Introduction
Despite recent advances in the development of
diagnostic tools for gastric cancer, many patients with gastric
cancer continue to be diagnosed at a late stage, and even following
curative surgery, recurrent tumors frequently occur. Therefore,
gastric cancer remains a major cause for cancer-associated
mortality worldwide (1) and the
development of novel drug therapies for gastric cancer is
important.
5FU is currently a key drug for adjuvant therapy
following curative surgery (2–4) and for
the treatment of metastatic gastric cancer (5,6). Three
mechanisms have been proposed for its action: Incorporation into
RNA (7), incorporation into DNA
(8) and the inhibition of thymidine
synthase (TS) leading to the inhibition of DNA de novo
synthesis by forming a ternary complex composed of TS,
5,10-methylenetetrahydrofolate (CH2THF) and fluoro-deoxyuridine
monophosphate (FdUMP) (9). The first
step in the activation of 5FU is the phosphorylation of 5FU by
orotate phosphoribosyltransferase (OPRT), which metabolizes 5FU to
5-fluorouridine monophosphate (FUMP). FUMP is then metabolized into
5-fluorouridine triphosphate (FUTP), which can be incorporated into
RNA and into 5-flurodeoxyuridine triphosphate (FdUTP), which can be
incorporated into DNA (10). Although
the incorporation into RNA and DNA is certainly an important aspect
of the mechanism of action of 5FU, the potential mechanism that has
received the most focus is the inhibition of TS by the formation of
ternary complexes, as a number of chemotherapeutic drugs similarly
inhibit TS, including methotrexate (11), pemetrexate and raltitrexed (12). TS is the enzyme required to convert
deoxyuridine monophosphate (dUMP) to thymidine monophosphate
(dTMP); the inhibition of TS causes a lack of dTMP, leading to the
inhibition of DNA de novo synthesis and cell death, a
phenomenon termed ‘thymineless death’ (13).
Decreased levels of OPRT and increased levels of TS
are thus considered major factors in the development of 5FU
resistance (14). However, analysis
of the expression of OPRT and TS in patients enrolled in the
ACTS-GC trial, which investigated the efficacy of S-1 (5FU
derivatives) for adjuvant therapy, revealed that increased TS was
associated with a good prognosis and that the expression of OPRT
was not associated with the prognosis at all (15).
These observations suggest that there may be unknown
mechanisms of action involved in the development of 5FU resistance.
Further investigation is therefore required to develop more
effective drug therapies for gastric cancer. As 5FU is considered
to be a TS inhibitor, 5FU resistance is associated with the
decreased intracellular concentration of FdUMP (16). Therefore, in the present study, the
mechanism of 5FU resistance was investigated, focusing on the
amount of TS ternary complex formed by FdUMP.
Materials and methods
Drugs
5FU was kindly provided by Kyowa Hakko Kirin Co.,
Ltd. (Tokyo, Japan). Raltitrexed, deoxyuridine (dU), and leucovorin
were purchased from Sigma-Aldrich (Merck KGaA, Darmstadt, Germany).
Thymidine (dT) was purchased from Wako Pure Chemical Industries,
Ltd. (Osaka, Japan).
Cell lines and cell culture
MKN45 cells (a poorly differentiated gastric cancer
cell line, kindly provided by Hiroshima Univiersity (Hiroshima,
Japan), were cultured in RPMI-1640 with 10% fetal bovine serum
(FBS; both from Wako Pure Chemical Industries, Ltd.) and sodium
pyruvate (Sigma-Aldrich; Merck KGaA). MKN45/F2R cells are a
5FU-resistant cell line that was established by continuously
exposing MKN45 cells to increasing concentrations (0.1–2 µM) of 5FU
over a year, as previously described (17). These cells were routinely maintained
in RPMI-1640 with 10% FBS containing 2 µM 5FU, and prior to the
study, the resistant cells were cultured in drug-free RPMI-1640
with 10% FBS for at least two weeks to eliminate the effects of 5FU
in the experiments. The two cell lines were incubated in a
humidified atmosphere of 5% CO2 at 37°C.
Western blot analysis and
antibodies
The cells were lysed in radioimmunoprecipitation
assay buffer (Sigma-Aldrich; Merck KGaA) for 15 min on ice. The
protein concentration of the lysates was measured using a Bio-Rad
Protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Inc.,
Hercules, CA, USA). The cell lysates were boiled in sample buffer
solution (Wako Pure Chemical Industries, Ltd.). Total cell protein
extracts (10 µg/lane) were separated by 10% SDS-PAGE using
SuperSep™ ACE (Wako Pure Chemical Industries, Ltd.) and
electrophoretically transferred onto polyvinyl difluoride (PVDF)
membranes. The membranes were blocked with PVDF blocking reagent
(Toyobo Co., Ltd., Osaka, Japan) for 1 h. The membranes were then
incubated with primary antibodies, such as β-actin (13E5) rabbit
mAb (cat. no. 4970; Cell Signaling Technology, Danvers, MA, USA;
1:5,000), anti-Thymidylate Synthase, clone TS106 (cat. no. MAB4130;
EMD Millipore, Billerica, MA, USA; 1:5,000) or anti-OPRT antibody
(kindly provided by Taiho Pharmaceutical Company, Tokyo, Japan;
1:10,000) overnight at 4°C. The primary antibodies were diluted
with Can Get Signal Solution 1 (Toyobo Co., Ltd.). The membranes
were then washed with Dako Washing Buffer (Agilent Technologies,
Inc., Santa Clara, CA, USA) and incubated with goat anti-Mouse IgG,
Peroxidase Conjugated, heavy chain + light chain (cat. no. AP124P;
EMD Millipore) or goat anti-rabbit IgG, Peroxidase Conjugate (cat.
no. AP132P; EMD Millipore) diluted to 1:25,000 with Can Get Signal
Solution 2 (Toyobo Co., Ltd.) for 1 h at room temperature.
