Introduction
Cervical cancer, characterized by the rapid and
uncontrolled viability of cervical cells, is the second most common
type of female carcinoma and the fifth most common type of cancer
worldwide (1,2), with ~510,000 novel cases and ~280,000
mortalities occurring worldwide each year (3). The traditional treatments for cervical
carcinoma, including surgical resection and chemotherapy, exhibit
adverse side effects, are not tolerated well by patients and
produce drug resistance following treatment for a prolonged time.
Therefore, determining a natural material to treat cervical
carcinoma which is low in cost and exhibits increased efficiency,
decreased resistance and limited side effects is required.
The concept of apoptosis has been studied for >40
years and was initially identified in 1972 by Keir et al
(4), which led to interest in this
field of science (5). Apoptosis is
the physiological process for nucleated cell death; it is distinct
from necrosis and involves typical morphological and biochemical
hallmarks, including the intactness of the cell membrane, cell
shrinkage, chromatin condensation and fragmentation, apoptotic body
formation and the overexpression of apoptosis-associated proteins
and genes (6,7). In the majority of cases, anticancer
therapies result in the activation of caspases which are a family
of cysteine proteases that serve functions in a variety of types of
cell death (8). There are two primary
apoptotic signaling pathways which lead to the activation of
caspases: The membrane receptor pathway (the extrinsic pathway) and
the mitochondrial pathway (the intrinsic pathway) (9,10). In the
intrinsic pathway, caspase activation is associated with
permeabilization of the outer mitochondrial membrane by
pro-apoptotic members of the B-cell lymphoma (Bcl) family (11). Upon disruption of the outer
mitochondrial membrane, a set of proteins, typically located in the
space between the inner and outer mitochondrial membranes, are
released, including cytochrome c (Cyt-c), second
mitochondria-derived activator of caspases/direct inhibitor of
apoptosis-binding protein with low pI and apoptosis-inducing factor
(12,13). The mitochondrial pathway served a
function in the death of tumor cells and is suggested to be the
primary underlying molecular mechanism of cancer cell death.
Ampelopsis megalophylla Diels et Gilg is
traditionally used in China as a folk medicine, termed ‘Mei Cha’,
for hypertensive disorders, bleeding and fever, and its tender
stems and leaves are used as experimental material. Previous
studies have demonstrated that ampelopsin (AMP), a primary
bioactive constituent of A. megalophylla, exhibits
hypoglycemic, antioxidant, antiviral and hepatoprotective
activities (14–18). In addition, AMP has been identified to
exhibit therapeutic effects on cancer; however, the association
between AMP and cervical cancer remains unclear, particularly the
underlying molecular anticancer mechanisms (19–22). In
the present study, three types of tumor cell, cervical carcinoma
(HeLa), human liver cancer (SMMC-7721) and human lung cancer (A549)
cells, were selected to determine the antitumor activity of AMP.
Subsequently, HeLa cells which were most sensitive to AMP were
selected to investigate the underlying molecular mechanisms of AMP.
The results of the present study demonstrated that AMP may treat
cervical cancer by inducing apoptosis in decreased concentrations
and provide an application of A. megalophylla in cervical
cancer therapy.
Materials and methods
Plant material
A. megalophylla was collected from Enshi
(China) and was identified by Professor Xiuqiao Zhang, School of
Pharmaceutical Sciences, Hubei University of Chinese Medicine
(Wuhan, China). A voucher specimen (no. 20130904002) was deposited
in the herbarium of Hubei University of Chinese Medicine.
AMP was separated and identified from the ethyl
acetate extract of A. megalophylla, as described in our
previous study (14,15). Soaked samples (500 g dry weight,
tender stems and leaves) were extracted twice with 95 and 75%
ethanol for 24 h at 25°C. Following filtration using filter paper,
the filtrate was concentrated using a vacuum at 60°C. The extract
was further extracted with petroleum ether 7 times to remove
chlorophyll, and was subsequently partitioned in ether, ethyl
acetate or water. The ethyl acetate extracts were fractionated with
a chloroform and methanol gradient of sequential silica gel column
chromatography. The compounds obtained in chloroform and methanol
were further purified by Sephadex LH-20 column chromatography.
Comparisons between infrared, ultraviolet, 1H nuclear
magnetic resonance (NMR), 13C NMR, 1D and 2D NMR, and
mass spectrometry were made to evaluate the structure of AMP.
Regents
MTT, Hoechst 33258, propidium iodide (PI), RNase A,
rhodamine 123 (Rh-123) and dimethyl sulfoxide (DMSO) were purchased
from Sigma-Aldrich; Merck KGaA (Darmstadt, Germany). Newborn bovine
serum (NBS) was purchased from Hangzhou Sijiqing Biological
Engineering Materials Co., Ltd. (Hangzhou, China). Dulbecco's
modified Eagle medium (DMEM), fetal bovine serum (FBS), trypsin,
modified RPMI-1640 medium, penicillin-streptomycin and PBS were all
obtained from Thermo Fisher Scientific, Inc. (Waltham, MA, USA). An
Annexin V-fluorescein isothiocyanate (FITC) apoptosis detection kit
was obtained from Beijing Zoman Biotechnology Co., Ltd. (Beijing,
China). Lysis buffer was purchased from JRDUN Biotechnology Co.,
Ltd. (Shanghai, China). Primary antibodies to Bcl-2 (no. ab117115),
Bax (no. ab32503), caspase 3 (no. ab2171), caspase 9 (no. ab32539),
Cyt-c (no. ab8245) and GAPDH (no. ab13575) were purchased
from Abcam (Cambridge, UK); secondary antibobodies, including goat
anti-mouse IgG-horseradish peroxidase (HRP; no. A0216) and goat
anti-rabbit IgG-HRP (no. A0208) were obtained from the Beyotime
Institute of Biotechnology (Haimen, China). All other chemicals and
reagents used in the present study were certified as analytical
grade.
Cell culture and treatment
HeLa, SMMC-7721 and A549 cells were obtained from
the China Center for Type Culture Collection (Wuhan, China). HeLa
cells and SMMC-7721 cells were cultured in sterile DMEM
supplemented with 10% NBS and 1% penicillin-streptomycin. A549
cells were cultured in sterile RPMI-1640 medium supplemented with
10% FBS and 1% penicillin-streptomycin. All the cells were
incubated under standard cell culture conditions at 37°C in an
atmosphere containing 5% CO2. AMP dissolved in DMSO was
used for the treatment of cells and the final concentration of DMSO
used was <0.1% (v/v) for each treatment. Cells (65–75%
confluence) were treated with AMP at various concentrations and
times, as specified in the subsequent sections, in complete growth
medium.
