Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide (1). Radiotherapy, alone or in combination
with either surgery or systematic therapies, is a key treatment
modality in the curative treatment of lung cancer patients,
particularly those with advanced-stage disease (2). With the advance of radiotherapy
technologies, precision radiotherapy has greatly improved the
outcome for patients with lung cancer, while reducing the toxicity
of an increased dose and alleviating the risk to adjacent organs.
Notably, the precision delineation of the gross target volume (GTV)
based on image data serves an essential role in precision
radiotherapy. Several non-invasive procedures, including
intensified computed tomography (CT) and positron emission
tomography (PET), have been widely used for the target volume
delineation of lung cancer (3).
Precision radiotherapy based on CT imaging generates a risk to the
organs due to its poor soft tissue contrast, particularly for
central lung cancer accompanied with pulmonary atelectasis or
mediastinal node metastasis (4).
Although a number of studies have demonstrated that
PET outperforms CT in diagnosing nodal involvement in lung cancer
(3,5,6), PET could
inevitably yield false-positive results (7). Recently, the combination of PET and CT
(PET/CT) has significantly improved the accuracy and sensitivity of
the lung cancer diagnosis (3,8) and has become a routine aspect of the
contemporary radiotherapy treatment planning process (9). However, the target delineation criteria
have not yet been fully optimized (10) due to low image resolution and the
difficulty in fusing the PET and CT images.
Diffusion-weighted magnetic resonance imaging
(DW-MRI) can detect the restricted diffusion of water molecules
through exploiting the random motion of water protons in biological
tissue (11). Compared with normal
tissues, malignant tumors exhibit a significantly decreased
diffusion of water molecules. The difference in the diffusion of
water molecules among tissues enables DW-MRI to detect malignant
tumors and differentiate them from benign masses (12). Previous studies have shown that DW-MRI
is comparable with PET/CT in detecting malignant lesions (13–15).
Numerous studies reported that DW-MRI provided more accurate
delineation for a number of cancer types, including prostate, head
and neck, and cranial tumors (16–18).
Compared with PET/CT, DW-MRI has demonstrated the same level of
sensitivity or higher in detecting the nodal and primary
malignancies in lung cancer (12).
Nevertheless, the implication of DW-MRI in lung cancer,
particularly lung cancer with pulmonary atelectasis, has not yet
been intensively investigated. In the present study, the potential
implication of DW-MRI in assessing central lung cancer with
pulmonary atelectasis was evaluated and the successful
implementation of DW-MRI in precision radiotherapy treatment
planning was demonstrated.
Patients and methods
Patient selection
The patients recruited in the present study were
histologically diagnosed with central lung cancer accompanied with
pulmonary atelectasis. In general, all patients exhibited a good
health condition with a Karnofsky performance status of ≥70
(19) and with no contraindications
to MRI examination. The images from CT, PET/CT and MRI scans for
each individual patient were collected within 1 week. The results
from the PET/CT scan were used as the standard for diagnosis. All
patients provided written informed consent prior to enrolling in
the study. The study was approved by the Institutional Review Board
and the Ethics Committee of Shandong Cancer Hospital and Institute
(Jinan, China).
Population
A total of 27 patients with central lung cancer
without any antitumor therapy scheduled to receive precision
radiotherapy were enrolled in the present study between October
2014 and June 2015. The cohort included 23 males and 4 females. The
patient ages ranged from 37 to 79 years, with a median of 61 years.
All patients were histologically confirmed with central lung cancer
via biopsy. The cohort included 12 cases of squamous cell
carcinoma, 6 cases of adenocarcinoma, 6 cases of small cell
carcinoma, 2 cases of atypical carcinoid and 1 case of adenoid
cystic carcinoma. The tumors were located in the upper left lung in
8 cases, the lower left lung in 4 cases, the upper right lung in 5
cases, the middle right lung in 4 cases and the lower right lung in
6 cases.
CT scan and image acquisition
Prior to radiotherapy, all patients underwent CT
simulation using immobilization by an evacuated vacuum-bag in the
supine position and a CT scanner (Brilliance CT Big Bore; Philips
Healthcare, DA Best, The Netherlands), with a 3-mm slice thickness
from the circular cartilage to the upper pole of the kidney.
Following the scan, each patient was injected with 90 ml ioversol
(320 mg/ml) for enhancement scanning.