Immunoreactive proteins were visualized with the ImmunoStar LD
reagent (Wako Pure Chemical Industries, Ltd.) and images were
captured using a GeneGnome HR system (Syngene Europe, Cambridge,
UK).
MTT assay for the effects of 5FU and
raltitrexed
Cell growth was assessed by an MTT assay. A total of
5×103 cells were seeded into each well of 96-well plates and
cultured for 24 h at 37°C. The cells were treated with raltitrexed,
dU or dT for 72 h, after which the culture medium was removed and
100 µl of a 0.5 mg/ml solution of MTT (Sigma-Aldrich; Merck KGaA)
was added to each well. The plates were then incubated for 4 h at
37°C. The MTT solution was replaced with 100 µl of dimethyl
sulfoxide (Wako Pure Chemical Industries, Ltd.) per well and the
absorbance at 540 nm was measured using an Envision 2104 Multilabel
Reader (PerkinElmer, Inc., Waltham, MA, USA). Each assay was
repeated three times.
Statistical analysis
The mean Half-maximal inhibitory concentration
(IC50) values were calculated based on each of the
results of the MTT assays using the Microsoft Excel 2010 (Microsoft
Corporation, Redmond, Washington, USA). Results are presented as
the mean ± standard error. The significance of differences in
IC50 values were determined by Student's t-test using
JMP 8 (SAS Institute Inc., Cary, NC, USA). P<0.05 was considered
to indicate a statistically significant difference.
Transfection and small interfering RNA
(siRNA) experiments for OPRT
MKN45 cells were cultured in RPMI-1640 with 10% FBS
without antibiotics for 24 h to 50–70% confluence prior to
transfection. Cells were then transfected with siRNA
oligonucleotides using Lipofectamine RNAiMAX (Invitrogen; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) at a concentration of 40
nmol/l siRNA in serum-free Opti-MEM (Invitrogen; Thermo Fisher
Scientific, Inc.) for 48 h. siRNA oligonucleotides for OPRT
(Stealth RNAi; cat. no. 10620319-281527 A02 and 10620318-281527
A03) and negative control oligonucleotides (Stealth RNAi siRNA
Negative Control; cat. no. 452002) were purchased from Invitrogen
(Thermo Fisher Scientific, Inc.).
Results
Decreased OPRT caused decreased