Measurement of HeLa cell
viability
An MTT assay was used to determine the antitumor
activities of AMP on HeLa, SMMC-7721 and A549 cells. Cells were
plated in 96-well culture plates at a density of 5×103
cells/well for 24 h and treated with AMP at various concentrations
(0, 10, 20, 40, 80, 160 and 320 µM) for 24, 48 and 72 h. At the end
of treatment, 20 µl MTT (5 mg/ml) in PBS was added and the cells
were further incubated at 37°C for 4 h. Subsequently, the
supernatant was discarded and 100 µl DMSO was added. Following
agitation using a micro-vibrator for 10 min, the optical density
(OD) of each well was measured at 490 nm using a microplate reader
(Bio-Rad Laboratories, Inc., Hercules, CA, USA). The cell viability
ratio was calculated using the following formula: Cell viability
ratio (%)=ODtreated/ODcontrol ×100.
Cytotoxicity was expressed as the half-maximal inhibitory
concentration (IC50) values of AMP.
Staining of cells with Hoechst
33258
Morphological changes of HeLa cells were detected
using Hoechst 33258 staining. HeLa cells were plated in 6-well
plates at a density of 3×105 cells/well. After 24 h of
treatment with various concentrations of AMP (0, 30, 40, 50, 60 and
70 µM), plates were washed with PBS and cells were fixed using a
3:1 ratio of methanol and acetic acid for 12 min at room
temperature. Subsequently, cells were stained with 500 µl Hoechst
33258 solution (5 µg/ml) in darkness for 30 min at room
temperature. Morphological features of apoptotic cells were
observed under a fluorescence microscope (IX51; Olympus
Corporation, Tokyo, Japan).
DNA content and cell cycle
analysis
Cell cycle distribution was determined using a PI
staining assay. HeLa cells were plated in 6-well plates at a
density of 3×105 cells/well and cultured at 37°C for 24
h. Following ~60% confluence, cells were treated with various
concentrations of AMP (0, 30, 40, 50, 60 or 70 µM) for 12 h.
Subsequently, cells were centrifuged at 500 × g for 5 min and the
sediment was resuspended with PBS and washed again. Cells were
fixed with 75% ethanol at −20°C overnight. Fixed cells were stained
with PI (50 µg/ml) and 0.1% RNase A in PBS away from direct
sunlight for 30 min. The samples were tested by flow cytometry
(FCM) on a BD FACSCalibur cytometer, the number of cells in each
phases during cell cycle was collected data by CellQuest software
version 3.3 (BD Biosciences, San Jose, CA, USA), and data was
analyzed by ModFit LT for Maclnel version 3.0 (Verity Software
House, Topsham, ME, USA).
Annexin V-FITC/PI double staining
An Annexin V-FITC/PI apoptosis detection kit was
used to determine the proportion of apoptotic cells induced by AMP.
HeLa cells were seeded at 3×105 cells/well in 6-well
plates and incubated for 24 h at 37°C with 5% CO2. Cells
were harvested following treatment with AMP (0, 30, 40, 50, 60 or
70 µM) for 8 h at 37°C and two washes in PBS. Subsequently, cells
were resuspended with 500 µl Annexin V-binding buffer and incubated
with 5 µl Annexin V-FITC and 10 µl PI in the dark for 10 min at
room temperature. Samples were tested by FCM and anaylzed by the
CellQuest.
Measurement of the mitochondrial
transmembrane potential
A Rh-123 staining assay was used to analyze the
mitochondrial membrane potential disruption. Following incubation
with various concentrations of AMP (0, 30, 40, 50, 60 or 70 µM) for
24 h, cells were harvested and incubated with 10 µM Rh-123 (10
mg/l) at 37°C for 30 min. Finally, the cells were washed twice with
PBS and analyzed using FCM.
Western blot analysis
Western blot analysis was used to analyze the
expression of apoptotic proteins following treatment with AMP of
HeLa cells. HeLa cells were harvested following 12 h of treatment
with AMP (30–70 µM), resuspended in a lysis buffer (JRDUN
Biotechnology Co., Ltd.) and centrifuged at 12,000 × g for 10 min.
A bicinchoninic acid assay kit (Thermo Fisher Scientific, Inc.) was
used to determine the protein concentration. Equal amounts of
protein (20 µg) were resuspended in Laemmli buffer, separated by
SDS-PAGE (10–15% gel) and transferred onto nitrocellulose membranes
(EMD Millipore, Billerica, MA, USA). Membranes were blocked in
Tris-buffered saline-Tween-20 (TBST) containing 5% (w/v) non-fat
milk powder for 1 h at room temperature and subsequently incubated
overnight at 4°C with primary antibodies [Bcl-2 (1:1,000), Bax
(1:1,000), caspase 3 (1:200), caspase 9 (1:2,000), Cyt-c
(1:1,000) and GAPDH (1:1,000)] diluted in TBST. Following three
washes with TBST, the membranes were incubated with secondary
antibodies (1:1,000) at 37°C for 1 h. Immunoreactive bands were
detected using an enhanced chemiluminescence reagent (EMD
Millipore). Bound antibodies were visualized by exposure to X-ray
film. Data were expressed as the relative density of the protein,
normalized to GAPDH.
Statistical analysis
All data are presented as the mean ± standard
deviation. Statistical analysis was performed with SPSS 17.0
statistical software (SPSS, Inc., Chicago, IL, USA) and an unpaired
Student's t-test was used to analyze differences between groups.
P<0.05 and P<0.01 were considered to indicate a statistically
significant difference.
Results
AMP affects the viability of tumor
cells
An MTT assay was used to investigate the viability
of HeLa, SMMC-7721 and A549 cells following treatment with AMP at
various concentrations. As presented in Table I, AMP exhibited decreased cytotoxicity
in SMMC-7721 and A549 cells, compared with that in HeLa cells. AMP
exhibited a potent cytotoxic effect in HeLa cells, particularly in
cells that were treated for 72 h. As HeLa cells exhibited the
lowest AMP IC50 values, they were selected for the
mechanistic study.
 | Table I.Cytotoxicity (IC50) values
for distinct tumor cell lines treated with ampelopsin (µM). |
Table I.
