard, 60 patients with
multiple myeloma were divided into 16 patients in stage I, 21
patients in stage II and 23 patients in stage III. The results
showed that BAFF mRNA expression in mononuclear cells of patients
in different stages had no significant difference (Table II and Fig.
2).
 | Table II.The expression of BAFF mRNA in BMMCs
of patients in different stages. |
Table II.
The expression of BAFF mRNA in BMMCs
of patients in different stages.
| Groups | Number | mRNA corrected
value |
|---|
| Stage I | 16 |
1.36±0.51 |
| Stage II | 21 |
1.62±0.58 |
| Stage III | 23 |
1.67±0.64 |
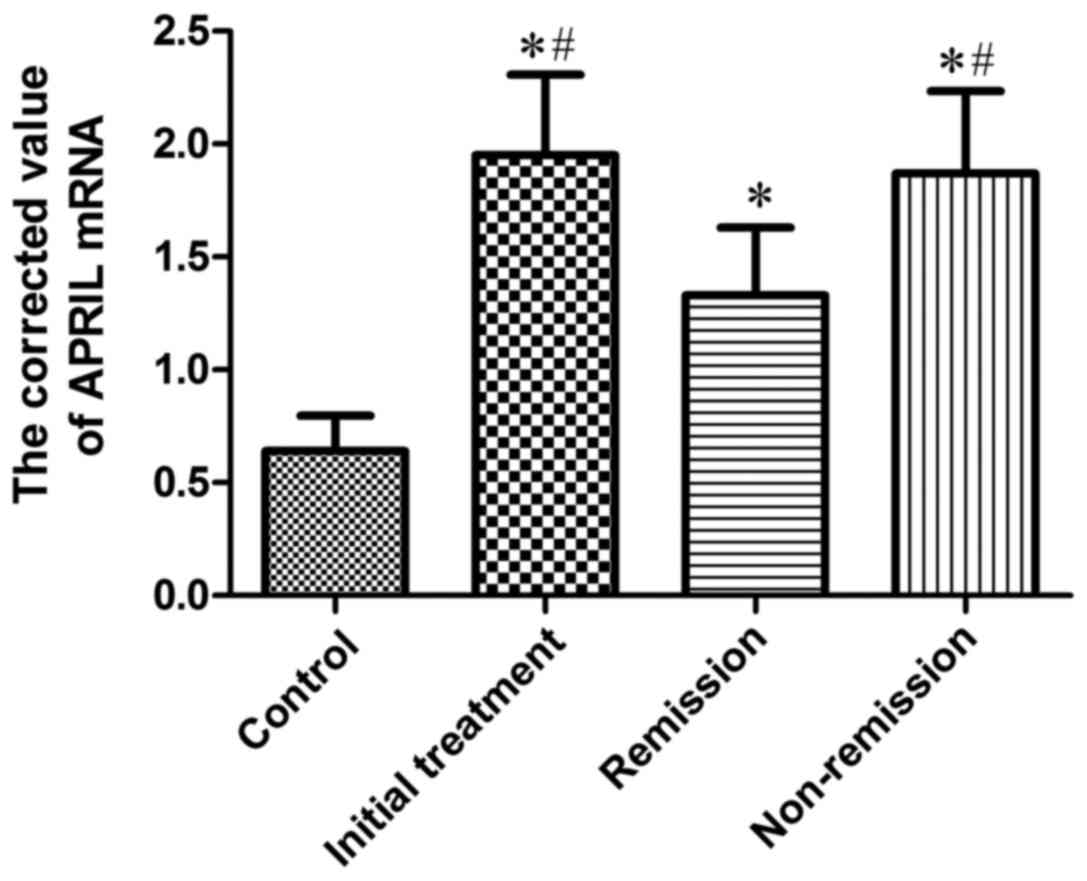
Expression of APRIL mRNA in BMMCs of
the four groups of patients
APRIL mRNA was positively expressed in patients with
MM and 10 cases of control group, and the corrected value is shown
in Table III and Fig. 3. The results suggested that: The
quantitative PCR corrected value of initial treatment group was
markedly higher than that of control group, which was statistically
different (P<0.05); the quantitative PCR corrected value in
remission group were significantly higher (P<0.05) than that of
the control group; the quantitative PCR corrected value in
non-remission group was obviously higher than that in the control
group, and the difference was significant (P<0.05); the
quantitative PCR corrected value in initial treatment group was
significantly higher than that in remission group, which was
statistically different (P<0.05); the quantitative PCR corrected
value in non-remission group was significantly higher than that in
remission group, and the difference was significant (P<0.05);
the quantitative PCR corrected value in initial treatment group and
in non-remission group showed no significant difference.