formation of ternary complexes, leading to 5FU resistance
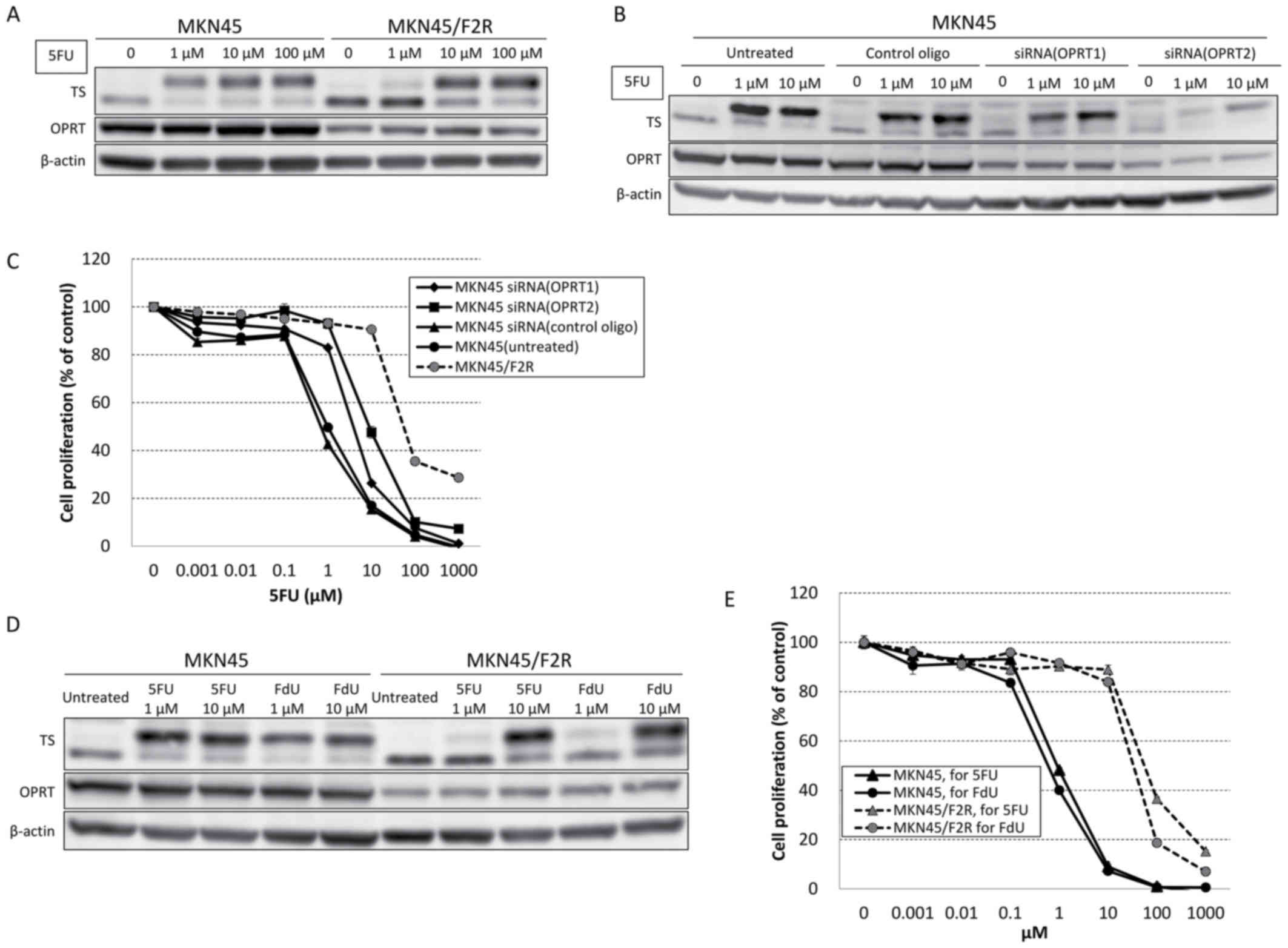
MKN45/F2R cells were previously established as
5FU-resistant cells with a demonstrated IC50 of
54.5±6.76 µM 5FU, representing a 56.2-fold increase in resistance
compared with parental MKN45 cells (IC50, 0.97±0.17 µM;
P<0.05) (17). A western blot
analysis demonstrated that the expression of TS was increased,
whereas the level of OPRT was decreased in MKN45/F2R cells compared
with MKN45 cells. Following treatment with 5FU (1, 10 or 100 µM)
for 24 h, the upper immunoreactive band of TS, which represents TS
in ternary complexes and is correlated with the intracellular
concentration of FdUMP (18), at 1 µM
5FU was decreased in MKN45/F2R cells compared with MKN45 cells
(Fig. 1A).
Following the transfection of two types of siRNA
against OPRT (OPRT 1 and 2) into MKN45 cells, a western blot
analysis demonstrated that the greater the extent of OPRT
knockdown, the greater the decrease in the upper band of TS at 1 µM
5FU (Fig. 1B). The results of an MTT
assay indicated an significantly increased 5FU IC50 in
MKN45 cells following siRNA transfection (OPRT1, 3.82±0.22 µM;
P<0.05); OPRT2, 8.85±2.93 µM; P<0.05; Fig. 1C), which was consistent with the
results of the western blot analysis. The results indicated that
decreased expression of OPRT may have caused a decrease in the
formation of ternary complexes, leading to 5FU resistance.
The formation of ternary complexes in
5FU-resistant cells decreased following treatment with 5FU or
FdU
Fluoro-deoxyuridine (FdU) may be converted to FdUMP
without OPRT and form ternary TS complexes (19). A western blot analysis revealed that
the upper band of TS at 1 µM of FdU in MKN45/F2R was decreased
compared with MKN45 cells, similar to the findings for 5FU
(Fig. 1D). An MTT assay demonstrated
there was a 56.1-fold increase in resistance to FdU
(IC50, 33.1±2.34 µM) compared with MKN45 cells
(IC50, 0.59±0.10 µM), which was similar to the extent of
5FU resistance (Fig. 1E). These
results indicated that the 5FU-resistant cells were able to reduce
the levels of intracellular FdUMP irrespective of decreased OPRT
levels.
