Introduction
At present, cancer threatening human health and life
is a major public problem and the lung caner is the most prevalent
malignant tumor. According to histological classification, lung
cancer was divided into non-small cell lung cancer (NSCLC) and
SCLC. The former captures more than 80% and the survival rate is
very low due to resistance of adjuvant chemotherapy (1). For many years, medical staffs have been
strived their best to find more effective and low cytotoxcity
chemotheraputics, especially the natural drugs.
Nature is rich, having provided us with a large
number of compounds derived from plants with tumor treatment
efficacy caused the attention of the world, because of their
availability and relatively low toxicity compared with
chemotherapy. Curcumin (diferuloylmethane)-(1,7-bis
(4-hydroxy-3-methoxyphenyl)-1,6-hepadiene-3,5-dione), a kind of
liposoluble polyphenol pigment extracted from rhizome of curcuma,
which has a long history as a dietary supplement is konwn with a
variety of biological activity such as anti-inflammatory,
anticoagulant, hypolipidemic, antioxidant, free radical scavenging,
anti-atherosclerosis, etc. It is the main efficacy components of
Chinese medicine turmeric playing a pharmacological effect
(2). Recently, clinical trials have
shown that curcumin is safe and well tolerated in humans (3). In addition, in vitro, curcumin
suppresses the growth of multiple cancer lines, especially in lung
cancer (4–6). These findings highly indicating its
potential clinic application speculation in cancer control.
Curcumin has been suggested to induce cell cycle
arrest and activate apoptosis-mediated cell death in several cancer
cells, including lung cancer (7–9). Although
it is strongly believed that apoptosis is the main toxic mechanism
of curcumin in tumor cells, it is not completely known whether
autophagy is induced in curcumin-caused cancer cell death.
Simultaneously, in recent years, the fact that curcumin can
upregulate autophagy to achieve its antitumor function arouse
strong interest.
As one of the three types of programmed cell death
(PCD), autophagy, a catabolic process for the degradation and
recycling of macromolecules and organelles which can be activated
during stress conditions was identified as an important point at
the tumor control procedure. It can be divided into macrophages,
micro-autophagy and molecular chaperone mediated autophagy. It was
found that curcumin induced autophagy by downregulating Akt/mTOR
and activating ERK1/2 pathway, and this effect was also mediated by
AMPK and related to the extensive degradation of P53 (10–13).
Autophagy is closely related to tumorigenesis affecting the
proliferation, migration, invasion and metastasis of tumor cells.
In recent years, an increasing number of research evidence that
autophagy may play a dual role in tumors (14). On the one hand, autophagic degradation
of amino acids, nucleotides and free fatty acids promote cell
survival. On the other hand, it is also an important mechanism of
tumor cell death. Autophagy has a dual role in cancer cells,
including curcumin-induced autophagy of tumor cells, likely this
could be a survival or cell death mechanism. Although the dual role
of autophagy has not yet reached a consensus, the role in tumor
development and treatment can't be ignored. So, exploring the role
of autophagy in tumorigenesis and development and the mechanism may
be able to reveal a new chapter in antitumor experience.
In order to investigate the curcumin induced
autophagy anticancer effects and the possible mechanism, a variety
of methods were used to observe the cytomorphology alteration,
viability, curcumin-induced autophagy and autophagy-specific
inhibition in A549 cell line. We prospect the results providing a
baseline information for better understanding the curcumin induced
anticancer mechanism.
Materials and methods
Materials
The A549 human lung adenocarcinoma cell line were
obtained from the First Affiliated Hospital of Xi'an Jiaotong
University (Xi'an, China) as a gift.
Curcumin was obtained from Sigma (St. Louis, MO,
USA) and dissolved into dimethyl sulphoxide (DMSO) stock solution,
kept in the dark, waiting to be used. RPMI-1640 (HyClone, Logan,
UT, USA). The MTT, DMSO, 3-methyladenine (3-MA), acridine orange
(AO) solution and monodansylcadaverine (MDC) are all obtained from
Sigma. Fetal bovine serum (FBS; Biological Industries, Kibbutz
Beit-Haemek, Israel). Penicillin-streptomycin (North China
Pharmaceutical Group Corp., Shijiazhuang, China). Anti-quencher
reagent (Hurt Biotechnology companies, Xi'an, China). The other
commonly used reagents are domestic analytical reagents. 3-MA
dissolved in PBS was added to the medium to final concentrations as
described in each experiment. DMSO is a control for the entire
study at a final concentration of <0.1%. Cells were pretreated
with 3-MA of 2.5 mM for 3 h and then incubated with curcumin for
scheduled time of experiment.
Methods
Cell culture
A549 cells maintained at 37°C, 5% CO2 and
saturated humidity in RPMI-1640 culture medium supplemented with
10% FBS and 1% penicillin-streptomycin. Changing the liquid every
day, and cells were passaged once every 2 to 3 days, taking the
logarithmic growth phase of the A549 cells for following tests.
Morphological observation
4×104/ml suspension of A549 cells were seeded in
24-well plates at 1 ml per well cultured overnight until the cells
adhered to the wall. The cells were exposed to curcumin (40 µM),
and the same volume of the consumption of DMSO for solubling
curcumin was considered a control group. After cultured for 24, 48,
72 and 96 h, A549 cell morphological changes were observed and
photographed under inverted phase contrast microscope (Nikon
Eclipse Ti; Nikon Corporation, Tokyo, Japan).
MTT cell viability assay
Collecting the logarithmic growth phase A549 cells
seeded at 2×104 cells per well in 96-well culture plates and at
least 3 wells were replated in each group. Each 96-well plate was
set up with control (cells-only) and zero-adjustment (medium only).
The following day, the medium was changed to RPMI-1640 supplemented
with 10% fetal bovine serum containing different concentrations of
curcumin or DMSO alone (control group). At 24, 48, 72 and 96 h
later, the medium was removed and the A549 cells were incubated
with 20 µl MTT (5 mg/ml) at 37°C for 4 h. Carefully aspirating the
liquid, adding 150 µl DMSO per well. Shaking 5 min and the
absorbance of each well was measured with the microplate reader at
570 nm. Cell viability (%)=(experimental group OD-zero adjustment
group OD)/(control group OD-zero adjustment group OD) × 100%. Cell
inhibitory ratio (%)=1-cell viability (%).