Cytotoxicity (IC50) values
for distinct tumor cell lines treated with ampelopsin (µM).
|
| Incubation time,
h |
|---|
|
|
|
|---|
| Tumor cell line | 24 | 48 | 72 |
|---|
| HeLa | 105.03±2.24 | 90.55±2.77 |
65.58±2.29 |
| SMMC-7721 | >320 | >320 | 248.86±1.89 |
| A549 | >320 | >320 | 296.28±2.23 |
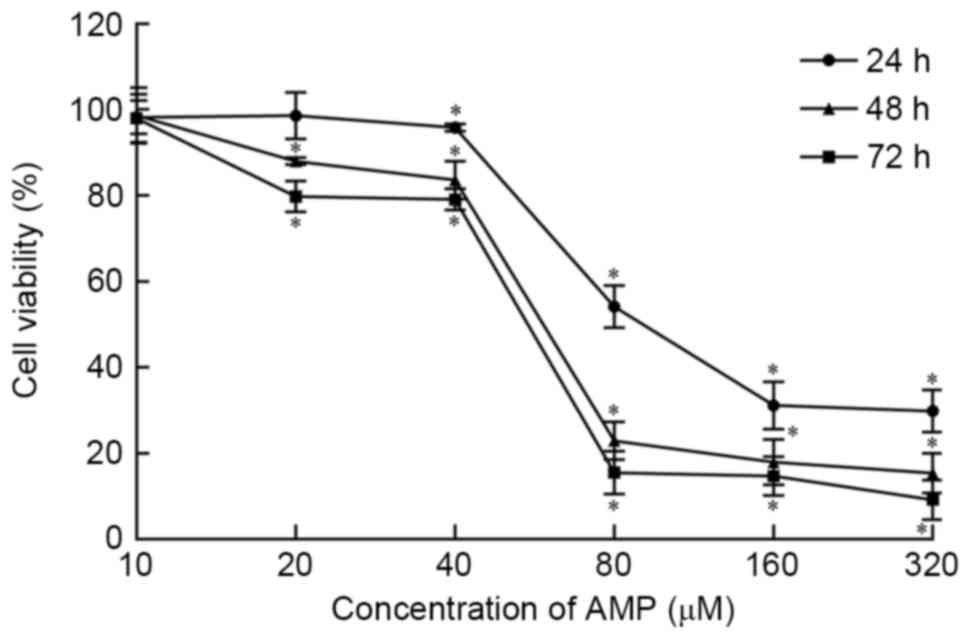
As presented in Fig.
1, the viability of HeLa cells is inhibited by AMP in a
dose-dependent manner at concentrations >10 and ≤320 µM.
Following exposure to AMP at concentrations between 40 and 80 µM
for 24, 48 and 72 h, cell viability was significantly decreased
(P<0.05). Viability was reduced the least in the 24 h treatment
group. Following treatment with 80 µM AMP for 24, 48 and 72 h, cell
viability was 54.10, 22.93 and 15.47%, respectively, According to
these data, AMP treatment concentrations of 30, 40, 50, 60 and 70
µM were used in subsequent experiments.
AMP induces apoptosis in HeLa
cells
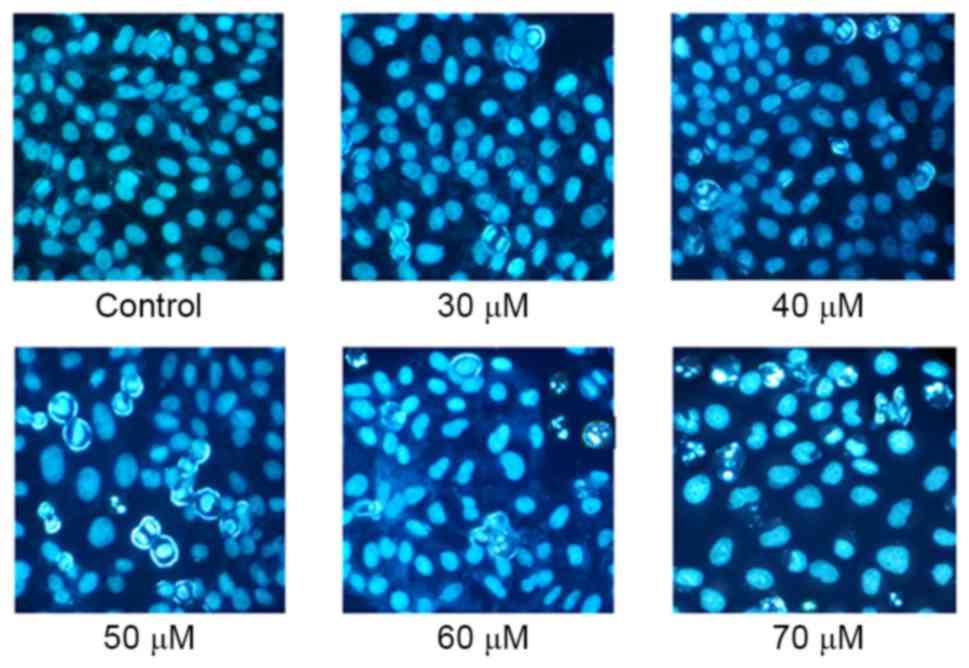
Hoechst 33258 staining may reveal alterations in
cell morphology, including nuclear shrinkage, chromatin
condensation and apoptotic bodies, and enables the occurrence of
apoptosis to be identified qualitatively. As presented in Fig. 2, chromatin condensation increased with
increasing concentrations of AMP. A number of apoptotic bodies,
including nuclear shrinkage, were observed following treatment with
between 50 and 70 µM AMP; however, these characteristics were not
observed in untreated controls.