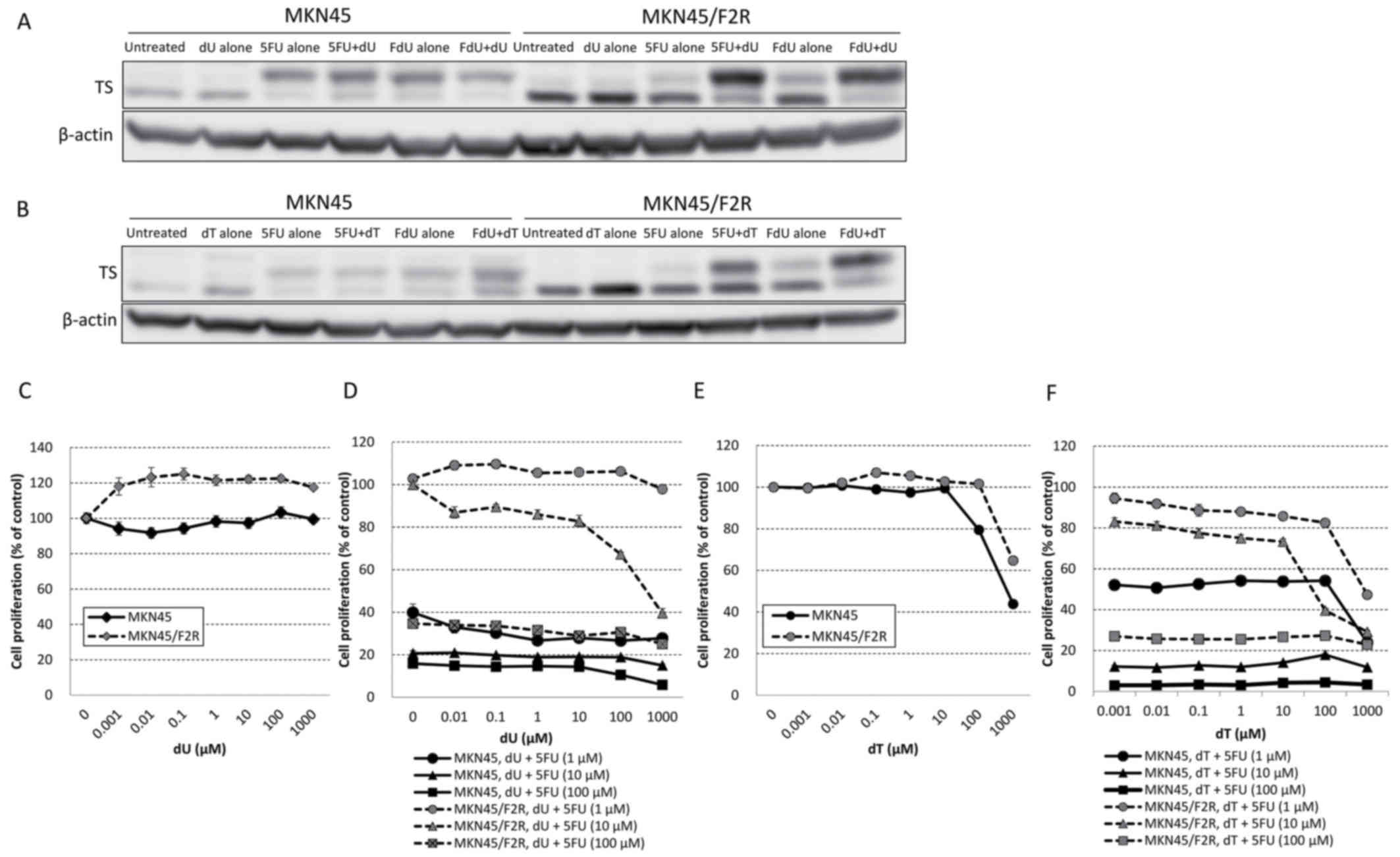
dU and dT promoted the formation of
ternary complexes and decreased 5FU resistance in MKN45/F2R
cells
High intracellular dUMP has been suggested to be the
causative factor for 5FU resistance, as dUMP may competitively
inhibit FdUMP (20). dTMP can also
inhibit TS inhibitors, as TS is the enzyme that catalyzes the
conversion of dUMP to dTMP. Therefore, dU and dT were used to
increase the intracellular concentrations of dUMP and dTMP and
investigate the influence of these molecules on 5FU.
A western blot analysis revealed that the
combination of 1,000 µM of dU with 1 µM of 5FU or FdU increased the
upper band of TS compared with 5FU or FdU alone (Fig. 2A), and similar results were observed
when 1,000 µM of dT was combined with 5FU or FdU (Fig. 2B). An MTT assay demonstrated that dU
alone did not have a cytotoxic effect on MKN45 or MKN45/F2R cells
(Fig. 2C), and the addition of dU did
not inhibit the cytotoxicity of 5FU; the addition of >1 µM of dU
actually decreased the 5FU resistance at 10 µM of 5FU in MKN45/F2R
cells (Fig. 2D). The combination of
dU with 5FU exhibited no effect in MKN45 cells (Fig. 2D). These results were consistent with
the results of the western blot analysis. Furthermore, dT alone
exerted a cytotoxic effect on MKN45 and MKN45/F2R cells
IC50 MKN45 cells, 671 µM; IC50 in MKN45/F2R
cells, >1,000 µM; Fig. 2E). The
addition of dT also did not inhibit the cytotoxicity of 5FU,
instead slightly decreasing the 5FU resistance at 10 µM of 5FU in
MKN45/F2R cells (Fig. 2F). These
results suggested that dU and dT did not inhibit the cytotoxicity
of 5FU, and may have reversed 5FU resistance in 5FU-resistant
cells.