AO staining
During authophagy, autophagosomes fuse with
lysosomes to form autophago-lysosomes which could be dyed by AO.
Collecting the logarithmic growth phase A549 cells seeded at 3×104
cells per well in 24-well culture plates. Exposed to different
concentrations of curcumin after 48 h, cells were stained with 10
µg/ml AO for 15 min. Following treatment, washed twice with PBS,
adding 30 µl anti-quencher per well and immediately observed under
an inverted fluorescence microscope (Nikon Eclipse Ti; Nikon
Corporation). The experiment was repeated three times.
Monodansylcadaverine (MDC) labeling
Usually, mature autophagic vacuoles which accumulate
in the autophagy are detected by MDC. The same as the AO staining
experiment, A549 cells were seeded into 24-well culture plates and
treated with 0 (DMSO), 10, 20 and 40 µM curcumin, 3-MA,
3-MA+curcumin (40 µM), respectively. At 48 h latter, the cells were
incubated with fresh medium containing MDC (50 µM) for 15 min at
37°C and 5% CO2. The anti-quencher was added after
washing twice with PBS and taking pictures with UV excitation by an
inverted fluorescence microscope (Nikon Eclipse Ti; Nikon
Corporation) quickly. The experiment was repeated three times.
Transmission electron microscopy (TEM)
A549 cells treated with 20 µM curcumin for 48 h were
rinsed with PBS twice. Firstly, the cells were fixed with 2.5%
glutaraldehyde at 4°C for 2 h and washed with 0.1 M phosphate
buffer for 30 min or more. Then post-fixed in 0.1 M sodium
phosphate buffer containing 1% osmium tetroxide for 2 h at 4°C.
After a series of ethanol dehydration, embedding, polymerization,
slicing and dyeing were carried out. Finally, the thin sections of
50–70 nm stained for 15 min with uranyl acetate and citric acid,
respectively. Images were obtained by electron microscopy
(JEM-1200EX; JEOL, Tokyo, Japan).
Statistical analysis
Each experiment was conducted at least three times.
SPSS 18.0 statistical software (SPSS, Inc., Chicago, IL, USA) was
used for data analysis. For the measurement data expressed as mean
± SEM, with a minimum of three separate experiments for each issue
addressed. Data were analyzed by one-way ANOVA or factorization
variance and comparisons between groups were done using the
Dunnett's or LSD t-test. P<0.05 was considered to indicate a
statistically significant difference; and P<0.01 was considered
to indicate a highly significant difference.
Results
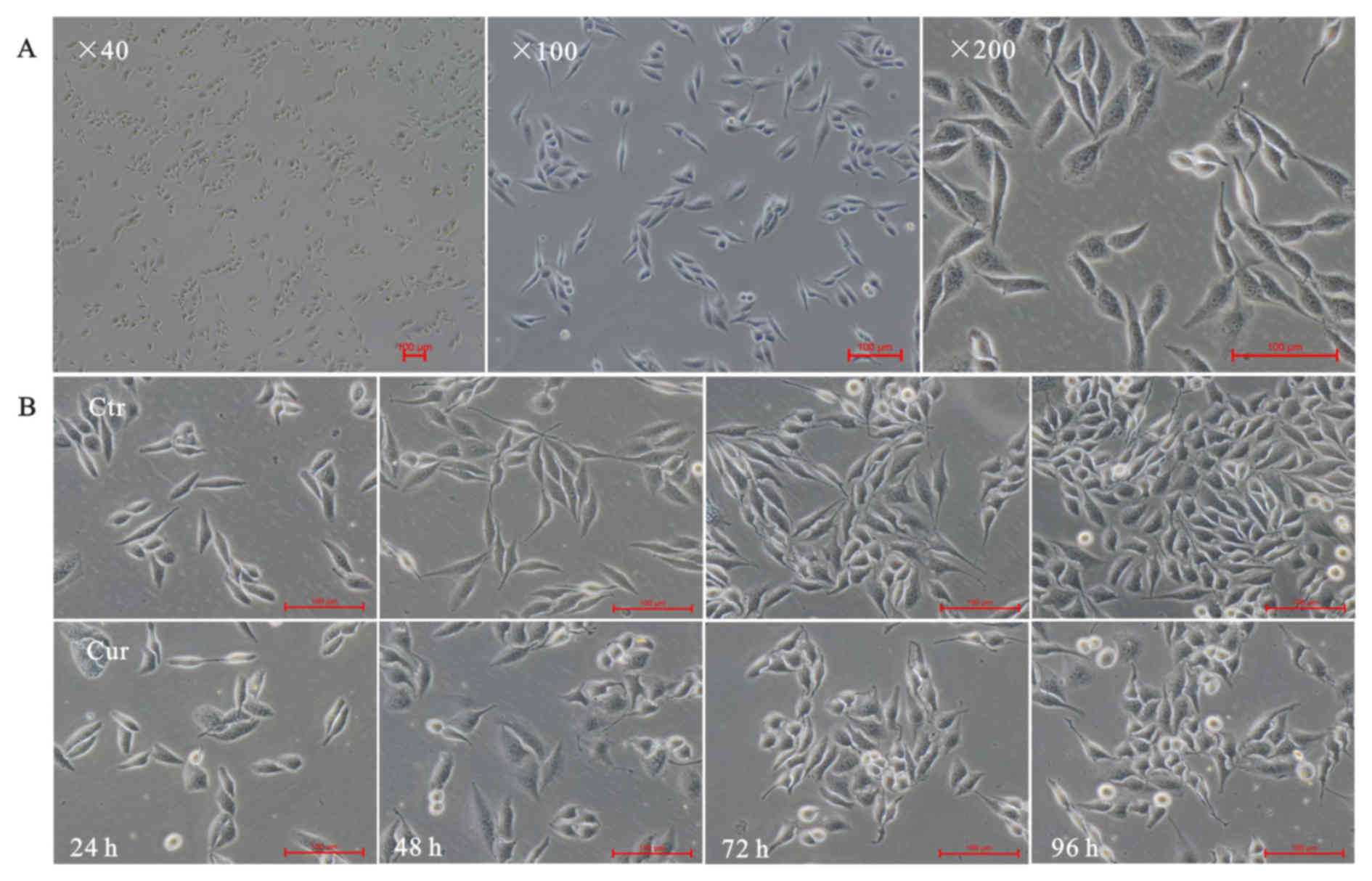
Cell morphology
The inverted microscope was used to exam A549 cells
morphology alteration. As expected, normal lung adenocarcinoma A549
cells showed a long fusiform shape, small size, clear cell
boundaries, well-adherent pebble-like growth, placental cytoplasm
and less cytoplasmic granules (Fig.