PET/CT scans and image
acquisition
The PET/CT scan was performed with the GE Discovery
LS PET/CT scanning system (GE Healthcare Life Sciences, Shanghai,
China). All patients were requested to fast at least 6 h and it was
necessary that their blood glucose should be within in the normal
range prior to the PET/CT examination. Between 40 and 60 min after
the intravenous injection of fluorodeoxyglucose
(18F-FDG; 5.55–7.40 MBq/kg), the CT scan was performed
and emission images were acquired. The patient was immobilized by
evacuated vacuum-bag in the supine position using the fixed-field
parameters based on the CT. The PET images were reconstructed using
the Ordered-Subset Expectation Maximization (OSEM) with the
built-in software on the scanning machine, and the PET images were
attenuation corrected with CT.
DW-MRI scan and image acquisition
The DW-MRI scan was performed by the Achieva 3.0T MR
PHILIPS scanner (Philips Healthcare). Patients were kept in the
same supine position as for the CT scan. The scanning parameters
were as follows: i) Cross sectional T1-weighted image (T1WI):
Repetition time/echo time TR/TE 10 sec/2 msec; slice
thickness/interslice gap, 3/0 mm; field of view (FOV), 375 mm;
matrix, 352×160; ii) cross-sectional T2WI:TR/TE 1.5 sec/80 msec;
slice thickness/interslice gap, 3/0 mm; FOV, 375 mm; matrix,
352×160; iii) coronal T2WI: TR/TE, 1.8 sec/80 msec; slice
thickness/interslice gap, 3/0 mm; FOV, 375 mm; matrix, 352×160; and
iv) DWI: TR/TE, 2.6 sec/52 msec; slice thickness/interslice gap,
3/0 mm; FOV, 375 mm; matrix, 352×160; diffusion-weighted sequence
(b=600 sec/mm2) was added in the axial plane.
Image fusion
To delineate GTVs, all CT, PET and DW-MRI images
were transferred to a treatment planning system (TPS, Eclipse
V11.5; Varian Medical Systems, Palo Alto, CA, USA). The PET and
DW-MRI images from each patient were fused with the corresponding
CT images. The accuracy of registration was visually inspected
using the software provided by the TPS.
Using the large aperture static image as the
reference, the rigid registration of the gray scale image method,
together with the correction based on the skeletal signs, was used
to locate the PET and DW-MRI images in the coordinate system.
GTV delineation on CT, PET/CT and
DW-MRI images
In total, 10 radiotherapists independently reviewed
the CT, PET and DW-MRI images, and delineated the contours of the
tumor following the standard procedure. The lung cancer usually
shows a heterogeneous and lobulated mass with rough edges in CT
image and the tumor edges were used as the reference on
GTVCT delineation.
The 18F-FDG concentration and
characteristics of the lung lesions were used to determine the
tumor tissue and the lung tissue on the PET/CT images. Using
Eclipse V11.5, the contour of the primary tumor target zone in
GTVPET was first automatically outlined if the
standardized uptake value was ≥2.5, while the non-tumor regions
could manually be removed by referencing the CT image. On DW-MRI
images, the solid region of the tumor appeared to have high signal
intensity. By contrast, the pulmonary atelectasis and the
obstructive inflammation had relatively low signal intensity. Only
the area with a high signal was used in contouring
GTVMRI. The volumes of the target area were
automatically provided using Eclipse V11.5 software.
Distance between centroids of
GTVs
The three directional coordinates of the
GTVCT, GTVPET and GTVMRI centroids
were determined using the Eclipse V11.5 software and were denoted
as (x, y, z). The relative coordinates between two GTV centroids
were denoted as (∆x, ∆y, ∆z), with ∆x the distance in the
left/right (LR) direction, ∆y the distance in the superior/inferior
direction direction and ∆z the distance in the anterior/posterior
direction. The formula V = √(∆x2 + ∆y2 +
∆z2) was used to calculate the distance between
centroids of GTVs (i.e., GTVCT vs. GTVPET;
GTVPET vs. GTVMRI; and GTVPET vs.
GTVMRI).