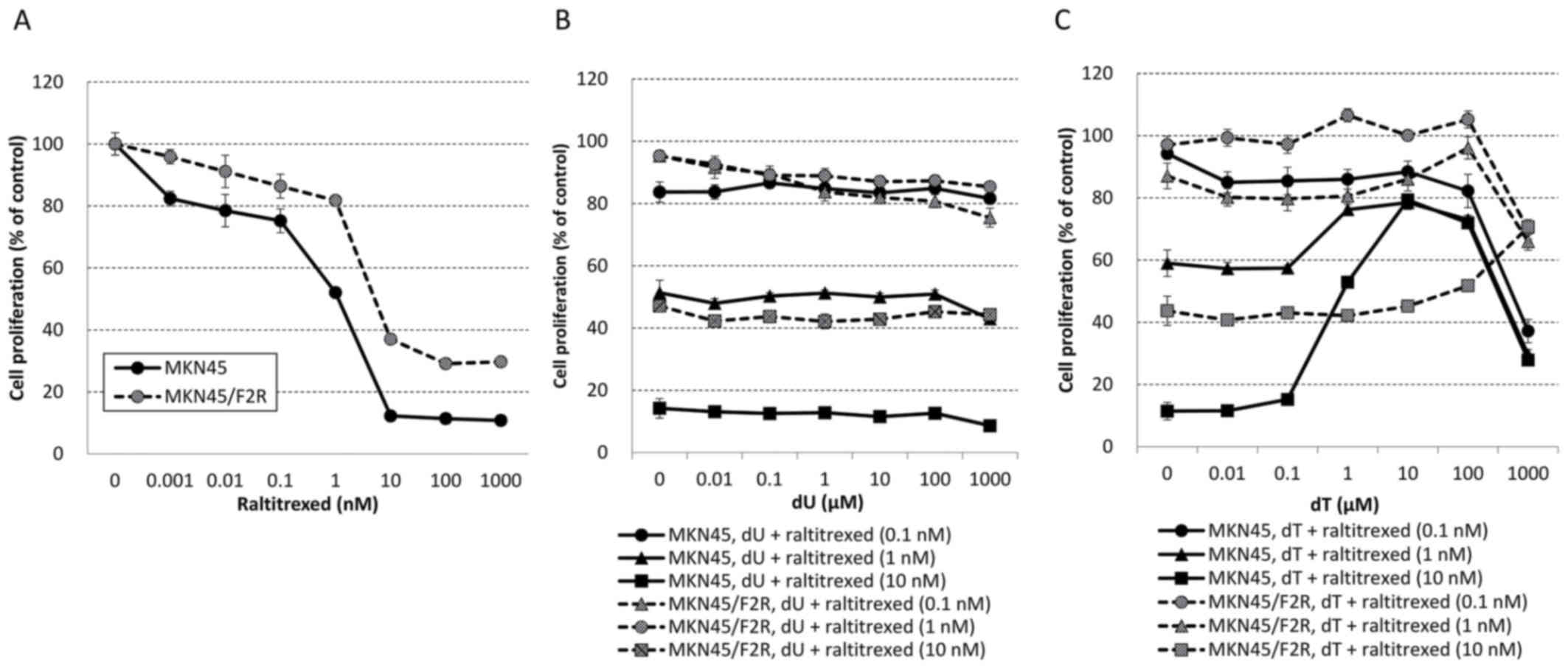
Thymidine decreased the cytotoxicity
of TS inhibitors
The cytotoxicity of TS inhibitors can be inhibited
by dT, as dT can be converted to dTMP without TS, a process known
as ‘thymidine rescue’ (21). As
aforementioned, 5FU was not inhibited by dT, although 5FU is
considered as a TS inhibitor. Therefore, dT treatment was combined
with raltitrexed, a specific TS inhibitor, to confirm if dT may
have inhibited TS inhibitors.
MKN45/F2R cells exhibited raltitrexed resistance and
the IC50s for raltitrexed alone in MKN45 and MKN45/F2R
cells were 1.12 and 5.13 nM, respectively (Fig. 3A). The addition of dU to raltitrexed
had no effect on MKN45 or MKN45/F2R cells (Fig. 3B), and the addition of dT inhibited
the cytotoxicity of raltitrexed at concentrations exceeding 0.1 µM
in MKN45 cells and exceeding 10 µM in MKN45/F2R cells (Fig. 3C). These results indicated that dT
exhibited the ability to inhibit TS inhibitors, and the cytotoxic
effect of 5FU is not mediated by ‘thymineless death’.
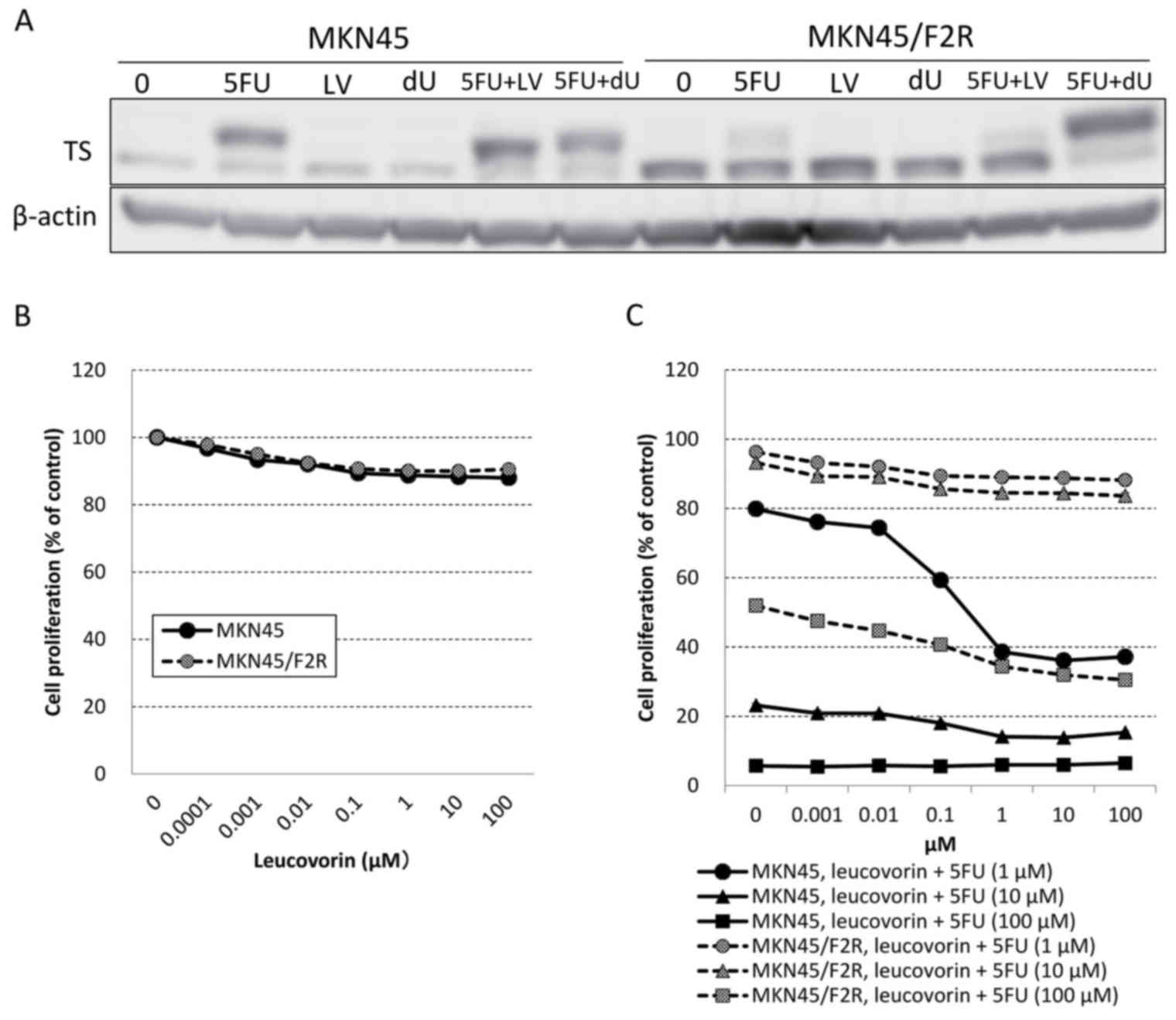
Leucovorin enhanced the cytotoxic
effect of 5FU in parental MKN45 cells
Leucovorin is easily metabolized to CH2THF, a
component of ternary complexes, and has been used to enhance the
efficacy of 5FU against colorectal cancer (22). To confirm the difference in the
mechanism between leucovorin and dU, leucovorin was combined with
5FU.
A western blot analysis demonstrated that the
addition of leucovorin to 5FU increased the upper band of TS in
parental MKN45 cells. However, it had no effect on MKN45/F2R cells
(Fig. 4A). An MTT assay demonstrated
that leucovorin alone did not have a cytotoxic effect on MKN45 or
MKN45/F2R cells (Fig. 4B), and
whereas combination of >0.01 µM of leucovorin increased the
cytotoxic effect of 5FU in parental MKN45 cells, there was no
effect on MKN45/F2R cells (Fig. 4C),
