Introduction
Prostate cancer (PCa) ranks as the most prevalent
type of cancer affecting male adults in the world (1). Despite local therapy, ~40% of patients
with PCa eventually develop metastases (2). Subsequent to the occurrence of
metastasis, PCa progresses to an aggressive disease and poses an
increased risk of mortality (3).
Thus, improved understanding of the molecular mechanisms involved
in PCa metastasis may be helpful for the effective control of
PCa.
One of the earliest events in the metastatic spread
of cancer is the proteolytic degradation of the extracellular
matrix proteins and invasion through the basement membrane. Matrix
metalloproteinases (MMPs) are a family of proteolytic enzymes that
degrade the extracellular matrix, as well as a variety of cell
surface receptors or signaling molecules (4,5). MMPs
perform a crucial role in the metastatic spread of cancer (6,7). MMP2 and
MMP9 are the most important cancer-associated zinc-dependent
endopeptidases in the invasion and metastasis of the majority of
carcinomas, including in brain neoplasms, human breast cancer and
colon cancer (8–10). High expression levels of activated
MMP2 or MMP9 have been associated with metastasis in patients with
PCa (11,12).
MMP16 (also termed MT3-MMP) belongs to the
membrane-type MMPs, a subgroup of the MMP family. MMP16 was
originally cloned from a human placenta cDNA library, and was
demonstrated to be located in the cell membrane (13). MMP16 exhibits a high expression level
in a variety of tumors tissues, including gastric cancer,
astrocytoma and melanoma, compared with normal tissues (14–16),
indicating its potential biological function. A previous study
reported that the migration and invasion of gliomas was mediated by
MMP16 (17), which indicated that the
expression of the MMP16 gene may be associated with tumor cell
invasion and metastasis. However, it is not clear whether MMP16 is
actually involved in the invasion and metastasis of PCa.
In the present study, the association of MMP16
expression with advanced prostate tumor stage and PCa metastasis
was first investigated. It was also examined whether the membrane
localization of MMP16 is required for its function. MMP16 may be
qualified to be a therapeutic target of PCa metastasis.
Materials and methods
Plasmids and cell culture
pcDNA3.1-MMP16 was kindly by Dr Stephen J. Weiss
from the University of Michigan (Ann Arbor, MI, USA) (18). psecTAGhygro-MMP16 [Δ533 aspartic acid
(Asp)] was provided by Dr Alyson E. Fournier from Montreal
Neurological Institute in McGill University (Montreal, QC, Canada)
(19).
LNCaP, PC3 and DU145 were purchased from American
Type Culture Collection, (Manassas, VA, USA). LNCaP was cultured in
T-medium (custom formula no. 02–0056DJ; Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), PC3 and DU145 cells were
cultured in Dulbecco's modified Eagle's medium (DMEM), supplemented
with 10% fetal bovine serum (FBS; Sijiqing Biological Engineering
Materials Co., Ltd., Hangzhou, China), 4 mM glutamine, 100 U/ml
penicillin and 100 µg/ml streptomycin in a humidified 5%
CO2 atmosphere at 37°C.
Synthetic small interfering RNAs
(siRNAs)
All siRNAs were purchased from Genepharma, Inc.
(Genepharma, Inc., Sunnyvale, CA, USA). The sequences for MMP16
siRNAs were: Forward, 5′-CGUGAUGUGGAUAUAACCAtt-3′ and reverse,
5′-UGGUUAUAUCCACAUCACGtt-3′, and the negative control sequence was
forward, 5′-UUCUCCGAACGUGUCACGUtt-3′, reverse,
5′-ACGUGACACGUUCGGAGAAtt-3′.
Transient transfection
LNCaP and PC3 cells were seeded onto 6-well plates,
and reached 50–60% confluency on the day of transfection. Each well
was transfected with 1 µg of DNA, including MMP16 and MMP16
(Δ533Asp), using polyethylenimine (Eurogentec, Liege, Belgium),
according to the manufacturer's protocol in LNCaP cells. To
knockdown MMP16, PC3 cells were transfected with 50 nM Dicer and
the negative control siRNA using RNAi-mate transfection reagent
(Genepharma, Inc.) at the final concentration of 50 nM, and
incubated for an additional 48 h.