Effect of AMP on the cell cycle in
HeLa cells
The induction of apoptosis has been associated with
cell cycle arrest (23,24) and inhibition of the cell cycle has
been regarded as one component of the apoptotic mechanism (25–27). To
investigate the effect of AMP on the progression of the cell cycle
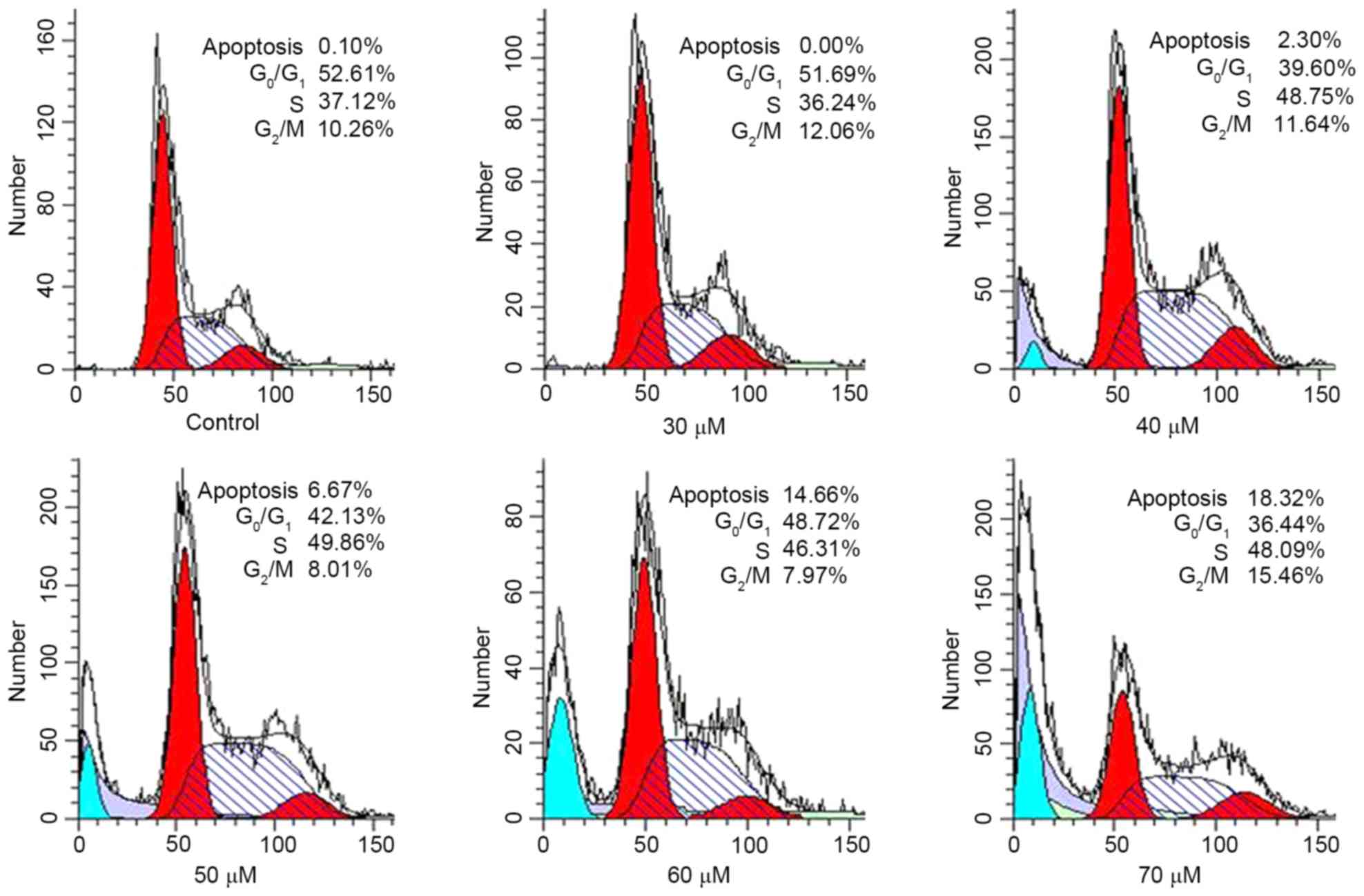
in HeLa cells, DNA content was determined using FCM (Fig. 3). As presented in Table II, compared with the control, an
increasing concentration of AMP resulted in an accumulation of
cells in the S phase of the cell cycle to 41.46, 47.32 and 49.09%
at 30, 40 and 50 µM AMP, respectively. In addition, at 70 µM AMP,
compared with the control, the proportion of cells in the S phase
of the cell cycle increased to 49.22%. The DNA content in the
G1 phase of the cell cycle decreased to between 38.24
and 49.15% at concentrations between 30 and 70 µM. Furthermore, the
subdiploid peak (sub-G1) rapidly increased in HeLa
cells, at 30 µM AMP the proportion of sub-G1 cells was
0.96%, whereas at 70 µM, the sub-G1 proportion was
17.59%. The increase in apoptosis of cells was validated using the
cell cycling histogram presented in Fig.
3. The results indicated that AMP-induced apoptosis may be
associated with the disruption of the cell cycle, which was
arrested in S phase.
 | Table II.Effect of AMP on the cell cycle
distribution in HeLa cells. |
Table II.
Effect of AMP on the cell cycle
distribution in HeLa cells.
|
| Cell cycle
phase |
|---|
|
|
|
|---|
| AMP concentration,
µM |
G0/G1, % | S, % | G2/M,