Statistical analysis
Statistical analysis was performed using the SAS 9.3
software (SAS Institute Inc., Cary, NC, USA). All the parameters
were descriptively summarized, including the mean and standard
deviation. The differences between the GTVs and the distance to the
centroids of the GTVs are presented as the mean and standard error
of the mean, and were assessed using Student's t-test. The
difference was considered as statistically significant if
P<0.05. Pearson's correlation coefficient values were summarized
for the GTVs measured by three different approaches. The comparison
between the group means was performed using one-way analysis of
variance. The variation in the target volume of the tumors measured
by different radiotherapists was assessed by the variation
coefficient (CV) as follows: CV = standard deviation/mean ×
100.
Results
Collection of CT, PET/CT and DW-MRI
images
To implement DW-MRI as a preferred lung cancer
radiotherapy procedure that is reproducible, non-invasive and
cost-effective, the present study evaluated and compared CT, PET/CT
and DW-MRI images of lung cancer. A total of 27 patients were
diagnosed with lung cancer and images were collected according to
the aforementioned methods. Using image fusions, GTVs for CT,
PET/CT and DW-MRI images were delineated. Images from 2 individual
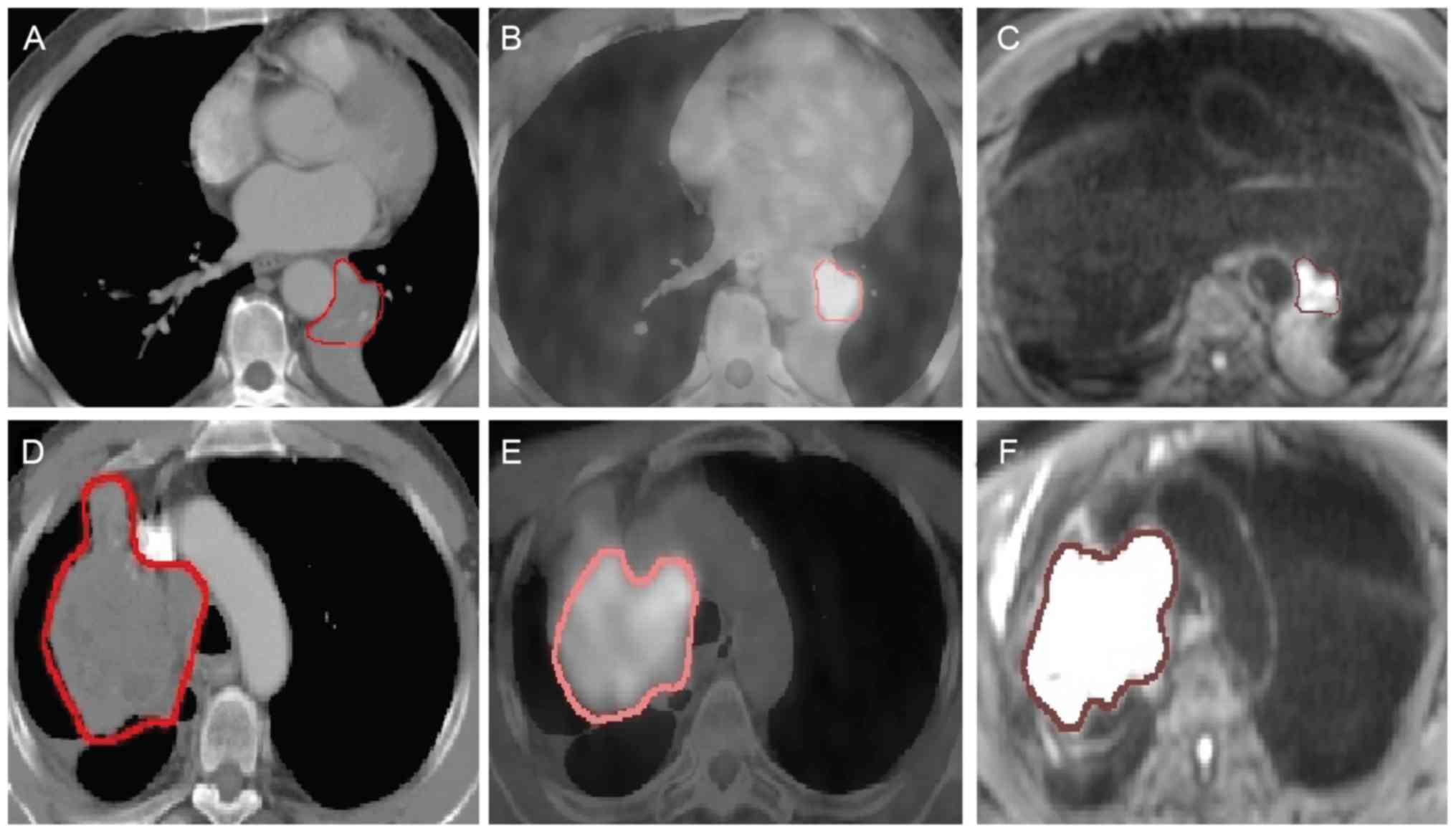
patients are presented as examples in Fig. 1.