Immunohistochemical staining
A total of 6 paraffin-embedded PCa tissue specimens
were acquired from the Jilin University Hospital (Jilin, China) and
sliced into 4-µm sections. Patients with PCa were divided into 2
groups: Non-metastasis cases [tumor (T)1–4 lymph-node
(N)0 metastasis (M)0] and metastasis cases
(T1–4 N1–2 M0 or T1–4
N0–2 M1). The present study received ethical
approval from the Commission for Scientific Research in Jilin
University, and was administered in accordance with the ethical
standards of the Declaration of Helsinki, second revision. Informed
consent was obtained from all individual patients involved in the
present study. The specimens were reactivated by heating for 3 mins
at 100°C, and 3% hydrogen peroxide in methanol was then added to
destroy the endogenous peroxidase activity. In total, 3 µg/ml of
anti-MMP16 polyclonal antibody (1:1,000 dilution; cat. no. BS1234;
Bioworld Technology, Inc., St. Louis Park, MN, USA) was applied
overnight at 4°C, and the secondary antibody (horseradish
peroxidase-conjugated anti-rabbit immunoglobulin G antibody;
1:1,000 dilution; cat. no. sc-53804; Santa Cruz Biotechnology,
Inc., Dallas, TX, USA) was then applied for 1 h at 25°C. For
immunohistochemical staining, an EliVision plus kit (Maixin Bio,
Fuzhou, China) was used according to the manufacturer's protocol.
Diaminobenzidine (cat. no. GK600505; Shanghai Gene Technology Co.,
Ltd., Shanghai, China) staining was performed followed by
counterstaining with hematoxylin (cat. no. AR-0711; Beijing Dingguo
Changsheng Biotechnology Co., Ltd., Beijing, China). ImagePro Plus
software (version IPP6.0; Media Cybernetics, Inc., Rockville, MD,
USA) was used to analyze the integrated optical density (IOD)
values of the positive areas of MMP16 immunohistochemical staining.
All brown-stained vessels were counted under 3 fields with ×200
power. The mean value of the vessels in the 3 fields was taken.
Immunoblotting
LNCaP, PC3 and DU145 cells, or the transfected cells
as previously described in the 6-well plate, were rinsed twice with
ice-cold PBS and lysed in radioimmunoprecipitation assay buffer
supplemented with the protease inhibitors dithiothreitol (cat. no.
CD116-1G) and phenylmethylsulfonyl fluoride (cat. no. LP250-10G)
(both from Beijing Dingguo Changsheng Biotechnology Co., Ltd.).
Equal amounts of protein were analyzed by western blotting using
antibodies against MMP16 (1:1,000 dilution; cat. no. BS1234;
Bioworld Technology, Inc.) and β-actin (mouse anti-human; 1:1,000
dilution; cat. no. sc-47778; Santa Cruz Biotechnology, Inc.).
Low-melt agarose drop migration
assay
The solution of melted 0.3% low melting-point
agarose with DMEM, without or with 10% FBS, was prepared. LNCaP,
PC3 and DU145 cells, or the transfected cells, were digested by
0.25% trypsinization, resuspended in the solution without FBS at a
concentration of 4×107 cells/ml, and warmed in at 37°C. Drops of
the cell suspension (2 µl) were plated at the center of the wells
in a 24-well tissue culture plate. The plate was stored at 4°C for
25 mins to allow the agarose drop to set. Once the drops were set,
the solution of DMEM with 10% FBS was added to melted 0.3% low
melting-point agarose, and the plate was stored at 4°C for 30 mins
to allow the agarose drop to set. Subsequently, the plate was moved
to a 37°C incubator. Images of cell migration were captured at 48
and 96 h.
In vitro wound healing assay
The transfected LNCaP and PC3 cells were cultured as
confluent monolayers on 6-well plates and synchronized in 1% FBS
for 24 h. The monolayer was wounded by removing a 300–500 µm strips
of cells across the well with a 200 µl pipette tip, and then washed
twice with cold PBS to remove non-adherent cells. Wound healing was
quantified at 0, 48 and 96 h. ImagePro Plus software (Media
Cybernetics, Inc.) was used to analyze the area of the wound
edge.
Transwell invasion assays
The transfected LNCaP and PC3 cells were seeded on
the top of a culture plate (Costar; Corning Incorporated, Corning,
NY, USA) containing a polycarbonate filter (diameter, 6.5 mm;
pores, 8 µm) pre-coated with fibronectin (0.5 mg/ml). The upper
chamber contained cells in T-medium/DMEM plus 1% FBS, and the lower
chamber contained T-medium/DMEM plus 10% (chemoattractant) or 1%
FBS (control). Cells were incubated for 12 h at 37°C in an
atmosphere containing 5% CO2. The cells that did not
migrate were wiped away from the top of the Transwell filter and
the migrated cells on the bottom surface were counted following
staining with coomassie blue (Beijing Dingguo Changsheng
Biotechnology Co., Ltd.). The cells were counted under a light
microscope (magnification, ×200).
Statistical analysis
SPSS 17.0 software (SPSS, Inc., Chicago, IL, USA)
was used to evaluate the statistical variability. All the
experiments were performed ≥3 times. The data were expressed as the
mean ± standard deviation. Two-tailed student's t-tests were used
to compare means of two independent groups. One-way analysis of
variance was applied to analyze the difference of means in ≥2
groups. P<0.05 was considered to indicate a statistically
significant difference.