% | sub-G1,
% |
|---|
| Control | 53.06±1.64 | 36.08±1.13 | 10.86±1.04 | 0.18±0.23 |
| 30 | 49.15±4.87 | 41.46±6.12 | 9.20±2.51 |
016±0.11b |
| 40 |
42.41±2.44b |
47.32±1.26a | 10.19±1.11 | 0.96±1.17 |
| 50 |
40.72±1.28b |
49.09±3.59b | 10.19±4.43 | 2.43±3.67 |
| 60 |
41.76±3.49b |
47.63±3.19b | 7.94±1.34 |
10.16±8.29b |
| 70 |
38.24±1.63b |
49.22±1.42b | 12.46±2.63 |
17.59±2.10b |
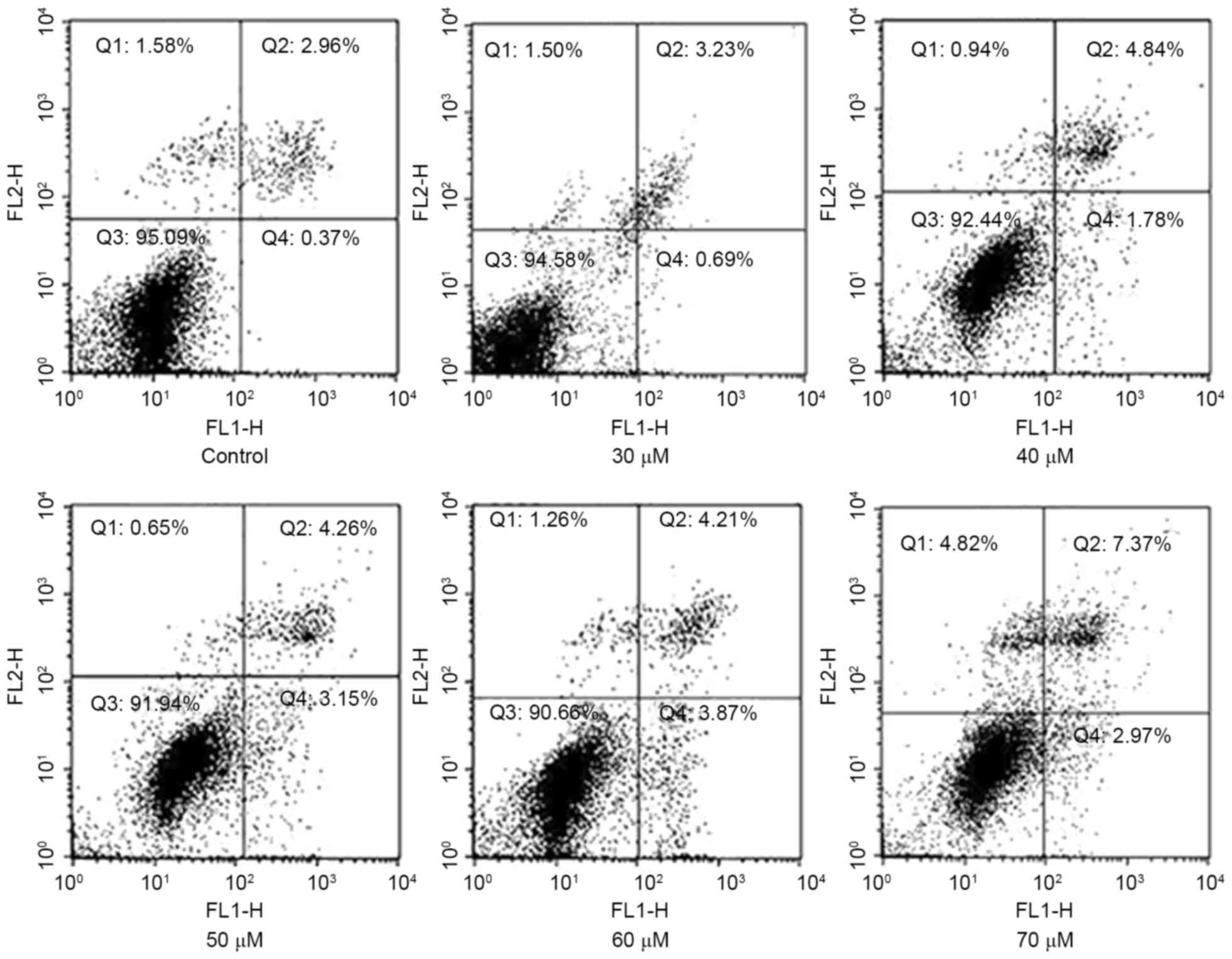
To validate that AMP may induce apoptosis in HeLa
cells, staining with Annexin V-FITC/PI was applied to examine the
apoptotic rate following treatment with AMP for 8 h. As presented
in Fig. 4, a limited proportion
(3.33±0.93%) of cells were stained with Annexin V-FITC in the group
untreated with AMP. The proportion of apoptotic cells increased to
6.62±1.05, 7.41±1.98 and 9.34±2.04% in a concentration-dependent
manner following treatment with 40, 50 and 70 µM AMP,
respectively.
Loss of mitochondrial membrane
potential
In order to explore the AMP-induced apoptotic
pathway in HeLa cells, potential changes in the mitochondrial
membrane were analyzed using a mitochondrion-specific fluorescent
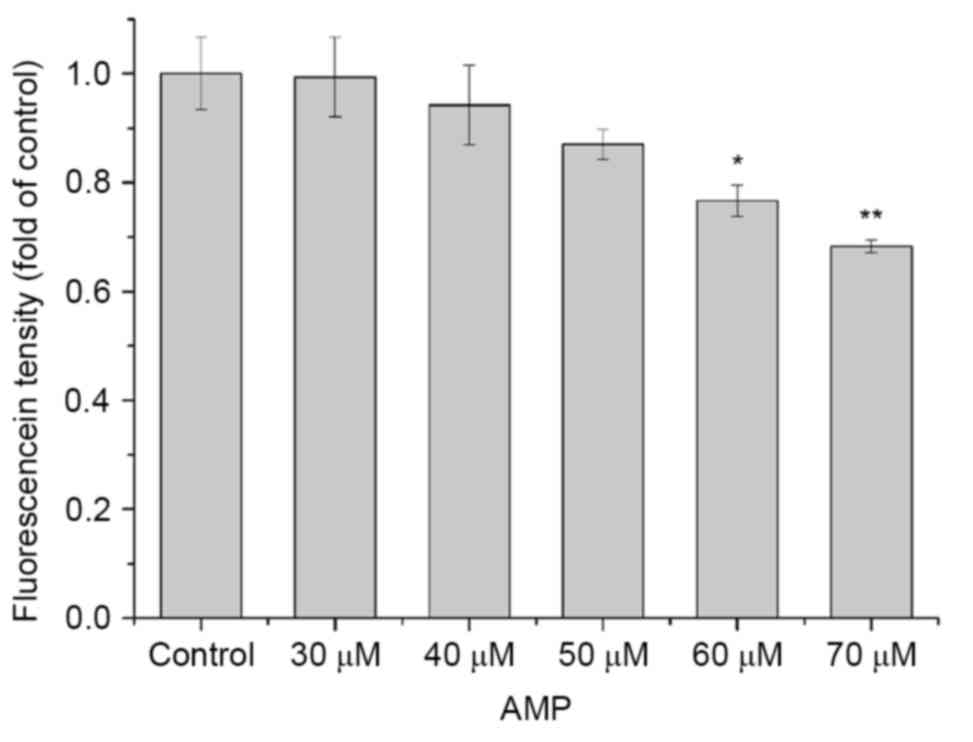
dye, Rh-123. As presented in Fig. 5,
a decrease in the mitochondrial membrane potential was identified
following treatment with various concentrations of AMP for 24 h.
Compared with the control, the mean fluorescence density decreased
from to 1, 6, 13, 23 and 32%. The results suggested that AMP may
induce mitochondrial membrane potential repression in a
dose-dependent manner.