 | Table III.The expression of APRIL mRNA in BMMCs
of the four groups of patients. |
Table III.
The expression of APRIL mRNA in BMMCs
of the four groups of patients.
| Groups | Number | mRNA corrected
value |
|---|
| Control | 20 |
0.64±0.27 |
| Initial
treatment | 18 |
1.95±0.62a,b |
| Remission | 25 |
1.33±0.52a |
| Non-remission | 17 |
1.87±0.63a,b |
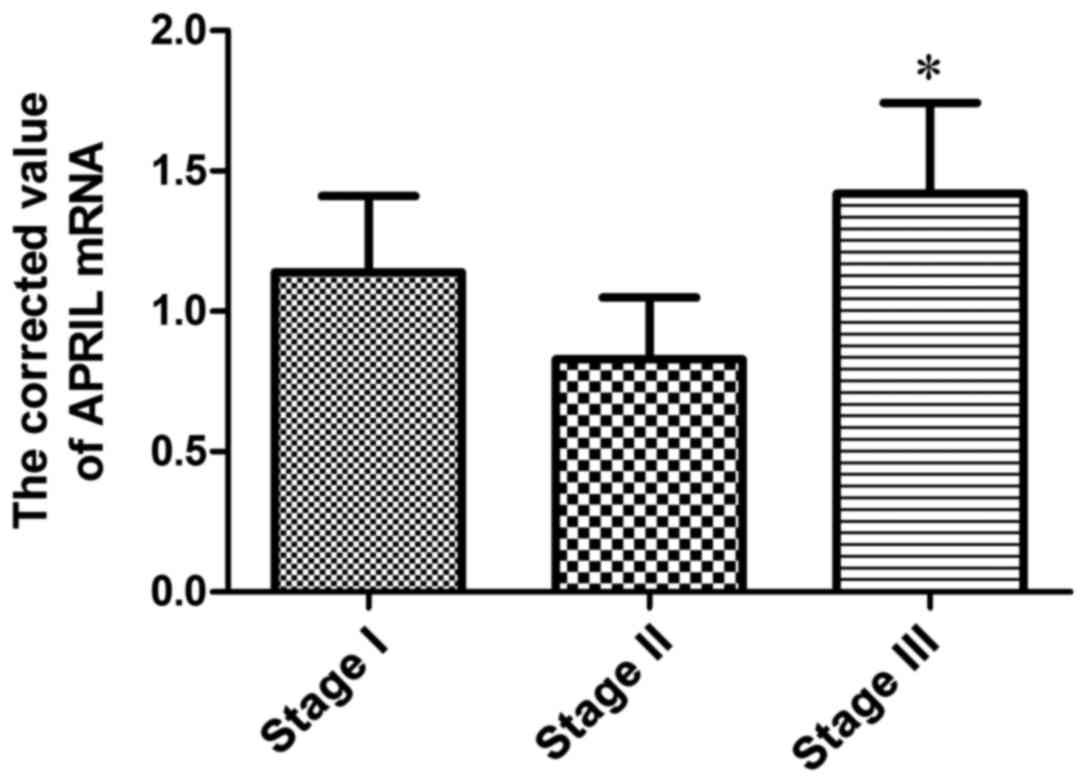
Expression of APRIL mRNA in BMMCs of
patients in different stages
APRIL mRNA expression in BMMCs in stage III patients
was significantly higher than that in stage II patients, and the
difference was significant (P<0.05); compared with stage I
patients, there was no significant difference (P>0.05) (Table IV and Fig.