Results
Increased MMP16 expression is
associated with PCa malignancy
Although the expression of MMP16 is high in a
variety of tumor tissues, the association between the expression
level and PCa malignancy remains to be elucidated.
To determine whether MMP16 is relevant to PCa
malignancy, MMP16 expression was evaluated by immunohistochemistry
in PCa tissue samples. The samples were classified into 2 groups
according to clinical stage (non-metastasis and metastasis). MMP16
expression level was quantified as the IOD values of the positive
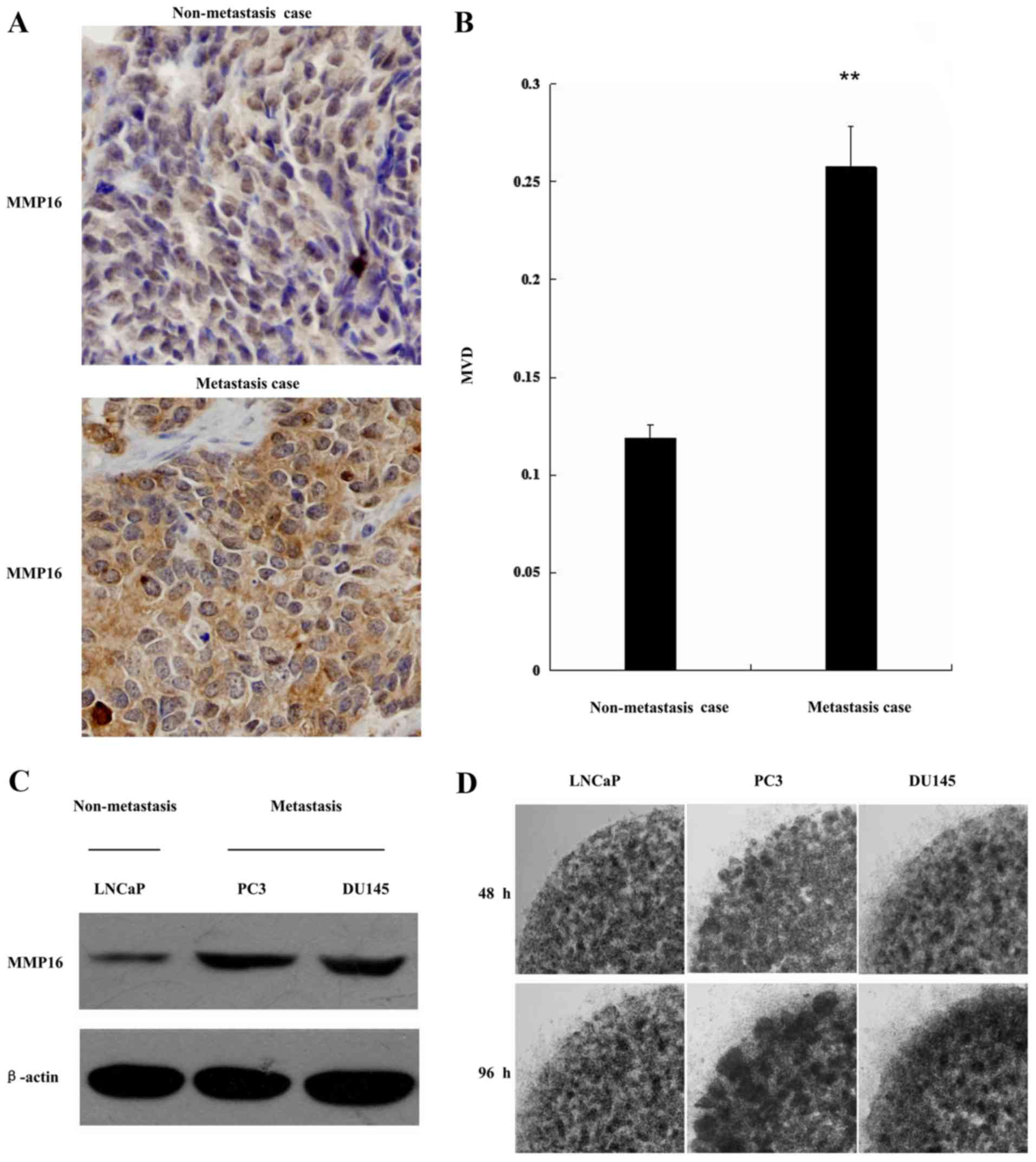
areas of MMP16. Representative immunostaining is shown in Fig. 1A. MMP16 expression was significantly
increased in the metastatic tissues when compared with the
non-metastatic tissues in Fig. 1B,
indicating that high levels of MMP16 may be associated with
advanced prostate tumor stage.