1A). Cells were treated with 40 µM curcumin, and pictures were
taken at indicated times (24, 48, 72 and 96 h). The results showed
that curcumin could inhibit growth of A549 cells. Curcumin made
A549 cells rounding and normal spindle shape disappeared, compared
with the corresponding time point of control group (Fig. 1B-Ctr), bright-circular dead cells
floating increased (Fig. 1B-Cur).
Curcumin affected the A549 cell
viability
Succinate dehydrogenase (SDH) in living cells can
reduce the exogenous MTT to insoluble formazan, a blue-violet
crystalline, and dead cells without this function. The formazan in
a cell can be dissolved in DMSO. Using a microplate reader to
measure the absorbance at 570 nm (OD value) can indirectly reflect
the number of living cells. Indirectly, absorbance at 570 nm (OD
value) which reflects the number of living cells, can be measured
by microplate reader (Infinire M200; Tecan, Männedorf,
Switzerland). The larger the OD value, the more live cells, the
stronger of activity.
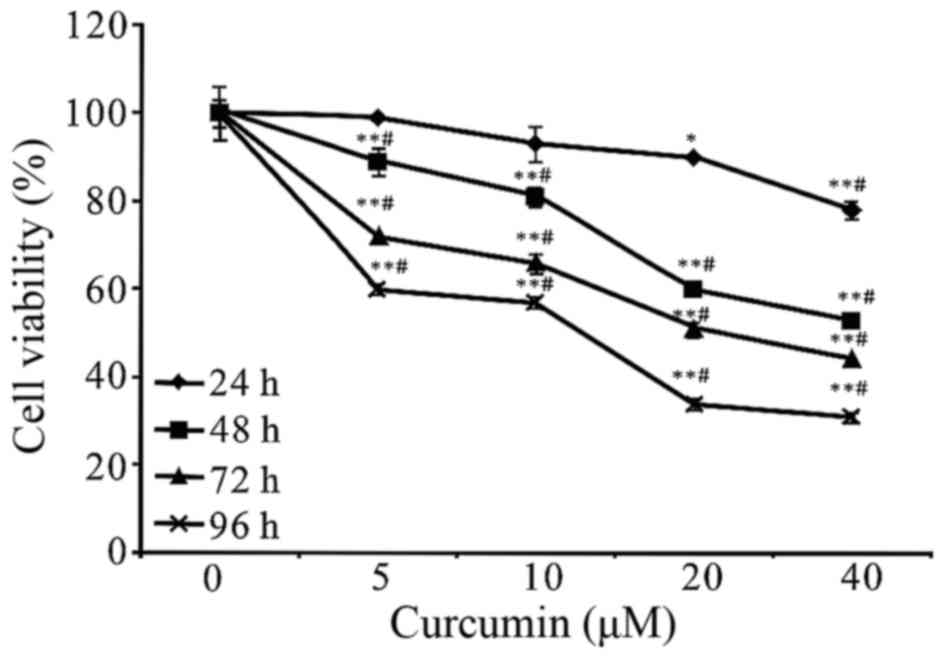
To assess the effects of curcumin on the cell
viability of A549 cells, the cells were treated with curcumin at
different concentrations (0, 5, 10, 20 and 40 µM) for different
time periods (24, 48, 72 and 96 h) and then followed by MTT assays.
It has been observed that the growth inhibition of curcumin on A549
cells was manifested in a concentration-and time-dependent manner
(Fig. 2). In addition, we found that
the cell viability could be significantly reduced with an
incubation period of 48 h at 20 and 40 µM. Therefore, the 48 h time
point was used for further studies. Treatment time of curcumin was
analyzed by single-effect analysis. As shown in Table I, the cell inhibitory rates were all
significantly different (P<0.01) in the 5, 10, 20 and 40 µM
groups (F=93.243; 39.36; 261.59 and 315.11, respectively) with
different time intervention. Similarly, there were statistical
significance (P<0.01) in 24, 48, 72 and 96 h groups (F=14.81;
62.92; 462.78 and 109.26, respectively) when the curcumin
concentrations were analyzed by single-effect analysis of the cell
inhibitory rates. In short, the results of a single-effect analysis
of the time and concentration of curcumin treatments were both
statistically significant in cell inhibition, P<0.01. Also,
there was an interaction between time and concentration of curcumin
treatments, P<0.01, both contributing to the inhibition of
proliferation of A549 cells.
 | Table I.The results of factorial analysis of
curcumin-treated cell viability. Interaction and single-effect
significance were found on treatment time and concentration of
curcumin. |
Table I.
The results of factorial analysis of
curcumin-treated cell viability. Interaction and single-effect
significance were found on treatment time and concentration of
curcumin.
|
| Curcumin (µM) |
|
|
|
|---|
|
|
|
|
|
|
|---|
| Time (h) | Control | 5 | 10 | 20 | 40 | Total | F | P-value |
|---|
| 24 | 0.00±0.01 | 0.01±0.01 | 0.07±0.04 | 0.10±0.01 | 0.22±0.02 | 0.08±0.02 | 14.81 | <0.01 |
| 48 | 0.00±0.03 | 0.11±0.03 | 0.19±0.02 | 0.40±0.02 | 0.47±0.01 | 0.23±0.03 | 62.92 | <0.01 |
| 72 | 0.00±0.01 | 0.28±0.00 | 0.34±0.02 | 0.49±0.01 | 0.56±0.01 | 0.34±0.03 | 462.78 | <0.01 |
| 96 | 0.00±0.06 | 0.40±0.01 | 0.43±0.01 | 0.66±0.01 | 0.69±0.01 | 0.44±0.04 | 109.26 | <0.01 |
| Total | 0.00±0.02 | 0.20±0.03 | 0.26±0.03 | 0.41±0.04 | 0.49±0.03 | 0.27±0.02 | 236.47a | <0.01a |
| F | 0.000 |
93.243 | 39.36 | 261.59 | 315.11 | 295.58a | 17.96b |
<0.01b |
| P | 1.000 | <0.01 | <0.01 |
<0.01 |
<0.01 |
<0.01a |
|
|
Curcumin triggered autophagy in A549
cells
To investigate whether curcumin triggers autophagy
in A549 cells, the morphological changes after curcumin treatment
were checked by AO, MDC staining and the TEM.