consistent with the results of the western blot analysis. These
results indicated that the mechanism for the reversal of 5FU
resistance by dU and dT differed from the mechanism for enhancing
the efficacy of 5FU by leucovorin. Furthermore, the results
confirmed that the cause of the decreased amount of ternary complex
in 5FU-resistant cells was unlikely to be a lack of CH2THF.
Discussion
In the present study, the mechanism of 5FU
resistance was investigated with a focus on the amount of TS
ternary complex, which correlates with the intracellular
concentration of FdUMP (16). It was
identified that 5FU-resistant cells may acquire the ability to
reduce intracellular FdUMP irrespective of decreased OPRT
levels.
A lack of dTTP causes ‘thymineless death’ (13); as 5FU inhibits TS by forming ternary
complexes, it has been hypothesized that the major mechanism of
action for 5FU is thymineless death caused by TS inhibition
(10). However, the data of the
present study suggest that cell death caused by 5FU was not induced
by thymineless death alone, as 5FU cytotoxicity could not be
inhibited by dT administration; dT actually somewhat decreased the
5FU resistance of 5FU-resistant cells.
OPRT has been reported as an important factor for
5FU resistance and there are a number of reports stating that
decreased OPRT levels cause 5FU resistance (17,23,24), which
does not contradict the results of the present study. OPRT is
required for the first step of 5FU activation; decreased OPRT
levels lead to decreased intracellular FdUMP. Therefore, decreased
OPRT levels lead to the reduced inhibition of TS. However, in the
present study, 5FU-resistant cells demonstrated decreased TS
ternary complex formation following treatment not only with 5FU,
but also with FdU, which can be converted to FdUMP without OPRT.
Therefore, the resistant cells may have the ability to reduce
intracellular FdUMP irrespective of decreased OPRT levels.