MMP16 expression was also examined in 3 typical PCa
cell lines: LNCaP, PC3 and DU145. These cell lines demonstrated
differential metastasis capacity; LNCaP cells had the lowest
metastasis capacity compared with the other two cell lines. The
level of MMP16 expression was examined by western blot analysis in
the 3 PCa cell lines. As shown in Fig.
1C, LNCaP cells had lower expression level of MMP16. By
contrast, the other two cell lines (PC3 and DU145) had increased
levels of MMP16 expression. The invasion ability of the three types
of cells was also examined using the low-melt agarose migration
assay. As shown in Fig. 1D, the LNCaP
cell line had a weaker ability in cell migration, while PC3 and
DU145 had high abilities in cell migration. This indicated that
there may be a positive association between MMP16 protein
expression level and PCa cell metastasis.
Endogenous MMP16 contributes to PC3
cells migration and invasion
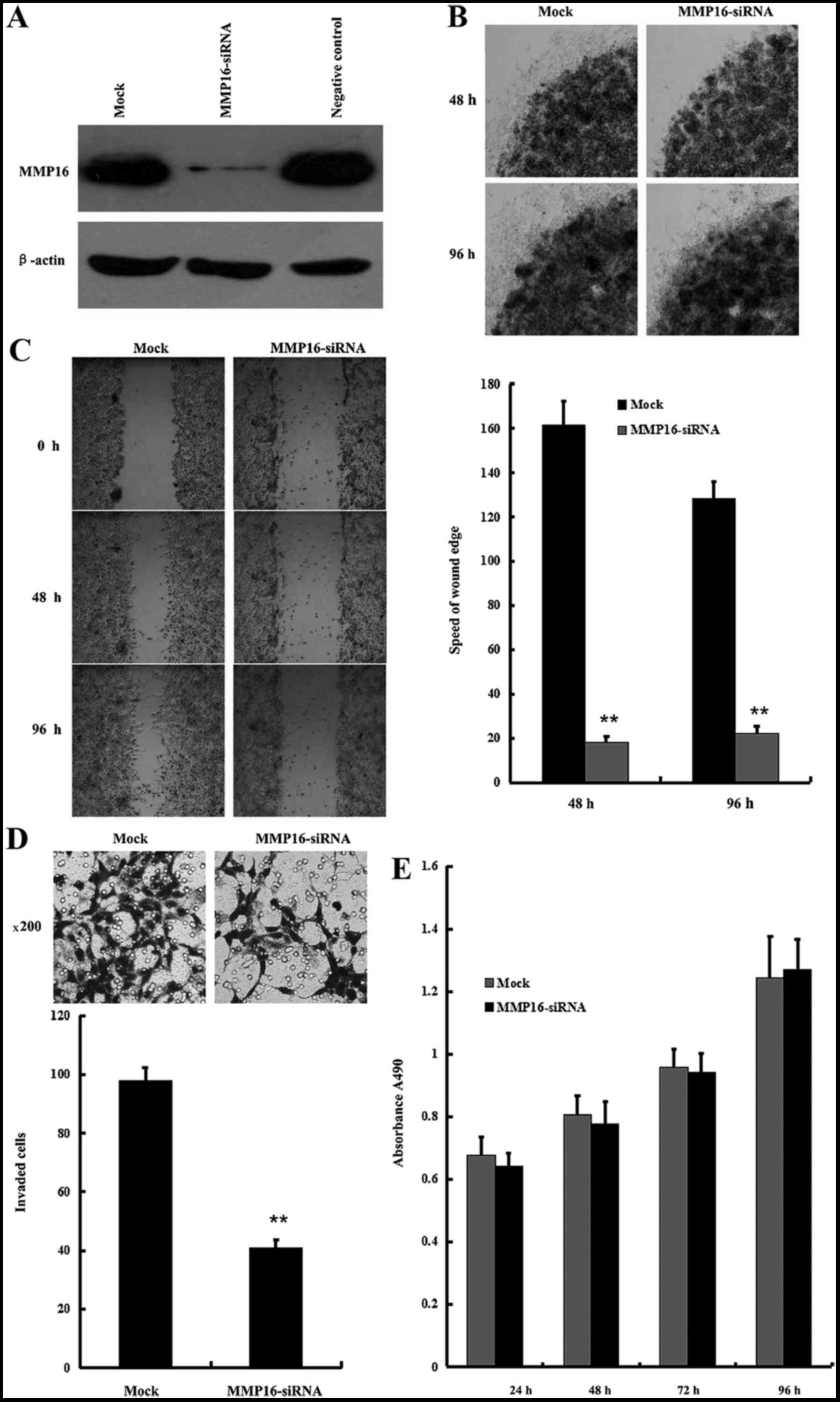
To test the association of MMP16 protein expression
level and PCa cell metastasis, the involvement of endogenous MMP16
in regulating the migration and invasion of PC3 cells was examined
with the RNA interference method, using the low-melt agarose
migration assay, the in vitro wound healing assay and the
Transwell cell migration assay. MMP16 can be significantly knocked
down by MMP16-siRNA (Fig. 2A). As
shown in Fig. 2B, MMP16 knockdown led
to inhibition of the migration of PC3 cells in the low-melt agarose
migration assay. The migration of PC3 cells was then examined with
the in vitro wound healing assay, and MMP16 siRNA was
revealed to decrease the migration ratio of PC3 cells (Fig. 2C). In addition, the Boyden chamber
assay was performed as an in vitro model of invasive
migration of PC3 transfected with MMP16-siRNA or control siRNA.