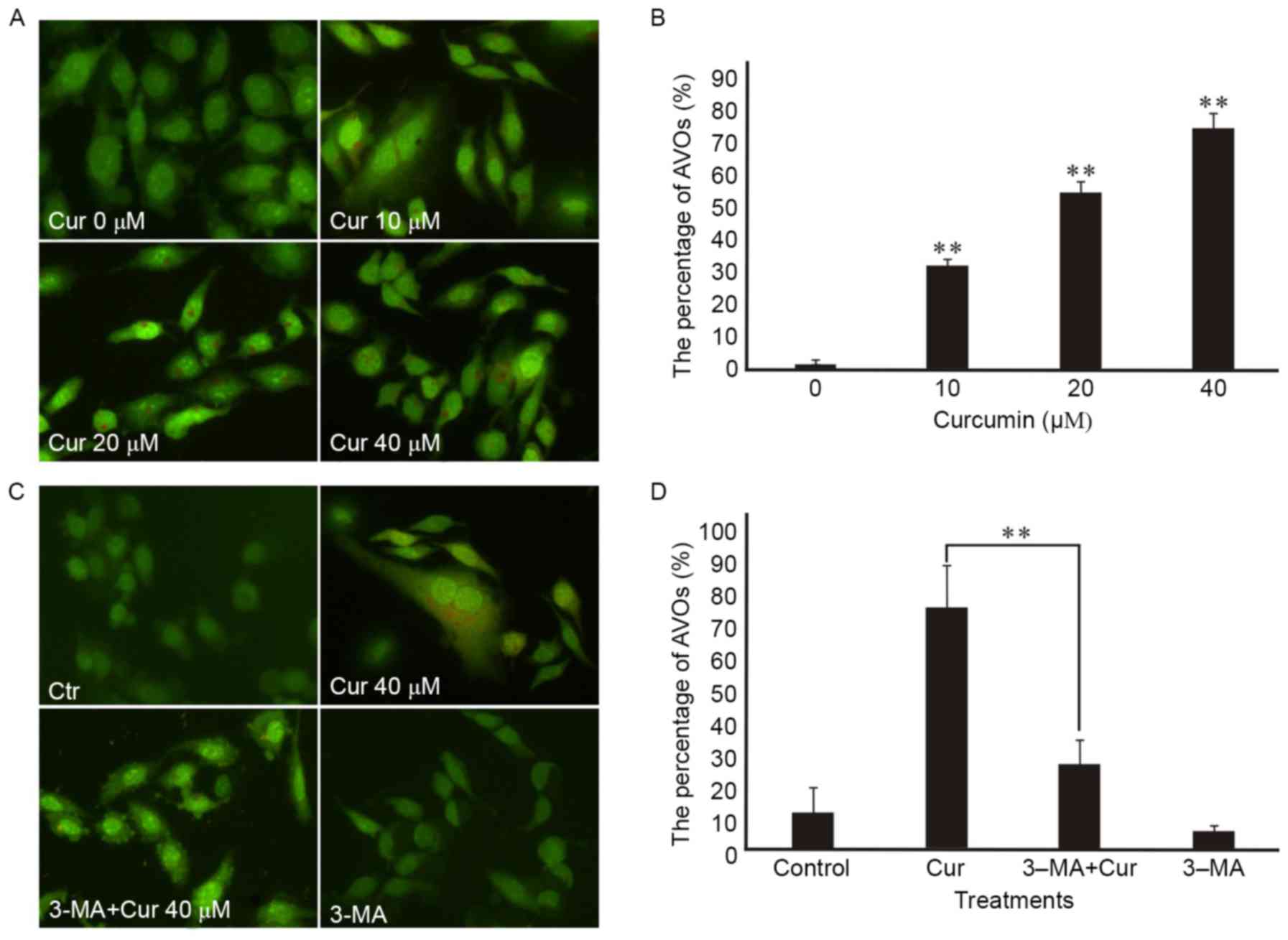
First, monitored the formation of autophagic
vesicles (AVs) by AO staining. Autophagosomes fuse with lysosomes
forming antophagolysosomes as acidic vesicular organelles (AVOs)
can bind AO in the process of cells autophagy (15). AO can stain nuclear and cytoplasm with
bright green and make AVOs with bright red. Formating and promoting
the AVOs is one of the characters of autophagy (16). The fluorescence microscope was used to
measure the changes of AVOs which could be investigated by the
appearance of red fluorescence. Our research showed that AVOs could
be detected in curcumin-treated A549 cells by AO staining analysis,
which suggested that curcumin may induce A549 cells autophagy. In
addition, we found that curcumin administration increased the
proportion of red-stained AVOs at 48 h in a dose-dependent manner
in the A549 cells by fluorescence microscopy (Fig. 3A). Also, the percentage of
curcumin-treated cells with AVOs was counted in the images, which
existed increasing in a concentration-dependent manner at 48 h
(Fig. 3B). In addition, the
pretreatment with 3-MA for 3 h attenuated curcumin-induced
autophagy in A549 cells according to the impaired red fluorescence
(Fig. 3C) which corresponded to the
percentage of AVOs in curcumin-treated cells (Fig. 3D).