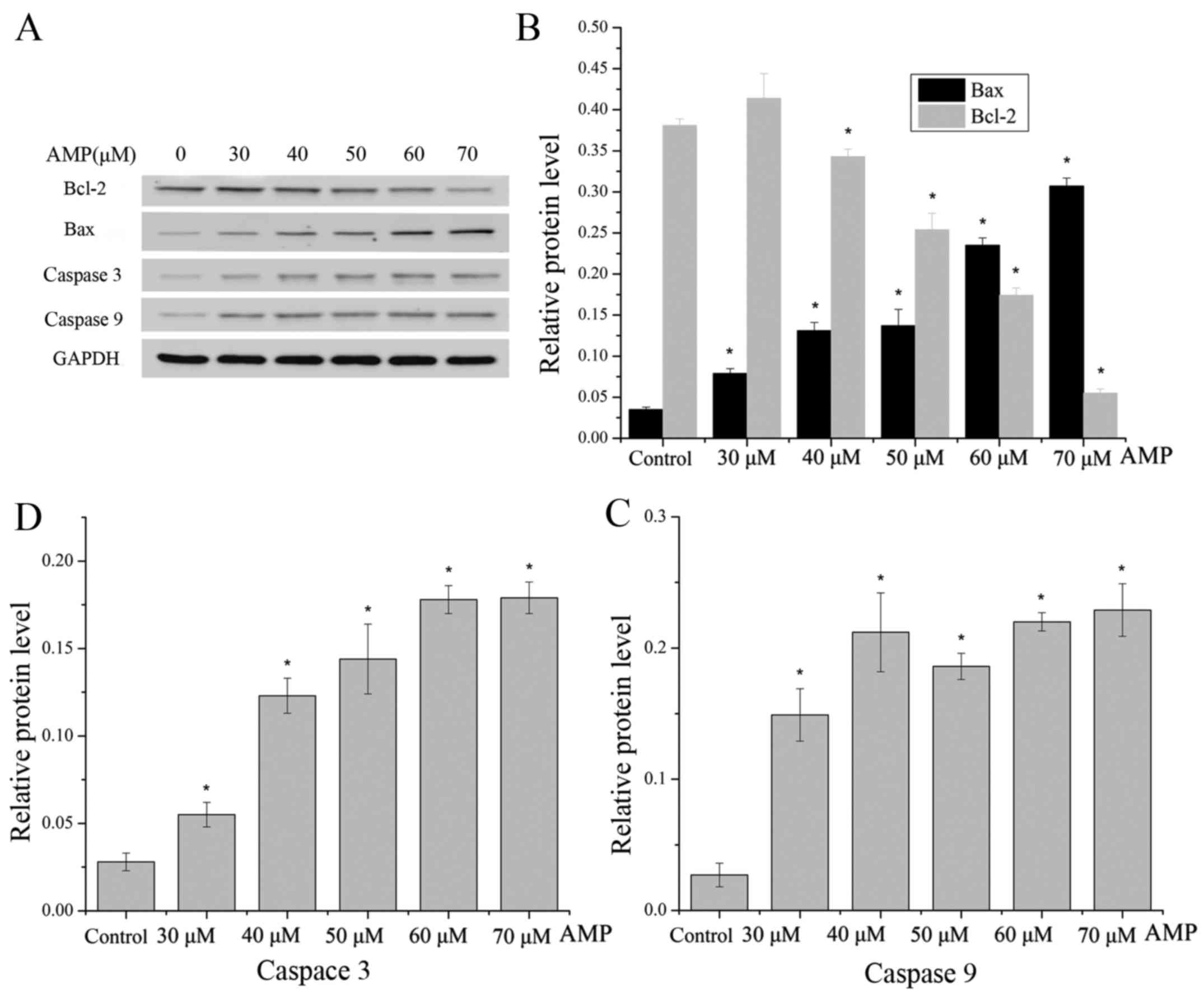
Effect of AMP on the expression of Bax
and Bcl-2 proteins
To validate whether the AMP-induced apoptosis of
HeLa cells may be associated with the mitochondrial pathway,
western blot analysis was used to detect the ratio of Bax/Bcl-2, as
this is typically considered to be a key factor in the regulation
of the apoptotic signal transduction pathway. AMP treatment of
cells resulted in a decrease in Bcl-2 expression, with a
concomitant increase in the protein level of Bax (Fig. 6A and B), leading to a marked increase
in the Bax/Bcl-2 ratio, which favors apoptosis.
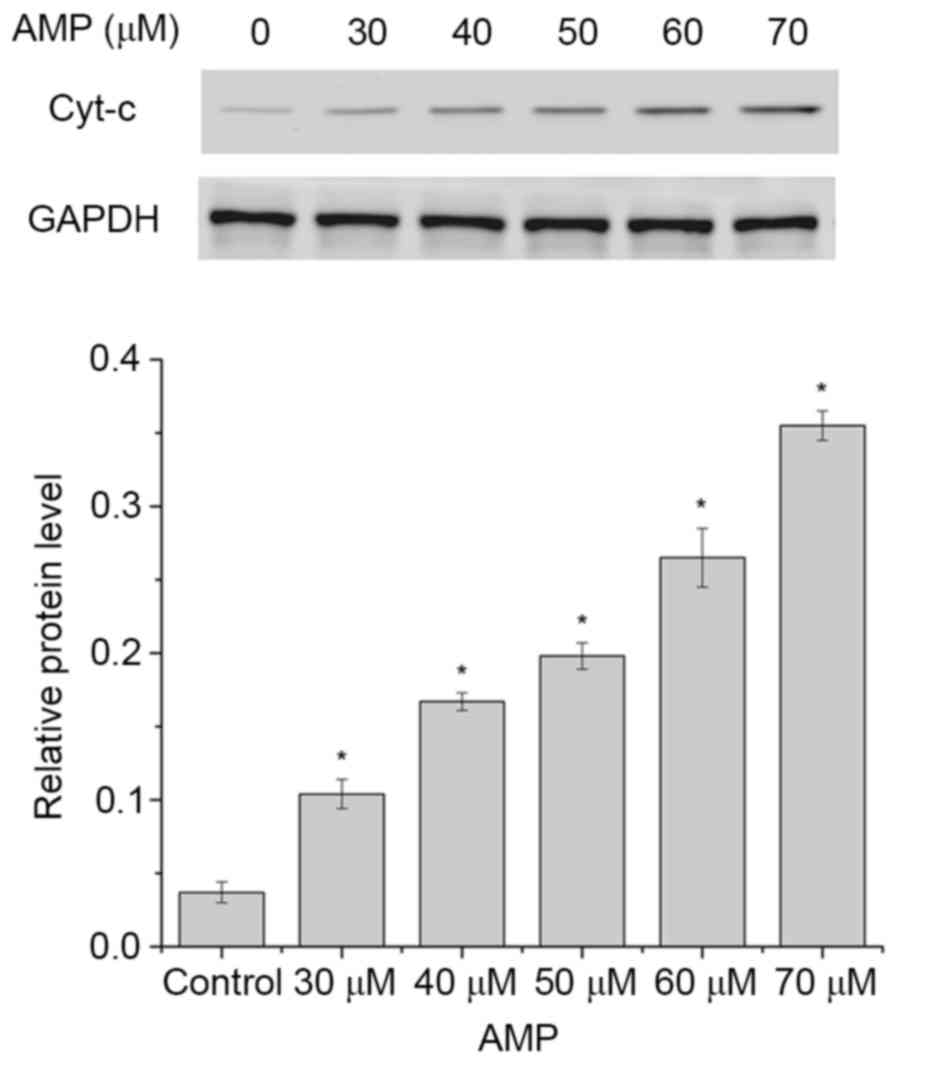
AMP induces the release of Cyt-c and
caspase activation in HeLa cells
The release of Cyt-c from the mitochondria to
the cytosol is a characteristic of the mitochondrial signaling
pathway. Following release, Cyt-c activates caspase 9,
combines with apoptotic protease-activating factor 1 (Apaf-1) and
subsequently activates caspase 3 (28). Therefore, the levels of Cyt-c
in the cytosol in HeLa cells following AMP treatment (between 30
and 70 µM) was determined. As presented in Fig. 7, the levels of Cyt-c in the
cytosol were significantly increased following treatment with 30,
40, 50, 60 or 70 µM AMP, compared with the control (P<0.05).