The delineated tumors among the 27 subjects varied
in shape and size. Noticeably and as expected, atelectasis
compromised the precise delineation of the tumors in the majority
of cases, as demonstrated by the blurry boundary in Fig. 1A and D. It is also worth noting that
compared with the GTVs of the CT and PET/CT images, the delineated
GTV of the DW-MRI images was often smaller in size with clear edges
(Fig. 1C and F).
Pairwise comparison between
GTVCT, GTVPET and GTVMRI
A total of 27 GTV measurements were obtained for all
patients based on CT, PET/CT and DW-MRI images, respectively.
GTVCT values ranged from 13.48 to 258.75 cm3,
with a mean of 109.45 cm3, whereas GTVPET
values ranged from 2.48 to 219.97 cm3, with a mean of
85.23 cm3, and GTVMRI values ranged from 3.88
to 246.95 cm3, with a mean of 83.10 cm3
(Table I). Among the 27 subjects
analyzed, 23 subjects presented with a larger mean GTVCT
than mean GTVMRI value, 22 subjects with a larger mean
GTVCT than GTVPET value, and 21 subjects with
a larger mean GTVPET than mean GTVMRI value
(data not shown).
 | Table I.GTV measurements using CT, PET/CT and
diffusion-weighted MRI (n=27). |
Table I.
GTV measurements using CT, PET/CT and
diffusion-weighted MRI (n=27).
| GTV measurements,
cm3 | Mean (standard
error of the mean) |
P-valuea |
|---|
|
GTVCT | 109.45 (14.90) | N/A |
| GTVPET | 85.23
(13.10) | N/A |
|
GTVMRI | 83.10
(14.26) | N/A |
|
GTVCT-GTVMRI | 26.34
(6.39) | <0.001 |
|
GTVCT-GTVPET | 24.22
(5.84) | 0.003 |
|
GTVPET-GTVMRI |
2.12 (2.46) | 0.395 |
The mean GTVCT was larger than the mean
GTVMRI and GTVPET by a value of 26.34
cm3 and 24.22 cm3, respectively (Table I). Student's t-test showed that the
differences in the mean GTV between CT and MRI, and between CT and
PET, were statistically significant (Table I), suggesting that CT contouring is
statistically different from PET and MRI contouring. By contrast,
the difference in the mean GTV between PET and MRI was negligible
and not significantly different (Table
I).