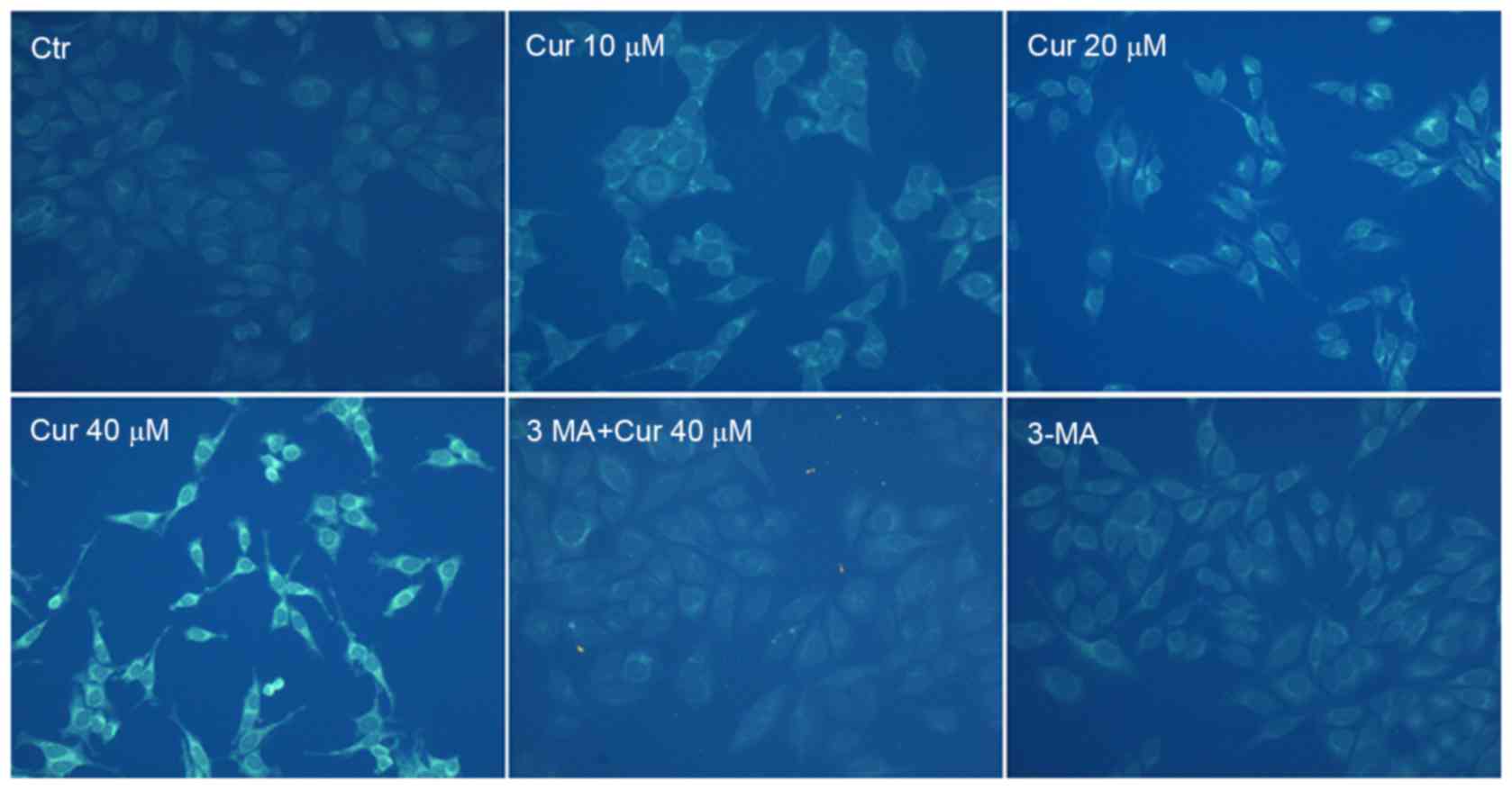
To confirm autophagy induction by curcumin, MDC
staining was performed. The activation of autophagy was inferred
from changes in fluorescent particles (AVs) infected with MDC. As
shown in Fig. 4, compared with
curcumin-untreated control, curcumin-treated A549 cells resulted in
an increase of fluorescence intensity and density at 48 h
indicating extensive MDC-positive autophagic vacuoles. We found
that curcumin administration increased the proportion of
MDC-infected AVs at 48 h in a dose-dependent manner. Moreover,
exposed to 40 µM curcumin after the pretreatment with 3-MA for 3 h,
MDC fluorescence particles were significantly reduced in A549
cells. As expected that the fluorescence intensity of the 3-MA
alone treatment group was the lowest. It was further demonstrated
that curcumin treatment induced autophagy which was inhibited by
3-MA in A549 cells.
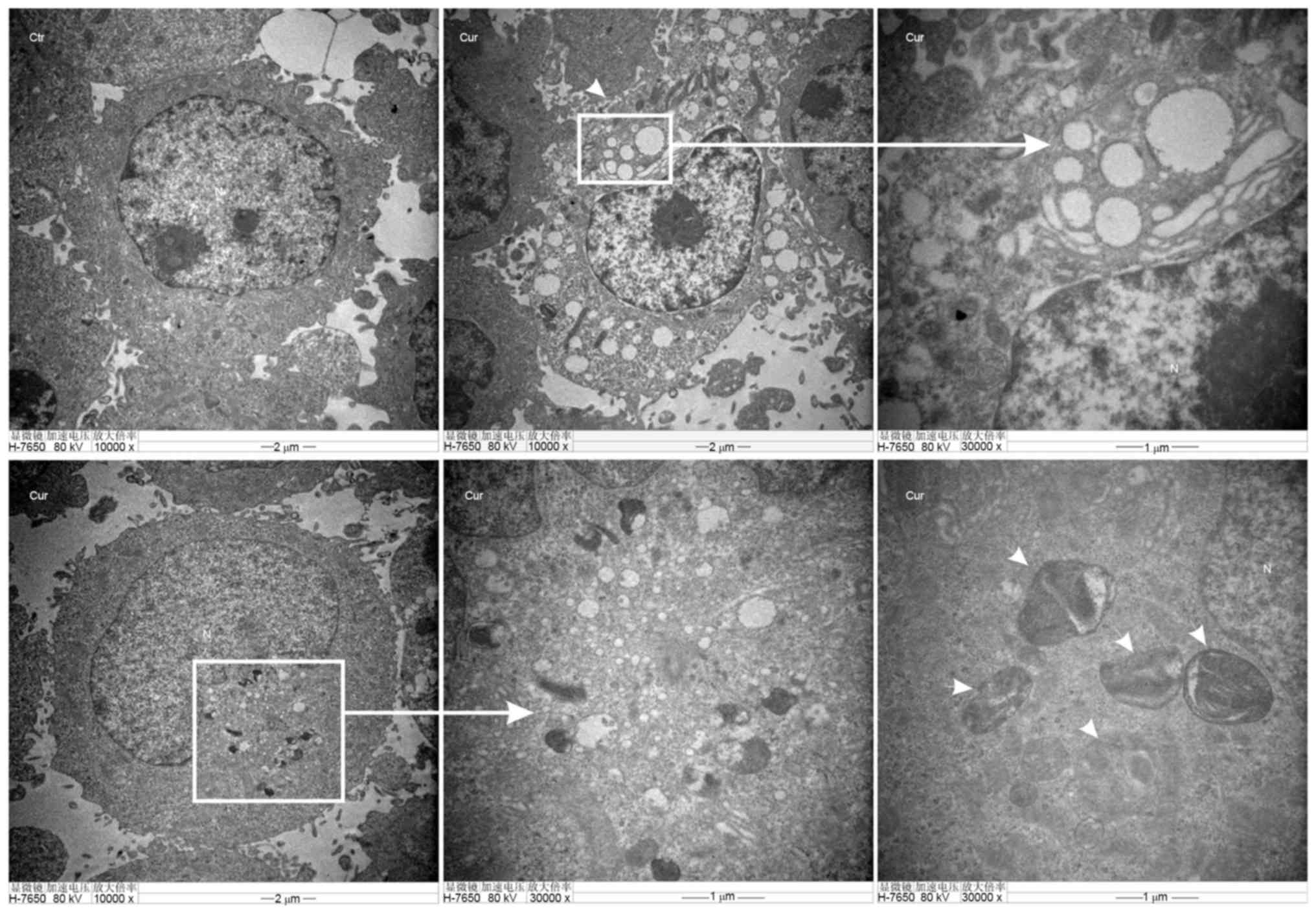
TEM of autophagosomes is the most direct way to
examine autophagy, which is a classical method to check autophagy
activation. Furthermore, the number of autophagic vacuoles
presenting in A549 cells were investigated using TEM analysis.