Cells migrated from the upper 1% of FBS to the lower 10% of FBS
through the 8 µm pores in the Boyden chamber. The migration rate of
cells transfected with MMP16-siRNA was significantly lower compared
with those transfected with MMP16-siRNA (Fig. 2D). In addition, MMP16-siRNA
transfection did not affect PC3 cellular proliferation in the MTT
assay (Fig. 2E).
Overexpressed membranous MMP16
promotes LNCaP cell migration and invasion
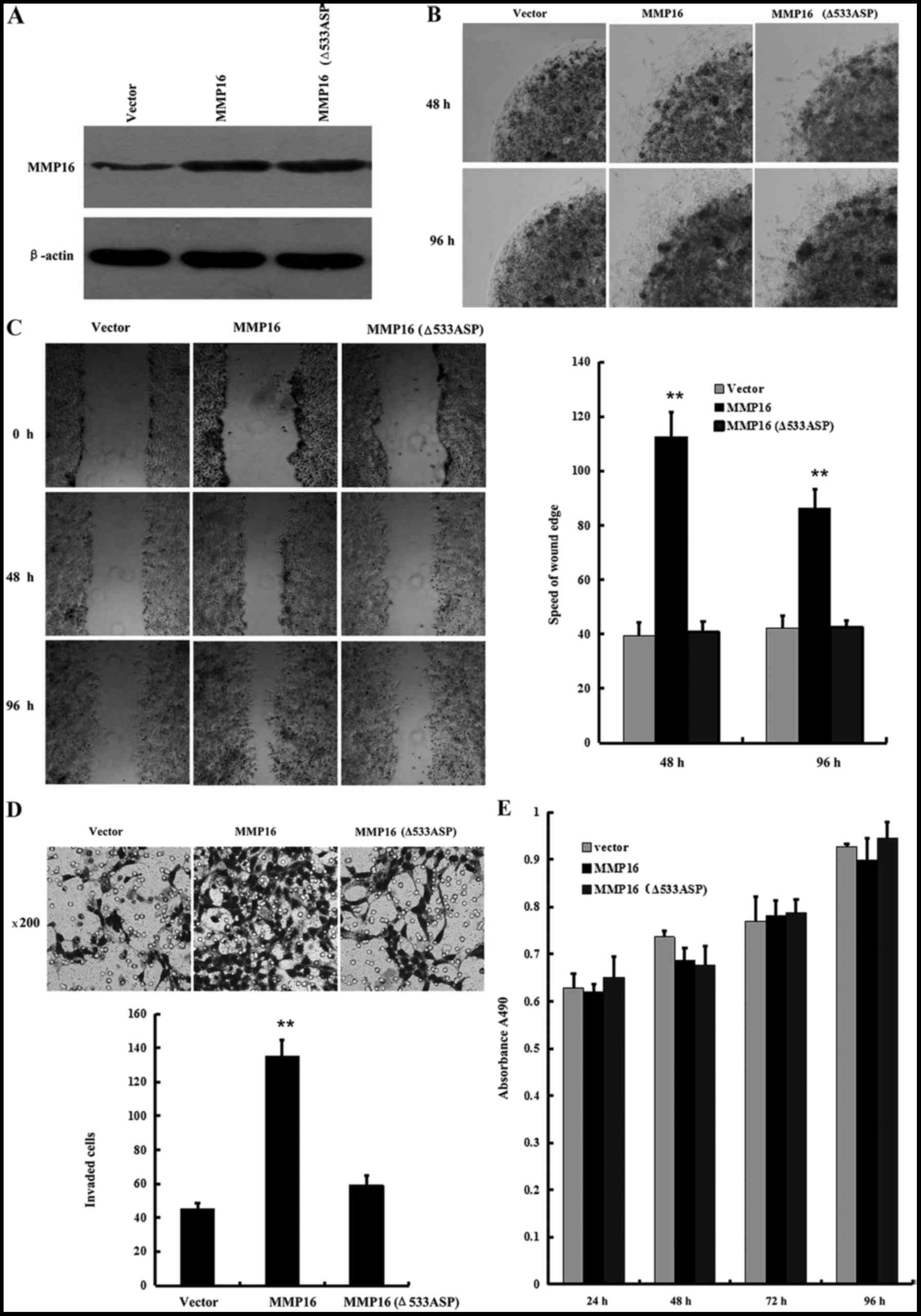
The effects of MMP16 on the migration and invasion
of LNCaP cells in which MMP16 protein level was low was then
examined. Wild-type MMP16 expression plasmid or MMP16-Δ533Asp
plasmid was transected with the deletion of 533 aspartic acid,
which is necessary for the membrane localization of MMP16. Western
blot analysis results revealed that the two plasmids expressed the
same size protein (Fig. 3A). Low
melting agarose cell migration assays, in vitro wound
healing assays and Transwell cell migration assays were then
performed to examine the differential impact of the two plasmids on
cell invasion. Overexpression of the membrane type MMP16
significantly promoted cell migration. However, when MMP16-Δ533Asp
was localized in the cytoplasm, it lost the promotion function in
cell migration as well as cell invasion (Fig. 3B-D), indicating that MMP16 expression
in the cell membrane performs an important role in promoting cell
migration and invasion. In addition, the effects of wild-type MMP16
and MMP16-Δ533Asp in cellular proliferation were also examined. No
significant changes in cellular proliferation ability were observed
for the two MMP16 plasmids when overexpressed in MTT assays
(Fig. 3E). These results indicated
that MMP16 is a new member of MMPS associated with cell metastasis
ability in PCa, and their function to promote cell metastasis is
dependent on its cell membrane localization.
Discussion
The expression of MMPs has been reported to be
upregulated in various types of cancer, including in lung,
pancreatic, breast and prostate cancer (20–26). While
the majority of studies were mainly focused on the collagenase and
gelatinase MMPs, studies on the membrane type MMPs are limited
(27–29). MMP16 is a new member of membrane-type
MMPs. Although the expression of MMP16 is high in a variety of
tumor tissues, the association between the expression level and PCa
malignancy remains to be elucidated.
The present results revealed that MMP16 expression