Correlations of GTVCT,
GTVPET and GTVMRI
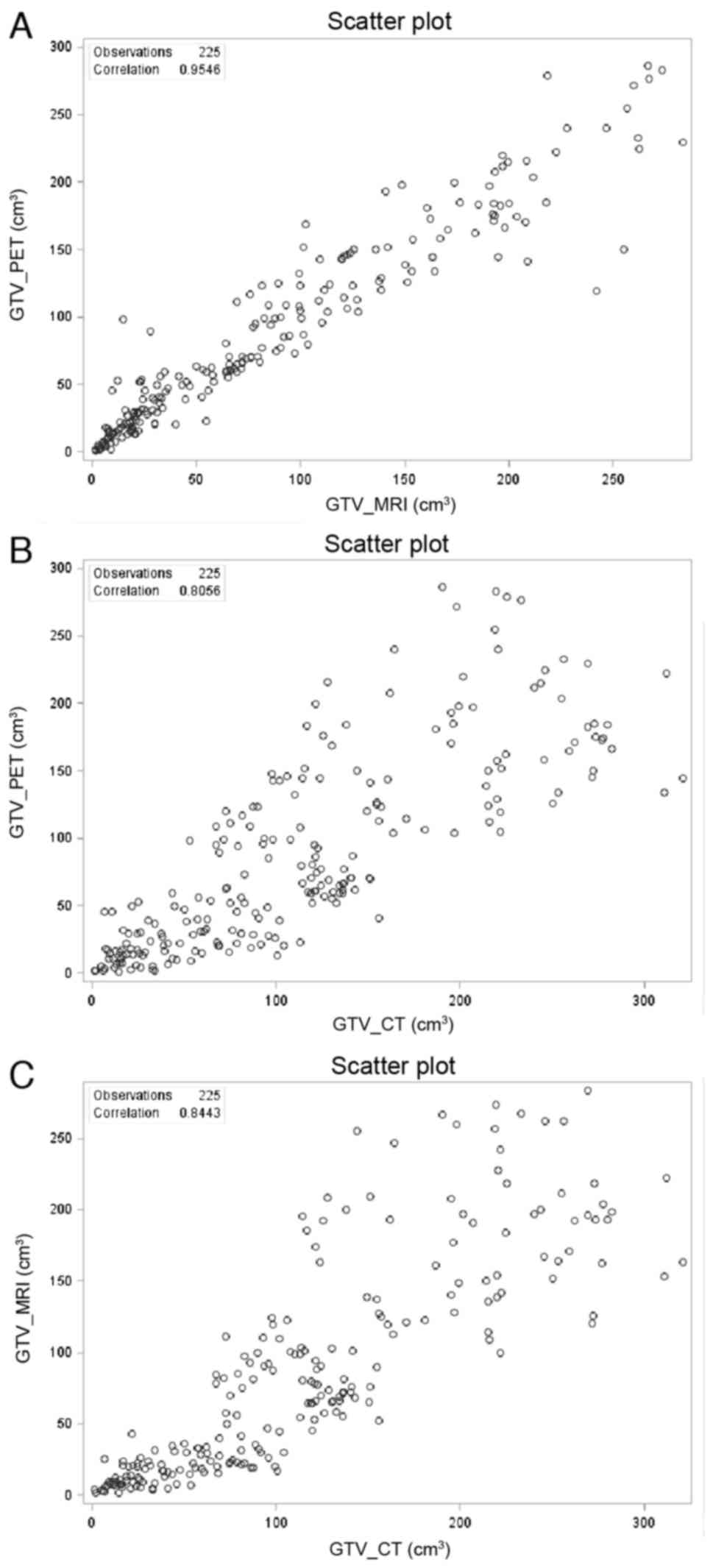
The correlations among GTVs were also examined by
measuring the Pearson's correlation coefficient in a pairwise
manner. Pearson's correlation values of the three comparisons were
all greater than 0.8 (Fig. 2),
suggesting that all three contour methods are coherently related.
However, Pearson's correlation between GTVPET and
GTVMRI (Fig. 2A, r=0.9546)
was significantly higher than those between GTVCT and
GTVPET (Fig. 2B,
r=0.8056), and between GTVCT and GTVMRI
(Fig. 2C, r=0.8443). This
demonstrates a direct dependency between PET and MRI contouring,
and a divergence of CT from the other contouring methods.
Pairwise comparison between CV of
GTVCT, GTVPET and GTVMRI
To evaluate the robustness and the reproducibility
of the DW-MRI procedure, cancer images of the 27 subjects were
randomly collected by 10 individual medical doctors and the CV was
calculated for CT, PET/CT and DW-MRI procedures, respectively. CV
is a standardized measure of dispersion in a distribution. The CV
among doctors for the GTVMRI procedure was 26.60%, which
is markedly smaller than the CVs for the GTVPET (32.00%)
and GTVCT (33.76%) procedures. These data suggested that
DW-MRI is the most robust and reproducible procedure in lung cancer
radiotherapy.
Distance between centroids of
GTVs
The mean distance between the centroids of
GTVCT and GTVPET was 0.87 cm (SD, 0.54 cm).
The mean distance between the centroids of GTVMRI and
GTVPET was 0.72 cm (SD, 0.34 cm). No statistically
significant differences in the distances were observed.