Images (Fig. 5-Ctr) showed normal
cytoplasm, organelles and nucleus morphology of control group, at
48 h in the cytoplasm. However, curcumin administration (20 µM)
induced the formation of multiple vacuole-like and
double-membrane-enclosed structures were considered to be
autophagosomes as shown by the black arrows in the images (Fig. 5-Cur).
All the results above have provided real evidence
for curcumin-induced autophagy of A549 cells.
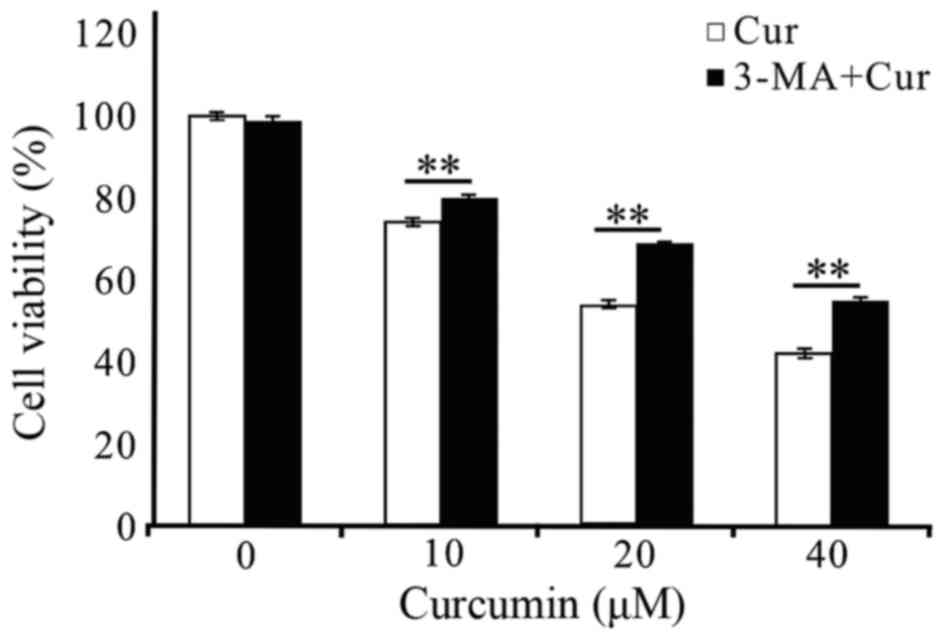
Autophagy inhibitor promoted the
curcumin-treated A549 cells survival
As above, curcumin could induced autophagy (Figs. 3A and B, and 4) and significant differences by AO staining
and MDC labeling analysis of treated A549 cells in the presence of
3-MA at 48 h implied autophagy did work at that time (Figs. 3C and D, and 4). What is the role of autophagy induced by
curcumin in inhibiting A549 cell growth? We pretreated the cells
with 3-MA for 3 h to block autophagy following MTT method to detect
the proliferation of cells. As shown in Fig 6, the treatment of A549 cells with 2.5
mM 3-MA for 48 h did not affect cell viability. When 3-MA was
pre-added (3 h) into the curcumin-treated (10, 20 and 40 µM) A549
cells at 48 h, the cell survival rates both increased significantly
at each treatment concentrations of curcumin. These results
suggested that autophagy was induced in A549 cells by curcumin
treatment, which was at least in part responsible for the reduction
in cell viability.
Discussion
Curcumin having a broad application prospects is
considered to be a safe and effective antitumor drug. However the
mechanism of antitumor of curcumin is still not fully elucidated.
In this study, the human lung adenocarcinoma A549 cell line were
selected as experimental cells. We aim to examine the
anti-proliferation mechanism of curcumin in A549 cells, especially
to confirm autophagy induced by curcumin. Morphologically, curcumin
(40 µM) administration caused the cells to lose their normal long
shuttle shape and resulted in the increase of floating suspected
dead cells, which appeared to be time dependent (Fig. 1). Curcumin could significantly inhibit
the proliferation of human lung adenocarcinoma A549 cells at the
concentration and time range we selected (Fig. 2). Previous studies have shown that
curcumin suppressed the growth of multiple cancer lines. Here,
evidence has been obtained that curcumin, even at very low
concentrations, could effectively inhibit the proliferation of lung
cancer A549 cells, but the specific mechanism need to be further
explored.
More than 3,000 plant species have been reported to
treat cancer and inducing apoptotic cell death is plant-derived
anticancer drugs mainly mechanism (17). Many natural plant compounds have a
shared molecular mechanism for the autophagy function of
anti-aging, anticancer and other diseases. Not surprisingly,
apoptosis has been recognized as the major toxic mechanism of
curcumin in tumor cells (8,18,19).