was increased in metastatic PCa tissues compared with in
non-metastatic PCa tissues, indicating that high level of MMP16 may
be associated with advanced prostate tumor stage. MMP16 expression
was also examined in the 3 typical PCa cell lines LNCaP, PC3 and
DU145. LNCaP cell lines with lowest metastasis capacity had
relatively lower expression level of MMP16. By contrast, the other
two cell lines (PC3 and DU145) had increased levels of MMP16
expression. This is consistent with the previous study by Daja
et al (30), in which MMP16
was shown to be increased in more invasive PCa sublines.
MMPs, including the two most important members MMP2
and MMP9, perform a crucial role in the invasion and metastasis of
cancer. A number of previous studies have reported that MMP16 was
associated with cell migration and cell invasion in certain
cancers, including colorectal cancer, glioma cancer and melanoma
(31–33). However, the role of MMP16 in
regulating PCa cell invasion and metastasis has yet to be
elucidated. In the present study, knockdown of MMP16 by siRNA was
shown to inhibit PC3 cell migration and invasion. Consistently,
overexpression of MMP16 also promoted LNCaP cell migration and
invasion without affecting cellular proliferation. These results
suggest that as a new member of MMPs, MMP16 is associated with cell
metastasis in PCa. In addition, MMP16 with a deletion of 533
aspartic acid, which is necessary for the membrane localization of
MMP16, lost the capability for promoting cell migration and
invasion. These results suggested that as an important mediator of
PCa cell metastasis, the transmembrane location of MMP16 is
required for its function. This is consistent with a previous
study, which demonstrated that the transmembrane domain of MT-MMPs
in the carboxyl-terminus of their molecules exhibits the function
of activating downstream pro-MMP2 (34).
To the best of our knowledge, the present study is
the first to show that MMP16 is associated with advanced prostate
tumor stage. As an important mediator of PCa cell metastasis, the
membrane localization of MMP16 is required for its function. These
results suggest that MMP16 may be qualified to be a therapeutic
target for PCa metastasis.
Acknowledgements
The present study was supported by the Ministry of
Science and Technology (grant no. 2016YFE0128500), National Natural
Science Foundation of China (grant nos. 30871301 and 30700827),
Jilin Provincial Science and Technology Department (grant nos.