In the present study, dT slightly decreased 5FU
resistance whereas dU greatly decreased 5FU resistance. There have
been previous reports describing the enhanced cytotoxicity of 5FU
due to deoxyribonucleosides, including dT and dU (25,26). In
these reports, the mechanism of this enhancement was speculated to
be the inhibition of ribonucleoside reductase (RR) by a high
concentration of deoxyribonucleosides, leading to an increase in
the incorporation of 5FU into RNA. However, the data of the present
study revealed that 5FU-resistant cells exhibited resistance to
FdU, which is not easily incorporated into RNA, similar to the
extent of 5FU resistance, suggesting that the incorporation into
RNA is not of high importance in 5FU resistance. Furthermore, dT
and dU appeared to inhibit the decrease in intracellular FdUMP in
5FU-resistant cells, as the addition of dT and dU increased the
ternary complex formation following treatment with 5FU or FdU.
To our knowledge, there have been no previous
reports describing a direct association between the ability of
cells to decrease intracellular FdUMP concentration and 5FU
resistance. We speculate that a potential mechanism for reducing
FdUMP may be dephosphorylation by 5′-nucleotidases and
phosphatases. The dephosphorylation of nucleosides, an important
component of nucleoside analog metabolism, and the overexpression
of nucleotidases is associated with an increased IC50
for the nucleoside analogs cytosine arabinoside and
chloro-deoxyadenosin (27). The
overexpression of nucleotidase may also be associated with an
increased IC50 for 5FU (27), but the association of the
dephosphorylation of FdUMP with 5FU resistance has not been
clarified. The present study hypothesized that a large amount of
deoxyribonucleosides, such as dU and dT, may inhibit the activity
of nucleotidases and phosphatases; this mechanism may be involved
in the reversal of 5FU resistance.
In conclusion, it was identified in the present
study that a reduction in the intracellular FdUMP level is likely
to be a major mechanism involved in the resistance to 5FU, and this
reduction could be inhibited by dT or dU treatment. Further
investigations, regarding the mechanism underlying the decrease in
intracellular FdUMP and the contribution of nucleotidase to 5FU
resistance, are required. The significance of these mechanisms also
requires confirmation in a clinical setting.
Acknowledgements
This study was supported by Grants-in-Aid for
Scientific Research (C) from the Ministry of Education, Culture,
Sports, Science and Technology (grant no. 26461973), and Taiho
Pharmaceutical Co., Ltd. The authors wish to disclose the following
potential conflicts of interest: Dr Kazuhiro Yoshida has received
grants and personal fees from Taiho Pharmaceutical Co., Ltd.,
Pfizer Inc., Chugai Pharmaceutical Co., Ltd., and Yakult Honsha
Co., Ltd.; grants from Bristol-Myers Squibb and Kyowa Hakko Kirin
Co., Ltd.; honoraria from Pfizer Inc., Chugai Pharmaceutical Co.,
Ltd., Kyowa Hakko Kirin Co., Ltd., Taiho Pharmaceutical Co., Ltd.
and Yakult Honsha Co., Ltd., and has had a consultant or advisory
relationship with La Roche, Ltd. and Taiho Pharmaceutical Co.,
Ltd.
Glossary
Abbreviations
Abbreviations:
|
5FU
|
5-fluorouracil
|
|
FdUMP
|
5-fluoro-deoxyuridine
monophosphate
|
|
dU
|
deoxyuridine
|
|
dUMP
|
deoxyuridine monophosphate
|
|
dTMP
|
thymidine monophosphate
|
|
dT
|
thymidine
|
|
TS
|
thymidylate synthase
|
|
OPRT
|
orotate phosphoribosyltransferase
|
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Sakuramoto S, Sasako M, Yamaguchi T,
Kinoshita T, Fujii M, Nashimoto A, Furukawa H, Nakajima T, Ohashi
Y, Imamura H, et al: Adjuvant chemotherapy for gastric cancer with
S-1, an oral fluoropyrimidine. N Engl J Med. 357:1810–1820. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Sasako M, Sakuramoto S, Katai H, Kinoshita
T, Furukawa H, Yamaguchi T, Nashimoto A, Fujii M, Nakajima T and
Ohashi Y: Five-year outcomes of a randomized phase III trial
comparing adjuvant chemotherapy with S-1 versus surgery alone in
stage II or III gastric cancer. J Clin Oncol. 29:4387–4393. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Tsuburaya A, Yoshida K, Kobayashi M,
Yoshino S, Takahashi M, Takiguchi N, Tanabe K, Takahashi N, Imamura
H, Tatsumoto N, et al: Sequential paclitaxel followed by tegafur
and uracil (UFT) or S-1 versus UFT or S-1 monotherapy as adjuvant
chemotherapy for T4a/b gastric cancer (SAMIT): A phase 3 factorial
randomised controlled trial. Lancet Oncol. 15:886–893. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Koizumi W, Narahara H, Hara T, Takagane A,
Akiya T, Takagi M, Miyashita K, Nishizaki T, Kobayashi O, Takiyama
W, et al: S-1 plus cisplatin versus S-1 alone for first-line
treatment of advanced gastric cancer (SPIRITS trial): A phase III
trial. Lancet Oncol. 9:215–221. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Koizumi W, Kim YH, Fujii M, Kim HK,
Imamura H, Lee KH, Hara T, Chung HC, Satoh T, Cho JY, et al:
Addition of docetaxel to S-1 without platinum prolongs survival of
patients with advanced gastric cancer: A randomized study (START).