Autophagy may play an important role in tumorigenesis and
development, and becomes a new hotspot in tumor research. Abnormal
autophagy exists in tumor cells in which autophagic death can play
an antitumor effect, which provides a new clue for the treatment of
antitumor. Recent studies have shown that autophagy can be
activated in tumor cells under the action of some natural botanical
ingredients (20,21). These findings highly suggested the
importance to further elucidate the relationship between the
potential autophagy state and curcumin induced anticancer
effect.
Therefore, a variety of methods have been used to
verify the relevance of curcumin and autophagy in human lung
adenocarcinoma A549 cells here. To elucidate the A549 cell death
pathway induced by curcumin, AO staining and MDC labeling
analysises were done to detect the AVOs which suggested a
concentration-dependent increase of the fluorescent structures for
48 h (Figs. 3A and 4). To be more intuitive, the percentage of
curcumin-treated cells with AVOs were counted in the pictures,
which was consistent with the above results (Fig. 3B). To further confirm the autophagy
induced by curcumin in A549, we performed the gold standard for
autophagy detection-TEM showing an accumulation of
double-membrane-enclosed autophagosome in A549 cells after exposure
to 20 µM of curcumin for 48 h, whereas no autophagosome was
observed in untreated cells (Fig. 5).
These results collectively indicated that autophagy was induced by
curcumin administration in A549 cells in a dose-dependent manner
within a certain range. Compared to previous studies (13), we have consistently reached a
conclusion that curcumin induced the formation of autophagosome in
tumor cells. It is worth mentioning that curcumin could induce
autophagy in A549 cells demonstrated in this experiment enriching
and contributing to the clinical treatment of lung cancer.
However, retrieving the present studies, it seems
still difficult to draw a positive convinciable conclusion that
curcumin induced autophagy did exert its anticancer effect on tumor
control. On the one hand, nucleotides and free fatty acids promote
cell survival (22). On the other
hand, autophagy may be capable of ultimately killing cells when
allowed to reach its limit (21).
Although the dual role of autophagy has not yet reached a
consensus, the role in tumor development and treatment can't be
ignored. So, what is the role of autophagy in the antitumor effect
of curcumin on the proliferation of lung adenocarcinoma A549 cell?
This is the focus of our research.
3-MA, used as a typical autophagy inhibitor,
interferences the initiation of autophagy by acting on Class III
PI3K. The effect of 3-MA on the viability of A549 cells has been
examined by MTT assay during the experiment. We found that
treatment of A549 cells with 0 and 2.5 mM 3-MA for 48 h did not
affect cell viability, while treatment with 5 and 10 mM 3-MA caused
different degrees of reduction in viability (data not shown).
Verifiably, 2.5 mM 3-MA blocked autophagosome formation in cells
treated with curcumin according to the results that AVOs stained
bright red and MDC fluorescence could be abrogated by pre-treating
cells with 3-MA (Figs. 3C and D, and
4). Come together, we selected 2.5 mM
of 3-MA, a safe concentration with a significant autophagic
inhibitory effect on A549 cells, which excluding the possible
interference effect of 3-MA on A549 cell viability (Fig. 6). What's more, the inhibitory effect
of curcumin on A549 cells was attenuated which can be drawn from
the significantly increased cell survival rates at each treatment
concentrations when pretreated with 3-MA at 2.5 mM (Fig. 6) suggesting that autophagy did take
part in the process of curcumin-induced cell death. In the limited
exposure time periods and concentration range of curcumin, we
support the views of most scholars (23–25) that
autophagy is the antitumor mechanism of curcumin rather than a
protective mechanism of the A549 cells itself which reminded that
the combination of the autophagy inhibitor with curcumin in
antitumor therapy requires caution.
Whether inhibition of autophagy has an effect on
cell apoptosis, we do not know. Recent studies have pointed towards
a closely interplay between autophagy and apoptosis involved in the
process of cell death (26–28). So, we apt to think that apoptosis and
autophagy share some common signaling pathways and are mutually
regulated. For our results, a large number of autophagic vacuoles
and characteristic autophagosomes were found in the
curcumin-treated group compared with the control group under TEM.
We have reason to suspect that prolonged inhibition of autophagy
stimulates apoptosis. Considering there is a complex interaction
between curcumin-induced apoptosis and autophagy of A549 cells,
which contributes to curcumin inhibiting cell survival, further
validation at the protein and gene levels should be undertaken.
In conclusion, evidences have been gained that
curcumin could significantly inhibit the proliferation of A549
cells in this study, while autophagy was gradually taking in charge
as the concentration of curcumin increased. Due to the wide range
of effects of natural compounds and the complexity of procedural
death we can only draw conclusions that the promotion of tumor cell
autophagy activity induced by curcumin even induced autophagic
death is a potential tumor treatment within a limited range. The
effects of curcumin may differ depending on cell types. There may
be having another role in anticancer effects on other exposuring
concentrations or treat-times of curcumin, wishing to be in-depth
studied later. The study of non-apoptotic cell death mechanisms
induced by curcumin would provide targets of plant-derived
compounds for future cancer therapeutics and become the new
direction of antitumor researches.
Acknowledgements
The project was supported by the National Natural
Science Foundation of China (grant no. 81172598).
References
|
1
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Gupta SC, Kismali G and Aggarwal BB:
Curcumin, a component of turmeric: From farm to pharmacy.
Biofactors. 39:2–13. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: Lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu D, You M, Xu Y, Li F, Zhang D, Li X
and Hou Y: Inhibition of curcumin on myeloid-derived suppressor
cells is requisite for controlling lung cancer. Int
Immunopharmacol. 39:265–272. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Watson JL, Greenshields A, Hill R, Hilchie
A, Lee PW, Giacomantonio CA and Hoskin DW: Curcumin-induced
apoptosis in ovarian carcinoma cells is p53-independent and
involves p38 mitogen-activated protein kinase activation and
downregulation of Bcl-2 and survivin expression and Akt signaling.