Discussion
It is not uncommon that central lung cancer occurs
with obstructive pulmonary atelectasis (20). Conceivably, accompanying atelectasis
often obscures the accuracy of a central lung cancer diagnosis
(21,22). The present study observed the
confounding effect of atelectasis on delineating the lung cancer
(Fig. 1). Distinguishing central lung
cancer from obstructive pulmonary atelectasis is critical in
clinical staging and target delineation. An incorrect delineation
of GTV could lead to decreased survival rates and increased side
effects from radiotherapy (2).
A hallmark feature of an intensified CT scan is the
superior spatial resolution of lung cancer. However, it is fairly
challenging to distinguish lung cancer from pulmonary atelectasis
due to inflammation and effusion (23,24).
Relatively low soft-tissue contrast prevents CT from providing
precise information on the GTV extension in the vast majority of
tissues. It has been widely recognized that this limitation of CT
has led to inter- and intra-observer variations in GTV delineation
in lung cancer (25,26).
PET/CT is an extremely important innovation in lung
cancer imaging, which is capable of explicitly differentiating
between the normal tissues and cancer tissues. PET/CT has been
proven to significantly enhance the accuracy of conventional
imaging in estimating the full spectrum of a number of tumors,
including lung cancer (27). Numerous
studies have reported that PET/CT has an influential contribution
on the radiotherapy of lung cancer patients (24,28), as it
can easily distinguish the central lung cancer from
atelectasis.
MRI has been rapidly and widely deployed in
radiotherapy planning due to its exquisite high contrast, high
resolution soft tissue visualization and functional imaging
modalities, which outperform PET/CT in terms of tumor visualization
capability (26). It has been
suggested that DWI combined with MRI can provide important
information in differentiating lung cancer and atelectasis
(20).
In the present study, the mean GTV measurements
based on DW-MRI were significantly smaller than the mean GTV based
on CT, and these were indistinguishable from the mean GTV based on
PET/CT (Table I), which is consistent
with previous studies (17,29–32).
DW-MRI is an excellent technique in differentiating the central
lung cancer from obstructive pulmonary atelectasis and can provide
accurate information for target delineation. In addition, it was
shown that the distance between the centroids of CT and PET images
was similar to that between the centroids of MRI and PET images,
suggesting that the difference in GTVs using different procedures
is largely attributable to the shape of the target delineation, but
not the location of the target centers. Notably, delineation based
on DW-MRI achieves similar clinical results with PET/CT, while it
can avoid the radiation for patients during the PET/CT scan, as
well as lowering the treatment cost.
The large variation among radiotherapists, and even
for the same doctor over a period of time, is not uncommon in the
target delineation (33,34). The methodology defects and poor
differentiation between normal tissues and tumors account for the
inter-observer variations (26). In
the present study, the variation based on DW-MRI was noticeably
lower than that based on PET/CT and CT. Overall, DW-MRI is much
more robust and reproducible compared with CT and PET/CT
procedures. Due to relatively poor soft-tissue contrast of the CT
image, it can only provide limited information for target
delineation. GTV based on CT varied enormously between
radiotherapists, although it was prophetically suggested that the
variation could be decreased via rigid training. By contrast, the
variations in estimating GTV using DW-MRI and PET/CT are much
smaller and more stable among radiotherapists than that in CT. In
other words, compared with PET/CT, DW-MRI has the highest target
delineation precision and lowest variation to the same extent, thus
significantly decreasing the toxicity of unintentional dosing and
impairment of adjacent tissues during the radiotherapy. DW-MRI is
highly recommended as it radiation-free and cost-effective, which
is of particular benefit in developing countries.
Acknowledgements
This study was supported by the National Natural
Science Foundation of China (grant nos. 81272699, 81301936 and
81472811), the Shandong Science and Technology Development Project
(grant no. 2014GGC03038) and the International Cooperation Project
of Science and Technology Department (grant no. 2012DFA31560).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Senan S and De Ruysscher D: Critical
review of PET-CT for radiotherapy planning in lung cancer. Crit Rev
Oncol Hematol. 56:345–351. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Dwamena BA, Sonnad SS, Angobaldo JO and
Wahl RL: Metastases from non-small cell lung cancer: Mediastinal
staging in the 1990s-meta-analytic comparison of PET and CT.