4).
 | Table IV.The expression of BAFF mRNA in BMMCs
of patients in different stages. |
Table IV.
The expression of BAFF mRNA in BMMCs
of patients in different stages.
| Groups | Number | mRNA corrected
value |
|---|
| Stage I | 16 |
1.14±0.47 |
| Stage II | 21 |
0.83±0.38 |
| Stage III | 23 |
1.42±0.56a |
Gene and protein expression of
BAFF/APRIL in KM3 cell line
Real-time fluorescent quantitative RT-PCR was
employed to determine BAFF and APRIL gene expression in KM3 cell
line. BAFF and APRIL protein concentration was detected with ELISA
(Table V).
 | Table V.The gene and protein expression of
BAFF/APRIL in KM3 cell line. |
Table V.
The gene and protein expression of
BAFF/APRIL in KM3 cell line.
| Cytokine | mRNA corrected
value | Protein
concentration (ng/ml) |
|---|
| BAFF | 0.8351 | 6.8204 |
| APRIL | 0.7134 | 3.5913 |
The relationship between BAFF/APRIL
expression and clinical parameters of patients with multiple
myeloma
ELISA was used to detect the expression of BAFF and
APRIL, and the clinical data of these patients were collected:
Peripheral blood hemoglobin (Hb) concentration, albumin (ALB)
content, Ig, A/G, β-2 microglobulin (β-2MG) and plasma cell ratio
in bone marrow. The relationship between BAFF and APRIL expression
and the parameters mentioned above was analyzed. We found that
there was negative correlation between APRIL concentration and ALB
in peripheral blood (P=0.0346). APRIL concentration was positively
correlated with β-2MG (P=0.0013), Ig (P=0.0082), plasma cell ratio
in bone marrow (P=0.0027). APRIL concentration had no significant
correlation with other parameters, such as Hb content. BAFF
concentration was negatively correlated to ALB (P=0.0024) and A/G
(P=0.0218), and was positively correlated to β-2MG (P=0.0402), Ig
(P=0.0384) and plasma cell ratio in bone marrow (P=0.0019). It had
no significant correlation with the content of Hb. APRIL was
positively correlated to BAFF in multiple myeloma (P=0.0027)
(Table VI).
 | Table VI.The relationship between BAFF/APRIL
expression and clinical parameters of patients with multiple
myeloma. |
Table VI.
The relationship between BAFF/APRIL
expression and clinical parameters of patients with multiple
myeloma.
|
| APRIL (ng/ml) | BAFF (ng/ml) |
|---|
|
|
|
|
|---|
| Clinical data | Correlation
coefficient | P-value | Correlation
coefficient | P-value |
|---|
| Hb (g/l) | −0.4013 | 0.1652 | −0.3615 | 0.2164 |
| ALB (g/l) | −0.4627 | 0.0346a | −0.6249 | 0.0024b |
| Ig (g/l) | 0.7036 | 0.0082b | 0.5932 | 0.0384a |
| A/G (%) | −0.3263 | 0.1935 | −0.5127 | 0.0218a |
| β-2MG (mg/l) | 0.8334 | 0.0013b | 0.4935 | 0.0402a |
| Plasma cell
(%) | 0.8052 | 0.0027b | 0.6814 | 0.0019b |
| APRIL (ng/ml) |
|
| 0.6013 | 0.0027b |
Discussion
BAFF and APRIL are newly-discovered members of the
TNF family. They promote the growth and proliferation of B
lymphocytes. Accordingly, exploring its relationship with blood B
lymphocyte malignancies has been the focus of research. It was
suggested that BAFF was necessary for the survival of normal
immature and mature B cells, and is also necessary for the growth
of the normal plasma blasts (11,12). BAFF
also plays a key role in the survival of the tumor cells in B-CLL
(13). In addition, APRIL can
stimulate some human and rat tumor cell growth in vivo and
in vitro (14). Therefore, we
chose the lymphoid proliferation factor BAFF and APRIL, and observe
their expression in patients with multiple myeloma.