Mol Carcinog. 49:13–24. 2010.PubMed/NCBI
|
|
6
|
Chang CC, Fu CF, Yang WT, Chen TY and Hsu
YC: The cellular uptake and cytotoxic effect of curcuminoids on
breast cancer cells. Taiwan J Obstet Gynecol. 51:368–374. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kang JH, Kang HS, Kim IK, Lee HY, Ha JH,
Yeo CD, Kang HH, Moon HS and Lee SH: Curcumin sensitizes human lung
cancer cells to apoptosis and metastasis synergistically combined
with carboplatin. Exp Biol Med (Maywood). 240:1416–1425. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Han X, Deng S, Wang N, Liu Y and Yang X:
Inhibitor effects and molecular mechanisms of tetraphydrocurcumin
against human breast cancer MCF-7 cell. Food Nutr Res.
60:306162016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Xia YQ, Wei XY, Li WL, Kanchana K, Xu CC,
Chen DH, Chou PH, Jin R, Wu JZ and Liang G: Curcumin analogue A501
induces G2/M arrest and apoptosis in non-small cell lung cancer
cells. Asian Pac J Cancer Prev. 15:6893–6898. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Shinojima N, Yokoyama T, Kondo Y and Kondo
S: Roles of the Akt/mTOR/p70S6K and ERK1/2 signaling pathways in
curcumin-induced autophagy. Autophagy. 3:635–637. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Guan F, Ding Y, Zhang Y, Zhou Y, Li M and
Wang C: Curcumin suppresses proliferation and migration of
MDA-MB-231 breast cancer cells through autophagy-dependent Akt
degradation. PLoS One. 11:e01465532016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Xiao K, Jiang J, Guan C, Dong C, Wang G,
Bai L, Sun J, Hu C and Bai C: Curcumin induces autophagy via
activating the AMPK signaling pathway in lung adenocarcinoma cells.
J Pharmacol Sci. 123:102–109. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Thongrakard V, Titone R, Follo C, Morani
F, Suksamrarn A, Tencomnao T and Isidoro C: Turmeric toxicity in
A431 epidermoid cancer cells associates with autophagy degradation
of anti-apoptotic and anti-autophagic p53 mutant. Phytother Res.
28:1761–1769. 2014. View
Article : Google Scholar : PubMed/NCBI
|
|
14
|
White E: Deconvoluting the
context-dependent role for autophagy in cancer. Nat Rev Cancer.
12:401–410. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Zhou GZ, Sun GC and Zhang SN: The
interplay between autophagy and apoptosis induced by one synthetic
curcumin derivative hydrazinobenzoylcurcumin in A549 lung cancer
cells. J Biochem Mol Toxicol. 29:267–273. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yu W, Tao Z, Hanyan S and Bei H: Study on
the relationship between autophagy and apoptosis in A549 cells
induced by curcumin analogue EF24. Chin J Cell Biol. 6:590–596.
2012.
|
|
17
|
Gali-Muhtasib H, Hmadi R, Kareh M, Tohme R
and Darwiche N: Cell death mechanisms of plant-derived anticancer
drugs: Beyond apoptosis. Apoptosis. 20:1531–1562. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Montazeri M, Sadeghizadeh M,
Pilehvar-Soltanahmadi Y, Zarghami F, Khodi S, Mohaghegh M,
Sadeghzadeh H and Zarghami N: Dendrosomal curcumin nanoformulation
modulate apoptosis-related genes and protein expression in
hepatocarcinoma cell lines. Int J Pharm. 509:244–254. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Guo S, Long M, Li X, Zhu S, Zhang M and
Yang Z: Curcumin activates autophagy and attenuates oxidative
damage in EA.hy926 cells via the Akt/mTOR pathway. Mol Med Rep.
13:2187–2193. 2016.PubMed/NCBI
|
|
20
|
Rigacci S, Miceli C, Nediani C, Berti A,
Cascella R, Pantano D, Nardiello P, Luccarini I, Casamenti F and
Stefani M: Oleuropein aglycone induces autophagy via the AMPK/mTOR
signalling pathway: A mechanistic insight. Oncotarget.
6:35344–35357. 2015.PubMed/NCBI
|
|
21
|
Selvaraj S, Sun Y, Sukumaran P and Singh
BB: Resveratrol activates autophagic cell death in prostate cancer
cells via downregulation of STIM1 and the mTOR pathway. Mol
Carcinog. 55:818–831. 2016. View
Article : Google Scholar : PubMed/NCBI
|
|
22
|
Jaroonwitchawan T, Chaicharoenaudomrung N,
Namkaew J and Noisa P: Curcumin attenuates paraquat-induced cell
death in human neuroblastoma cells through modulating oxidative
stress and autophagy. Neurosci Lett. 636:40–47. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Wang K, Zhang C, Bao J, Jia X, Liang Y,
Wang X, Chen M, Su H, Li P, Wan JB and He C: Synergistic
chemopreventive effects of curcumin and berberine on human breast
cancer cells through induction of apoptosis and autophagic cell
death. Sci Rep. 6:260642016. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Zanotto-Filho A, Braganhol E, Klafke K,
Figueiró F, Terra SR, Paludo FJ, Morrone M, Bristot IJ, Battastini
AM, Forcelini CM, et al: Autophagy inhibition improves the efficacy
of curcumin/temozolomide combination therapy in glioblastomas.
Cancer Lett. 358:220–231. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Papandreou I, Verras M, McNeil B, Koong AC
and Denko NC: Plant stilbenes induce endoplasmic reticulum stress
and their anti-cancer activity can be enhanced by inhibitors of
autophagy. Exp Cell Res. 339:147–153. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Jing K, Song KS, Shin S, Kim N, Jeong S,
Oh HR, Park JH, Seo KS, Heo JY, Han J, et al: Docosahexaenoic acid
induces autophagy through p53/AMPK/mTOR signaling and promotes
apoptosis in human cancer cells harboring wild-type p53. Autophagy.
7:1348–1358. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Kumar D, Das B, Sen R, Kundu P, Manna A,
Sarkar A, Chowdhury C, Chatterjee M and Das P: Andrographolide
analogue induces apoptosis and autophagy mediated cell death in
U937 cells by inhibition of PI3K/Akt/mTOR pathway. PLoS One.
10:e01396572015. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ge J, Liu Y, Li Q, Guo X, Gu L, Ma ZG and
Zhu YP: Resveratrol induces apoptosis and autophagy in T-cell acute
lymphoblastic leukemia cells by inhibiting Akt/mTOR and activating
p38-MAPK. Biomed Environ Sci. 26:902–911. 2013.PubMed/NCBI
|