Radiology. 213:530–536. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Devic S: MRI simulation for radiotherapy
treatment planning. Med Phys. 39:6701–6711. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Toloza EM, Harpole L and McCrory DC:
Noninvasive staging of non-small cell lung cancer: A review of the
current evidence. Chest. 123 1 Suppl:137S–146S. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Gould MK, Kuschner WG, Rydzak CE, Maclean
CC, Demas AN, Shigemitsu H, Chan JK and Owens DK: Test performance
of positron emission tomography and computed tomography for
mediastinal staging in patients with non-small-cell lung cancer: A
meta-analysis. Ann Intern Med. 139:879–892. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Roberts PF, Follette DM, von Haag D, Park
JA, Valk PE, Pounds TR and Hopkins DM: Factors associated with
false-positive staging of lung cancer by positron emission
tomography. Ann Thorac Surg. 70:1154–1160. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Silvestri GA, Gould MK, Margolis ML,
Tanoue LT, McCrory D, Toloza E and Detterbeck F: American College
of Chest Physicians: Noninvasive staging of non-small cell lung
cancer: ACCP evidenced-based clinical practice guidelines (2nd
edition). Chest. 132 3 Suppl:178S–201S. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Al-Jahdali H, Khan AN, Loutfi S and
Al-Harbi AS: Guidelines for the role of FDG-PET/CT in lung cancer
management. J Infect Public Health. 5 Suppl 1:S35–S40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Aristei C, Falcinelli L, Palumbo B and
Tarducci R: PET and PET-CT in radiation treatment planning for lung
cancer. Expert Rev Anticancer Ther. 10:571–584. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Le Bihan D, Breton E, Lallemand D, Aubin
ML, Vignaud J and Laval-Jeantet M: Separation of diffusion and
perfusion in intravoxel incoherent motion MR imaging. Radiology.
168:497–505. 1988. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Usuda K, Zhao XT, Sagawa M, Matoba M,
Kuginuki Y, Taniguchi M, Ueda Y and Sakuma T: Diffusion-weighted
imaging is superior to positron emission tomography in the
detection and nodal assessment of lung cancers. Ann Thorac Surg.
91:1689–1695. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Komori T, Narabayashi I, Matsumura K,
Matsuki M, Akagi H, Ogura Y, Aga F and Adachi I:
2-[Fluorine-18]-fluoro-2-deoxy-D-glucose positron emission
tomography/computed tomography versus whole-body diffusion-weighted
MRI for detection of malignant lesions: Initial experience. Ann
Nucl Med. 21:209–215. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nomori H, Mori T, Ikeda K, Kawanaka K,
Shiraishi S, Katahira K and Yamashita Y: Diffusion-weighted
magnetic resonance imaging can be used in place of positron
emission tomography for N staging of non-small cell lung cancer
with fewer false-positive results. J Thorac Cardiovasc Surg.
135:816–822. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Klingensmith WC III, Perlman D and Baum K:
Intrapatient comparison of 2-deoxy-2-[F-18]fluoro-D-glucose with
positron emissiontomography/computed tomography to Tc-99 m
fanolesomab (NeutroSpec) for localization of infection. Mol Imaging
Biol. 9:295–299. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Subesinghe M, Scarsbrook AF, Sourbron S,
Wilson DJ, McDermott G, Speight R, Roberts N, Carey B, Forrester R,
Gopal SV, et al: Alterations in anatomic and functional imaging
parameters with repeated FDG PET-CT and MRI during radiotherapy for
head and neck cancer: A pilot study. BMC Cancer. 15:1372015.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sander L, Langkilde NC, Holmberg M and
Carl J: MRI target delineation may reduce long-term toxicity after
prostate radiotherapy. Acta Oncol. 53:809–814. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Navarria P, Reggiori G, Pessina F,
Ascolese AM, Tomatis S, Mancosu P, Lobefalo F, Clerici E, Lopci E,
Bizzi A, et al: Investigation on the role of integrated PET/MRI for
target volume definition and radiotherapy planning in patients with
high grade glioma. Radiother Oncol. 112:425–429. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Karnofsky DA, Abelman WH, Craver LF and
Burchenal JH: The use of nitrogen mustards in the palliative
treatment of carcinoma. With particular reference to bronchogenic
carcinoma. Cancer. 1:634–656. 1948. View Article : Google Scholar
|
|
20
|
Qi LP, Zhang XP, Tang L, Li J, Sun YS and
Zhu GY: Using diffusion-weighted MR imaging for tumor detection in
the collapsed lung: A preliminary study. Eur Radiol. 19:333–341.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Yang RM, Li L, Wei XH, Guo YM, Huang YH,
Lai LS, Chen AM, Liu GS, Xiong WF, Luo LP and Jiang XQ:
Differentiation of central lung cancer from atelectasis: Comparison
of diffusion-weighted MRI with PET/CT. PLoS One. 8:e602792013.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Onitsuka H, Tsukuda M, Araki A, Murakami
J, Torii Y and Masuda K: Differentiation of central lung tumor from
postobstructive lobar collapse by rapid sequence computed
tomography. J Thorac Imaging. 6:28–31. 1991. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
McAdams HP, Erasums JJ, Patz EF, Goodman
PC and Coleman RE: Evaluation of patients with round atelectasis
using 2-[18F]-fluoro-2-deoxy-D-glucose PET. J Comput Assist Tomogr.