These results suggested that AMP may have induced the apoptosis of
HeLa cells.
The caspase family of cysteine proteases serve key
roles in apoptosis. The activities of caspases 9 and 3 were
measured following AMP treatment at between 30 and 70 µM for 12 h.
As presented in Fig. 6A, C and D, the
levels of caspase 9 were increased following treatment with AMP at
between 30 and 40 µM, and the levels were additionally increased
following treatment with AMP at between 60 and 70 µM. Furthermore,
levels of caspase 9 were decreased following treatment with 50 µM
AMP, whereas the levels of caspase 3 markedly increased with a
dose-dependent manner.
Discussion
The results of the present study demonstrated that
AMP exhibited the most marked suppressive effect in HeLa cells,
among three tumor cell lines investigated, and it decreased the
survival rate of HeLa cells in a dose- or time-dependent manner
within a certain concentration range. In addition, AMP was
associated with the induction of apoptosis in HeLa cells.
First, the apoptosis evoked by AMP was validated by
the alterations in nuclear morphology observed with the Hoechst
33258 staining assay. Morphological characteristics of apoptosis
include plasma membrane blebbing, cell shrinkage, nuclear
condensation, chromosomal DNA fragmentation and the formation of
apoptotic bodies. Compared with the control, chromatin condensation
in HeLa cells treated with AMP at between 30 and 70 µM for 24 h was
observed, and the fluorescence intensities of chromatin were more
marked. Additionally, the number of apoptotic bodies, a typical
characteristic of apoptotic cells, increased in a dose-dependent
manner. Secondly, Annexin V-FITC/PI double staining suggested that
apoptosis was the primary cause of HeLa cell death following AMP
treatment and the effect was dose-dependent. Furthermore, HeLa
cells were observed to be arrested in S phase of the cell cycle
following treatment with AMP and subsequent accumulation in the
sub-G1 phase of cell cycle. On the basis of the results
of the present study, AMP was identified to induce apoptosis in
HeLa cells. Therefore, the signaling pathway which was involved in
AMP-induced apoptosis was investigated.
Apoptosis is an ordered and orchestrated cellular
process that occurs in physiological and pathological conditions.
The mechanisms of apoptosis primarily involve two signaling
pathways: The death receptor-mediated extrinsic pathway and the
mitochondrion-mediated intrinsic pathway. The extrinsic pathway
involves the activation of the tumor necrosis factor/Fas death
receptor family, whereas the intrinsic pathway involves the
permeability of mitochondria and the efflux of Cyt-c.
Mitochondria serve a crucial function in the intrinsic pathway of
apoptosis, a topic that has attracted a lot of research attention.
Upon stimulation of apoptosis-inducing factors, the Bcl-2 family,
including pro-apoptotic proteins [Bax, Bcl-2-associated death
promoter (Bad), Bcl homology domain 3-interacting domain death
agonist (Bid), Bcl-2-like protein 11 (Bim), Bcl-2-interacting
killer and activator of apoptosis harakiri], and anti-apoptotic
proteins [Bcl-2, Bcl extra-large (Bcl-xL), Bcl-2-related protein A1
and induced myeloid leukemia cell differentiation protein],
regulate apoptosis by maintaining mitochondrial permeability and
inducing the release of Cyt-c. The anti-apoptotic proteins
Bcl-2 and Bcl-xL reside in the outer mitochondrial wall and inhibit
Cyt-c release; whereas the pro-apoptotic proteins Bax, Bad,
Bid and Bim reside in the cytosol, translocate to the mitochondria
following cell death signaling and promote the release of
Cyt-c (29–31). In the present study, the results
demonstrated that AMP markedly downregulated the expression of
Bcl-2 protein and upregulated Bax protein simultaneously in HeLa
cells, which resulted in an increased intracellular Bax/Bcl-2
ratio, suggesting the involvement of the intrinsic apoptotic
pathway with AMP-induced apoptosis in HeLa cells.
A key function in the mitochondrial pathway is the
decrease in mitochondrial membrane potential, which directly causes
the release of Cyt-c into the cytoplasm. In the present
study, the mitochondrial membrane potential decreased in a
dose-dependent manner following AMP treatment, suggesting that the
intrinsic apoptotic pathway was mediated by AMP. Cyt-c, a
primary respiratory chain protein, is an indicator of cell
apoptosis. Following release, Cyt-c binds Apaf-1 and forms
an activation complex with caspase 9. The release of Cyt-c
and the activation of caspase 9 are essential for activating other
caspase proteins, including the executioner caspase 3, and leads to
DNA fragmentation, which results in apoptosis (32). Western blot analysis demonstrated that
the level of Cyt-c was markedly increased in the cytosol.
Furthermore, the expression of caspase 9 protein was markedly
upregulated and caspase 3 protein increased simultaneously
following AMP treatment. Thus, it is hypothesized that the
induction of apoptosis by AMP in HeLa cells was due to the
activation of the mitochondrion-mediated intrinsic apoptosis
pathway.
The present study demonstrated that AMP inhibited
cell viability and induced apoptosis in HeLa cells. The increased
ratio of Bax/Bcl-2, a marked decrease in mitochondrial membrane
potential, Cyt-c release from the mitochondria and the
activation of caspases 9 and 3 all indicated that the
mitochondrion-mediated apoptotic pathway was involved in
AMP-induced HeLa cell death. The results of the present study
validated the potential of AMP as a chemotherapeutic and cytostatic
agent in HeLa cells. However, additional in vivo studies are
required.
Acknowledgements
The present study was supported by the National
Natural Science Foundation of China (grant no. 31170335) and the
Key Projects of Hubei Province Natural Science Foundation (grant
no. 2014CFA083).
References
|
1
|
Parkin DM, Bray F, Ferlay J and Pisani P:
Global cancer statistics, 2002. CA Cancer J Clin. 55:74–108. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Li Y, Wang SJ, Xia W, Rahman K, Zhang Y,
Peng H, Zhang H and Qin LP: Effects of tatariside G isolated from
fagopyrum tataricum roots on apoptosis in human cervical cancer
HeLa cells. Molecules. 19:11145–11159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Su JH, Wu A, Scotney E, Ma B, Monie A,
Hung CF and Wu TC: Immunotherapy for cervical cancer: Research
status and clinical potential. Biodrugs. 24:109–129. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Keir JF, Wyllie AH and Currie AR:
Apoptosis: A basic biological phenomenon with wide-ranging
implications in tissue kinetics. Br J Cancer. 26:239–257. 1972.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Peter ME, Heufelder AE and Hengartner MO:
Advances in apoptosis research. Proc Natl Acad USA. 94:12736–12737.