Srinivasan and Schiffer firstly found that myeloma
cell expressed BAFF and APRIL. They also detected the concentration
of soluble BAFF and APRIL in the serum of patients with multiple
myeloma, which was 5 times that of normal people of the same age
(15).
With ELISA, Ilić et al analyzed the average
concentration of BAFF level in 51 patients with myeloma patients
and 11 normal people. The result was 968 and 417 pg/ml,
respectively, which was statistically different (16).
Fluorescent quantitative PCR was employed to detect
the expression of BAFF and APRIL mRNA in BMMCs in 60 cases with
multiple myeloma and control group. The results showed that the
expression of BAFF and APRIL mRNA of initial treatment group,
non-remission group and remission group patients were higher than
that in control group. The initial treatment group and
non-remission group were higher than the remission group, which
suggested that BAFF and APRIL were highly expressed in multiple
myeloma and the two factors decreased after treatment. Such changes
of BAFF and APRIL existed in the whole process of MM, which are
useful indicators to judge the remission state of multiple myeloma
and may be the main tumor promoter in multiple myeloma.
Mechanisms by which BAFF and APRIL promote the
survival and proliferation of B lymphocytes are closely related to
BCMA, TACI and BAFF-R9 (17). BAFF-R
has high affinity for BAFF, which mainly regulates the growth of
normal B cells (18). BCMA and TACI
are main receptors which maintain the multiple myeloma cell
proliferation and survival (19,20). The
main mechanisms are: BAFF/APRlL binds to its receptor BCMA, TACI
and induces NF-κB, P13/AKT and MAPK signal pathway to increase the
expression of anti-apoptotic protein Mcl-1 and Bcl-2, inhibiting
the apoptosis of myeloma cells and promoting their proliferation
and survival (21,22). Ho et al found that bone marrow
stromal cells in the bone marrow microenvironment could produce
BAFF, and found that the concentration of BAFF in bone marrow
stromal cells was 3–10 times that of myeloma cells by flow
cytometry (23). When myeloma cell
adheres to bone marrow stromal cells, BAFF produced by bone marrow
stromal cell increased 2–5 times compared with BAFF produced by
single bone marrow stromal cells (24,25). At
the same time, the increase of BAFF expression in turn promotes
myeloma cell adhesion to bone marrow stromal cells, which has
dose-effect relationship (26).
Osteoclasts in multiple myeloma microenvironment produce a large
number of APRIL by paracrine to promote the occurrence and
development of myeloma (27).
In order to understand whether the expression of
BAFF/APRIL is associated with the severity of multiple myeloma,
patients with multiple myeloma were divided into stage I, II and
III according to the clinical stage of ISS. The results suggest
that expression of APRIL mRNA in BMMCs in stage III patients was
significantly higher than that in stage II patients, which is
statistically different. We may consider that the expression of
BAFF and APRIL mRNA in MM clinical staging has the tendency for the
expression to increase with the increasing of staging. The main
cause is that Patients with high clinical stage get less
opportunity to be relieved, and the bone marrow microenvironment
often appears as a neoplastic proliferation alteration, resulting
in the increase of BAFF/APRIL expression. However, APRIL is widely
produced by the bone marrow microenvironment cells and
extramicroenvironment cells, so the plasma concentration is often
higher than BAFF and is sensitive when detected.