22:601–604. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Schmidt S, Nestle U, Walter K, Licht N,
Ukena D, Schnabel K and Kirsch CM: Optimization of radiotherapy
planning for non-small cell lung cancer (NSCLC) using 18FDG-PET.
Nuklearmedizin. 41:217–220. 2002.(In German). PubMed/NCBI
|
|
25
|
Senan S, van Sörnsen de Koste J, Samson M,
Tankink H, Jansen P, Nowak PJ, Krol AD, Schmitz P and Lagerwaard
FJ: Evaluation of a target contouring protocol for 3D conformal
radiotherapy in non-small cell lung cancer. Radiother Oncol.
53:247–255. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Van de Steene J, Linthout N, de Mey J,
Vinh-Hung V, Claassens C, Noppen M, Bel A and Storme G: Definition
of gross tumor volume in lung cancer: Inter-observer variability.
Radiother Oncol. 62:37–49. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Al-Jahdali H, Khan AN, Loutfi S and
Al-Harbi AS: Guidelines for the role of FDG-PET/CT in lung cancer
management. J Infect Public Health. 5 Suppl 1:S35–S40. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ling CC, Humm J, Larson S, Amols H, Fuks
Z, Leibel S and Koutcher JA: Towards multidimensional radiotherapy
(MD-CRT): Biological imaging and biological conformality. Int J
Radiat Oncol Biol Phys. 47:551–560. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Gao Z, Wilkins D, Eapen L, Morash C,
Wassef Y and Gerig L: A study of prostate delineation referenced
against a gold standard created from the visible human data.
Radiother Oncol. 85:239–246. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
McLaughlin PW, Evans C, Feng M and
Narayana V: Radiographic and anatomic basis for prostate contouring
errors and methods to improve prostate contouring accuracy. Int J
Radiat Oncol Biol Phys. 76:369–378. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Seppälä T, Visapää H, Collan J, Kapanen M,
Beule A, Kouri M, Tenhunen M and Saarilahti K: Converting from CT-
to MRI-only-based target definition in radiotherapy of localized
prostate cancer: A comparison between two modalities. Strahlenther
Onkol. 191:862–868. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Bradley J, Bae K, Choi N, Forster K,
Siegel BA, Brunetti J, Purdy J, Faria S, Vu T, Thorstad W and Choy
H: A phase II comparative study of gross tumor volume definition
with or without PET/CT fusion in dosimetric planning for
non-small-cell lung cancer (NSCLC): Primary analysis of radiation
therapy oncology group (RTOG) 0515. Int J Radiat Oncol Biol Phys.
82:435–41.e1. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Tyng CJ, Chojniak R, Pinto PN, Borba MA,
Bitencourt AG, Fogaroli RC, Castro DG and Novaes PE: Conformal
radiotherapy for lung cancer: Interobservers' variability in the
definition of gross tumor volume between radiologists and
radiotherapists. Radiat Oncol. 4:282009. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Rodríguez N, Sanz X, Trampal C, Foro P,
Reig A, Lacruz M, Membrive I, Lozano J, Quera J and Algara M:
18F-FDG PET definition of gross tumor volume for radiotherapy of
lung cancer: Is the tumor uptake value-based approach appropriate
for lymph node delineation? Int J Radiat Oncol Biol Phys.
78:659–666. 2010. View Article : Google Scholar : PubMed/NCBI
|