20170204009YY, 20150101187JC and 20150414007GH), Jilin Province
Education Department (grant nos. 2015–526 and 2015–551) the
Fundamental Research Funds for the Central Universities (grant nos.
2412016KJ037, 130017507 and 130028633). University S and T
Innovation Platform of Jilin Province for Economic Fungi (grant no.
2014B-1), and the Program for Introducing Talents to Universities
(grant no. B07017). The authors would like to thank the
organizations mentioned above for their support.
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Grubb RL III and Kibel AS: Prostate
cancer: Screening, diagnosis and management in 2007. Mo Med.
104:408–413; quiz 413–404. 2007.PubMed/NCBI
|
|
3
|
Feldman BJ and Feldman D: The development
of androgen-independent prostate cancer. Nat Rev Cancer. 1:34–45.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
4
|
Bejarano PA, Noelken ME, Suzuki K, Hudson
BG and Nagase H: Degradation of basement membranes by human matrix
metalloproteinase 3 (stromelysin). Biochem J. 256:413–419. 1988.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Pei D: Matrix metalloproteinases target
protease-activated receptors on the tumor cell surface. Cancer
Cell. 7:207–208. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Verma RP and Hansch C: Matrix
metalloproteinases (MMPs): Chemical-biological functions and
(Q)SARs. Bioorg Med Chem. 15:2223–2268. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Vihinen P and Kähäri VM: Matrix
metalloproteinases in cancer: Prognostic markers and therapeutic
targets. Int J Cancer. 99:157–166. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Jäälinojä J, Herva R, Korpela M, Höyhtyä M
and Turpeenniemi-Hujanen T: Matrix metalloproteinase 2 (MMP-2)
immunoreactive protein is associated with poor grade and survival
in brain neoplasms. J Neurooncol. 46:81–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Lu N, Ling Y, Gao Y, Chen Y, Mu R, Qi Q,
Liu W, Zhang H, Gu H, Wang S, et al: Endostar suppresses invasion
through downregulating the expression of matrix
metalloproteinase-2/9 in MDA-MB-435 human breast cancer cells. Exp
Biol Med (Maywood). 233:1013–1020. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang B, Tang F, Zhang B, Zhao Y, Feng J
and Rao Z: Matrix metalloproteinase-9 overexpression is closely
related to poor prognosis in patients with colon cancer. World J
Surg Oncol. 12:242014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Gohji K, Fujimoto N, Hara I, Fujii A,
Gotoh A, Okada H, Arakawa S, Kitazawa S, Miyake H, Kamidono S and
Nakajima M: Serum matrix metalloproteinase-2 and its density in men
with prostate cancer as a new predictor of disease extension. Int J
Cancer. 79:96–101. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Moses MA, Wiederschain D, Loughlin KR,
Zurakowski D, Lamb CC and Freeman MR: Increased incidence of matrix
metalloproteinases in urine of cancer patients. Cancer Res.
58:1395–1399. 1998.PubMed/NCBI
|
|
13
|
Takino T, Sato H, Shinagawa A and Seiki M:
Identification of the second membrane-type matrix metalloproteinase
(MT-MMP-2) gene from a human placenta cDNA library. MT-MMPs form a
unique membrane-type subclass in the MMP family. J Biol Chem.
270:23013–23020. 1995. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Lowy AM, Clements WM, Bishop J, Kong L,
Bonney T, Sisco K, Aronow B, Fenoglio-Preiser C and Groden J:
beta-Catenin/Wnt signaling regulates expression of the membrane
type 3 matrix metalloproteinase in gastric cancer. Cancer Res.
66:4734–4741. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Nakada M, Nakamura H, Ikeda E, Fujimoto N,
Yamashita J, Sato H, Seiki M and Okada Y: Expression and tissue
localization of membrane-type 1, 2 and 3 matrix metalloproteinases
in human astrocytic tumors. Am J Pathol. 154:417–428. 1999.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Tatti O, Arjama M, Ranki A, Weiss SJ,
Keski-Oja J and Lehti K: Membrane-type-3 matrix metalloproteinase
(MT3-MMP) functions as a matrix composition-dependent effector of
melanoma cell invasion. PLoS One. 6:e283252011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Li Y, Wang Y, Yu L, Sun C, Cheng D, Yu S,
Wang Q, Yan Y, Kang C, Jin S, et al: miR-146b-5p inhibits glioma
migration and invasion by targeting MMP16. Cancer Lett.