References
|
1
|
Sanchez E, Li M, Kitto A, Li J, Wang CS,
Kirk DT, Yellin O, Nichols CM, Dreyer MP, Ahles CP, et al: Serum
B-cell maturation antigen is elevated in multiple myeloma and
correlates with disease status and survival. Br J Haematol.
158:727–738. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Fragioudaki M, Boula A, Tsirakis G,
Psarakis F, Spanoudakis M, Papadakis IS, Pappa CA and Alexandrakis
MG: B cell-activating factor: its clinical significance in multiple
myeloma patients. Ann Hematol. 91:1413–1418. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Tsukada Y, Hattori Y, Nakajima H, Yokoyama
K, Murata M, Shimizu N, Kondo N and Okamoto S: B-cell acute
lymphoblastic leukemia developed 5 years after autologous stem cell
transplantation for multiple myeloma. Rinsho Ketsueki. 53:219–223.
2012.(In Japanese). PubMed/NCBI
|
|
4
|
Jöhrer K, Hofbauer SW, Zelle-Rieser C,
Greil R and Hartmann TN: Chemokine-dependent B cell-T cell
interactions in chronic lymphocytic leukemia and multiple myeloma -
targets for therapeutic intervention? Expert Opin Biol Ther.
12:425–441. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Kurzrock R, Voorhees PM, Casper C, Furman
RR, Fayad L, Lonial S, Borghaei H, Jagannath S, Sokol L, Usmani SZ,
et al: A phase I, open-label study of siltuximab, an anti-IL-6
monoclonal antibody, in patients with B-cell non-Hodgkin lymphoma,
multiple myeloma, or Castleman disease. Clin Cancer Res.
19:3659–3670. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Reijmers RM, Spaargaren M and Pals ST:
Heparan sulfate proteoglycans in the control of B cell development
and the pathogenesis of multiple myeloma. FEBS J. 280:2180–2193.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Wang SM, Zhang TL, Jiang YM, Wu HY, Hao LM
and Xing XH: Expression and significance of B cell-activating
factor of TNF family (BAFF) and B cell lymphoma/leukemia-2 (BCL-2)
in multiple myeloma. Zhongguo Shi Yan Xue Ye Xue Za Zhi.
19:395–398. 2011.(In Chinese). PubMed/NCBI
|
|
8
|
Carpenter RO, Evbuomwan MO, Pittaluga S,
Rose JJ, Raffeld M, Yang S, Gress RE, Hakim FT and Kochenderfer JN:
B-cell maturation antigen is a promising target for adoptive T-cell
therapy of multiple myeloma. Clin Cancer Res. 19:2048–2060. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Boucher K, Parquet N, Widen R, Shain K,
Baz R, Alsina M, Koomen J, Anasetti C, Dalton W and Perez LE:
Stemness of B-cell progenitors in multiple myeloma bone marrow.
Clin Cancer Res. 18:6155–6168. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Goel A, Spitz DR and Weiner GJ:
Manipulation of cellular redox parameters for improving therapeutic
responses in B-cell lymphoma and multiple myeloma. J Cell Biochem.
113:419–425. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Mahtouk K, Tjin EP, Spaargaren M and Pals
ST: The HGF/MET pathway as target for the treatment of multiple
myeloma and B-cell lymphomas. Biochim Biophys Acta. 1806:208–219.
2010.PubMed/NCBI
|
|
12
|
Hollander N: Current vaccination
strategies for the treatment of B-cell lymphoma and multiple
myeloma. Crit Rev Immunol. 29:399–418. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Chen P, Li B, Zhuang W, Huang H, Zhang H
and Fu J: Multiple bone lesions and hypercalcemia presented in
diffuse large B cell lymphoma: mimicking multiple myeloma? Int J
Hematol. 91:716–722. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Harding SJ, Mead GP, Bradwell AR and
Berard AM: Serum free light chain immunoassay as an adjunct to
serum protein electrophoresis and immunofixation electrophoresis in
the detection of multiple myeloma and other B-cell malignancies.