1997. View Article : Google Scholar
|
|
6
|
Kroemer G, Petit P, Zamzami N, Vayssière
JL and Mignotte B: The biochemistry of programmed cell death. FASEB
J. 9:1277–1287. 1995.PubMed/NCBI
|
|
7
|
Nagata S: Apoptotic DNA fragmentation. Exp
Cell Res. 256:12–18. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Degterev A, Boyce M and Yuan J: A decade
of caspases. Oncogene. 22:8543–8567. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Hengartner MO: The biochemistry of
apoptosis. Nature. 407:770–776. 2000. View
Article : Google Scholar : PubMed/NCBI
|
|
10
|
Lei JC, Yu JQ, Yin Y, Liu YW and Zou GL:
Alantolactone induces activation of apoptosis in human hepatoma
cells. Food Chem Toxicol. 50:3313–3319. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Fulda S and Debatin KM: Extrinsic versus
intrinsic apoptosis pathways in anticancer chemotherapy. Oncogene.
25:4798–4811. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Deng X, Zhao X, Lan Z, Jiang J, Yin W and
Chen L: Anti-tumor effects of flavonoids from the ethnic medicine
Docynia delavayi (Franch.) Schneid. and its possible mechanism. J
Med Food. 17:787–794. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Saelens X, Festjens N, Vande Walle L, van
Gurp M, van Loo G and Vandenabeele P: Toxic proteins released from
mitochondria in cell death. Oncogene. 23:2861–2874. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Zhang XQ, Shen W, Chen SH and Liu ZW:
Chemical constituents of Ampelopsis Megalophylla. Chin Trad
Herb Drugs. 39:1135–1137. 2008.
|
|
15
|
Fang Y, Cheng PP, Xia Y, Huang J, Da GZ
and Zhang XQ: HPLC fingerprint of total flavonoid extracts from
Ampelopsis megalophylla. Medicinal Plant. 5:49–52. 2014.
|
|
16
|
Gui C, Cheng PP, Xia Y, Fang Y and Zhang
XQ: Anti-HBV active of flavonoids from Ampelopsis
Megalophylla. Jokull Journal. 64:134–139. 2014.
|
|
17
|
Liu J, Shu Y, Zhang Q, Liu B, Xia J, Qiu
M, Miao H, Li M and Zhu R: Dihydromyricetin induces apoptosis and
inhibits proliferation in hepatocellular carcinoma cells. Oncol
Lett. 8:1645–1651. 2014.PubMed/NCBI
|
|
18
|
Shen Y, Lindemeyer AK, Gonzalez C, Shao
XM, Spigelman I, Olsen RW and Liang J: Dihydromyricetin as a novel
anti-alcohol intoxication medication. J Neurosci. 32:390–401. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wu S, Liu B, Zhang Q, Liu J, Zhou W, Wang
C, Li M, Bao S and Zhu R: Dihydromyricetin Reduced Bcl-2 Expression
via p53 in Human Hepatoma HepG2 Cells. PLoS One. 8:e768862013.
View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Xia J, Guo S, Fang T, Feng D, Zhang X,
Zhang Q, Liu J, Liu B, Li M and Zhu R: Dihydromyricetin induces
autophagy in HepG2 cells involved in inhibition of mTOR and
regulating its upstream pathways. Food Chem Toxicol. 66:7–13. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Zhou Y, Shu F, Liang X, Chang H, Shi L,
Peng X, Zhu J and Mi M: Ampelopsin induces cell growth inhibition
and apoptosis in breast cancer cells through ROS generation and
endoplasmic reticulum stress pathway. PLoS One. 9:e890212014.
View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang Q, Liu J, Liu B, Xia J, Chen N, Chen
X, Cao Y, Zhang C, Lu C, Li M and Zhu R: Dihydromyricetin promotes
hepatocellular carcinoma regression via a p53 activation-dependent
mechanism. Sci Rep. 4:46282014. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Danno K and Horio T: Formation of
UV-induced apoptosis relates to the cell cycle. Br J Dermatol.
107:423–428. 1982. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jiang W, Zhu Z, Bhatia N, Agarwal R and
Thompson HJ: Mechanisms of energy restriction: Effects of
corticosterone on cell growth, cell cycle machinery, and apoptosis.
Cancer Res. 62:5280–5287. 2002.PubMed/NCBI
|
|
25
|
Shi Q, Zuo G, Feng Z, Zhao L, Luo N, You
Z, Xia J, Li D, Li J and Chen D: Inhibitory effect of trichostatin
A on HepG2 cell proliferation and the mechanisms. Nan Fang Yi Ke Da
Xue Xue Bao. 34:917–922. 2014.(In Chinese). PubMed/NCBI
|
|
26
|
Liu B, Zhou Z, Zhou W, Liu J, Zhang Q, Xia
J, Liu J, Chen N, Li M and Zhu R: Resveratrol inhibits
proliferation in human colorectal carcinoma cells by inducing
G1/S-phase cell cycle arrest and apoptosis through
caspase/cyclin-CDK pathways. Mol Med Rep. 10:1697–1702.
2014.PubMed/NCBI
|
|
27
|
Grogan PT, Sarkaria JN, Timmermann BN and
Cohen MS: Oxidative cytotoxic agent withaferin A resensitizes
temozolomide-resistant glioblastomas via MGMT depletion and induces
apoptosis through Akt/mTOR pathway inhibitory modulation. Invest
New Drugs. 32:604–617. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kidd VJ: Proteolytic activities that
mediate apoptosis. Annu Rev Physiol. 60:533–573. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Zou H, Li Y, Liu X and Wang X: An APAF-1.
cytochrome c multimeric complex is a functional apoptosome that
activates procaspase-9. J Biol Chem. 274:11549–11556. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Li P, Nijhawan D, Budihardjo I,
Srinivasula SM, Ahmad M, Alnemri ES and Wang X: Cytochrome c and
dATP-dependent formation of Apaf-1/caspase-9 complex initiates an
apoptotic protease cascade. Cell. 91:479–489. 1997. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Bounda GA, Zhou W, Wang DD and Yu F: Rhein
elicits in vitro cytotoxicity in primary human liver HL-7702 cells
by inducing apoptosis through mitochondria-mediated pathway. Evid
Based Complement and Alternat Med. 2015:3298312015. View Article : Google Scholar
|
|
32
|
Green DR: Apoptotic pathways: Ten min to
dead. Cell. 121:671–674. 2005. View Article : Google Scholar : PubMed/NCBI
|