339:260–269. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Hotary K, Allen E, Punturieri A, Yana I
and Weiss SJ: Regulation of cell invasion and morphogenesis in a
three-dimensional type I collagen matrix by membrane-type matrix
metalloproteinases 1, 2 and 3. J Cell Biol. 149:1309–1323. 2000.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ferraro GB, Morrison CJ, Overall CM,
Strittmatter SM and Fournier AE: Membrane-type matrix
metalloproteinase-3 regulates neuronal responsiveness to myelin
through Nogo-66 receptor 1 cleavage. J Biol Chem. 286:31418–31424.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Koc M, Ediger D, Budak F, Karadağ M, Oral
HB, Uzaslan E, Ege E and Gözü RO: Matrix metalloproteinase-9
(MMP-9) elevated in serum but not in bronchial lavage fluid in
patients with lung cancer. Tumori. 92:149–154. 2006.PubMed/NCBI
|
|
21
|
Kuhlmann KF, van Till JW, Boermeester MA,
de Reuver PR, Tzvetanova ID, Offerhaus GJ, Ten Kate FJ, Busch OR,
van Gulik TM, Gouma DJ and Crawford HC: Evaluation of matrix
metalloproteinase 7 in plasma and pancreatic juice as a biomarker
for pancreatic cancer. Cancer Epidemiol Biomarkers Prev.
16:886–891. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Poola I, DeWitty RL, Marshalleck JJ,
Bhatnagar R, Abraham J and Leffall LD: Identification of MMP-1 as a
putative breast cancer predictive marker by global gene expression
analysis. Nat Med. 11:481–483. 2005. View
Article : Google Scholar : PubMed/NCBI
|
|
23
|
Sauer CG, Kappeler A, Späth M, Kaden JJ,
Michel MS, Mayer D, Bleyl U and Grobholz R: Expression and activity
of matrix metalloproteinases-2 and −9 in serum, core needle
biopsies and tissue specimens of prostate cancer patients. Virchows
Arch. 444:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Tetu B, Brisson J, Wang CS, Lapointe H,
Beaudry G, Blanchette C and Trudel D: The influence of MMP-14,
TIMP-2 and MMP-2 expression on breast cancer prognosis. Breast
Cancer Res. 8:R282006. View
Article : Google Scholar : PubMed/NCBI
|
|
25
|
Wood M, Fudge K, Mohler JL, Frost AR,
Garcia F, Wang M and Stearns ME: In situ hybridization studies of
metalloproteinases 2 and 9 and TIMP-1 and TIMP-2 expression in
human prostate cancer. Clin Exp Metastasis. 15:246–258. 1997.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Wu ZS, Wu Q, Yang JH, Wang HQ, Ding XD,
Yang F and Xu XC: Prognostic significance of MMP-9 and TIMP-1 serum
and tissue expression in breast cancer. Int J Cancer.
122:2050–2056. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Nagakawa O, Murakami K, Yamaura T,
Fujiuchi Y, Murata J, Fuse H and Saiki I: Expression of
membrane-type 1 matrix metalloproteinase (MT1-MMP) on prostate
cancer cell lines. Cancer Lett. 155:173–179. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Ota I, Li XY, Hu Y and Weiss SJ: Induction
of a MT1-MMP and MT2-MMP-dependent basement membrane transmigration
program in cancer cells by Snail1. Proc Natl Acad Sci USA.
106:20318–20323. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Sato H, Takino T and Miyamori H: Roles of
membrane-type matrix metalloproteinase-1 in tumor invasion and
metastasis. Cancer Sci. 96:212–217. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Daja MM, Niu X, Zhao Z, Brown JM and
Russell PJ: Characterization of expression of matrix
metalloproteinases and tissue inhibitors of metalloproteinases in
prostate cancer cell lines. Prostate Cancer Prostatic Dis. 6:15–26.
2003. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Moon JW, Choi JH, Lee SK, Lee YW, Lee JO,
Kim N, Lee HJ, Seo JS, Kim J, Kim HS, et al: Promoter
hypermethylation of membrane type 3 matrix metalloproteinase is
associated with cell migration in colorectal adenocarcinoma. Cancer
Genet. 208:261–270. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Tatti O, Gucciardo E, Pekkonen P,
Holopainen T, Louhimo R, Repo P, Maliniemi P, Lohi J, Rantanen V,
Hautaniemi S, et al: MMP16 mediates a proteolytic switch to promote
cell-cell adhesion, collagen alignment, and lymphatic invasion in
melanoma. Cancer Res. 75:2083–2094. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Wang H, Li XT, Wu C, Wu ZW, Li YY, Yang
TQ, Chen GL, Xie XS, Huang YL, Du ZW and Zhou YX: miR-132 can
inhibit glioma cells invasion and migration by target MMP16 in
vitro. Onco Targets Ther. 8:3211–3218. 2015.PubMed/NCBI
|
|
34
|
Sato H and Seiki M: Membrane-type matrix
metalloproteinases (MT-MMPs) in tumor metastasis. J Biochem.
119:209–215. 1996. View Article : Google Scholar : PubMed/NCBI
|