Clin Chem Lab Med. 47:302–304. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Srinivasan S and Schiffer CA: Concurrent
B-cell chronic lymphocytic leukemia and multiple myeloma treated
successfully with lenalidomide. Leuk Res. 33:561–564. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Ilić V, Milosević-Jovcić N, Petrović S,
Marković D, Stefanović G and Ristić T: Glycosylation of IgG B cell
receptor (IgG BCR) in multiple myeloma: relationship between
sialylation and the signal activity of IgG BCR. Glycoconj J.
25:383–392. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Fragioudaki M, Tsirakis G, Pappa CA,
Aristeidou I, Tsioutis C, Alegakis A, Kyriakou DS, Stathopoulos EN
and Alexandrakis MG: Serum BAFF levels are related to angiogenesis
and prognosis in patients with multiple myeloma. Leuk Res.
36:1004–1008. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Shen X, Zhang X, Xu G and Ju S: BAFF-R
gene induced by IFN-γ in multiple myeloma cells is related to NF-κB
signals. Cell Biochem Funct. 29:513–520. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Shen X, Zhu W, Zhang X, Xu G and Ju S: A
role of both NF-κB pathways in expression and transcription
regulation of BAFF-R gene in multiple myeloma cells. Mol Cell
Biochem. 357:21–30. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Li LS, Shen JK and Zhang GS: Effect of
BAFF/APRIL mRNA expression induced by glucocorticoid and bortezomib
in multiple myeloma cells in vitro. Zhongguo Shi Yan Xue Ye Xue Za
Zhi. 19:1419–1423. 2011.(In Chinese). PubMed/NCBI
|
|
21
|
Quinn J, Glassford J, Percy L, Munson P,
Marafioti T, Rodriguez-Justo M and Yong K: APRIL promotes
cell-cycle progression in primary multiple myeloma cells: influence
of D-type cyclin group and translocation status. Blood.
117:890–901. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Moreaux J, Sprynski AC, Dillon SR, Mahtouk
K, Jourdan M, Ythier A, Moine P, Robert N, Jourdan E, Rossi JF, et
al: APRIL and TACI interact with syndecan-1 on the surface of
multiple myeloma cells to form an essential survival loop. Eur J
Haematol. 83:119–129. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Ho J, Yang L, Banihashemi B, Martin L,
Halpenny M, Atkins H, Sabloff M, McDiarmid SA, Huebsch LB,
Bence-Bruckler I, et al: Contaminating tumour cells in autologous
PBSC grafts do not influence survival or relapse following
transplant for multiple myeloma or B-cell non-Hodgkin's lymphoma.
Bone Marrow Transplant. 43:223–228. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Peh SC, Gan GG, Lee LK and Eow GI:
Clinical relevance of CD10, BCL-6 and multiple myeloma-1 expression
in diffuse large B-cell lymphomas in Malaysia. Pathol Int.
58:572–579. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Ianotto JC, Tempescul A, Eveillard JR,
Marion V, Quintin-Roué I and Berthou C: Tri-lineage disease
involving sideroblastic anaemia, multiple myeloma and B-cell
non-Hodgkin's lymphoma in the same patient. Ann Hematol.
88:273–274. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Kuiper R, Broyl A, de Knegt Y, van Vliet
MH, van Beers EH, van der Holt B, el Jarari L, Mulligan G, Gregory
W, Morgan G, et al: A gene expression signature for high-risk
multiple myeloma. Leukemia. 26:2406–2413. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Novak AJ, Grote DM, Ziesmer SC, Rajkumar
V, Doyle SE and Ansell SM: A role for IFN-lambda1 in multiple
myeloma B cell growth. Leukemia. 22:2240–2246. 2008. View Article : Google Scholar : PubMed/NCBI
|