J Cancer Res Clin Oncol. 140:319–328. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Kufe DW and Major PP: 5-Fluorouracil
incorporation into human breast carcinoma RNA correlates with
cytotoxicity. J Biol Chem. 256:9802–9805. 1981.PubMed/NCBI
|
|
8
|
Saito K, Nagashima H, Noguchi K, Yoshisue
K, Yokogawa T, Matsushima E, Tahara T and Takagi S: First-in-human,
phase I dose-escalation study of single and multiple doses of a
first-in-class enhancer of fluoropyrimidines, a dUTPase inhibitor
(TAS-114) in healthy male volunteers. Cancer Chemother Pharmacol.
73:577–583. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Park JG, Collins JM, Gazdar AF, Allegra
CJ, Steinberg SM, Greene RF and Kramer BS: Enhancement of
fluorinated pyrimidine-induced cytotoxicity by leucovorin in human
colorectal carcinoma cell lines. J Natl Cancer Inst. 80:1560–1564.
1988. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Longley DB, Harkin DP and Johnston PG:
5-fluorouracil: Mechanisms of action and clinical strategies. Nat
Rev Cancer. 3:330–338. 2003. View
Article : Google Scholar : PubMed/NCBI
|
|
11
|
Goodsell DS: The molecular perspective:
Methotrexate. Oncologist. 4:340–341. 1999.PubMed/NCBI
|
|
12
|
Rose MG, Farrell MP and Schmitz JC:
Thymidylate synthase: A critical target for cancer chemotherapy.
Clin Colorectal Cancer. 1:220–229. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Ahmad SI, Kirk SH and Eisenstark A:
Thymine metabolism and thymineless death in prokaryotes and
eukaryotes. Annu Rev Microbiol. 52:591–625. 1998. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Peters GJ, Laurensse E, Leyva A, Lankelma
J and Pinedo HM: Sensitivity of human, murine and rat cells to
5-fluorouracil and 5′-deoxy-5-fluorouridine in relation to
drug-metabolizing enzymes. Cancer Res. 46:20–28. 1986.PubMed/NCBI
|
|
15
|
Sasako M, Terashima M, Ichikawa W, Ochiai
A, Kitada K, Kurahashi I, Sakuramoto S, Katai H, Sano T and Imamura
H: Impact of the expression of thymidylate synthase and
dihydropyrimidine dehydrogenase genes on survival in stage II/III
gastric cancer. Gastric Cancer. 18:538–548. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Patel K, Yerram SR, Azad NA and Kern SE: A
thymidylate synthase ternary complex-specific antibody, FTS,
permits functional monitoring of fluoropyrimidines dosing.
Oncotarget. 3:678–685. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Tsutani Y, Yoshida K, Sanada Y, Wada Y,
Konishi K, Fukushima M and Okada M: Decreased orotate
phosphoribosyltransferase activity produces 5-fluorouracil
resistance in a human gastric cancer cell line. Oncol Rep.
20:1545–1551. 2008.PubMed/NCBI
|
|
18
|
Drake JC, Allegra CJ and Johnston PG:
Immunological quantitation of thymidylate
synthase-FdUMP-5,10-methylenetetrahydrofolate ternary complex with
the monoclonal antibody TS 106. Anticancer Drugs. 4:431–435. 1993.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Levin RD and Gordon JH: Fluorodeoxyuridine
with continuous leucovorin infusion. A phase II clinical trial in
patients with metastatic colorectal cancer. Cancer. 72:2895–2901.
1993. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Myers CE, Young RC and Chabner BA:
Biochemical determinants of 5-fluorouracil response in vivo. The
role of deoxyuridylate pool expansion. J Clin Invest. 56:1231–1238.
1975. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Howell SB, Ensminger WD, Krishan A and
Frei E III: Thymidine rescue of high-dose methotrexate in humans.
Cancer Res. 38:325–330. 1978.PubMed/NCBI
|
|
22
|
Francini G, Petrioli R, Lorenzini L,
Mancini S, Armenio S, Tanzini G, Marsili S, Aquino A, Marzocca G,
Civitelli S, et al: Folinic acid and 5-fluorouracil as adjuvant
chemotherapy in colon cancer. Gastroenterology. 106:899–906. 1994.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Kodera Y, Ito S, Fujiwara M, Mochizuki Y,
Nakayama G, Ohashi N, Koike M, Yamamura Y and Nakao A: Gene
expression of 5-fluorouracil metabolic enzymes in primary gastric
cancer: Correlation with drug sensitivity against 5-fluorouracil.
Cancer Lett. 252:307–313. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Isshi K, Sakuyama T, Gen T, Nakamura Y,
Kuroda T, Katuyama T and Maekawa Y: Predicting 5-FU sensitivity
using human colorectal cancer specimens: Comparison of tumor
dihydropyrimidine dehydrogenase and orotate phosphoribosyl
transferase activities with in vitro chemosensitivity to 5-FU. Int
J Clin Oncol. 7:335–342. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Santelli G and Valeriote F: In vivo
potentiation of 5-fluorouracil cytotoxicity against AKR leukemia by
purines, pyrimidines, and their nucleosides and deoxynucleosides. J
Natl Cancer Inst. 64:69–72. 1980.PubMed/NCBI
|
|
26
|
Spiegelman S, Sawyer R, Nayak R, Ritzi E,
Stolfi R and Martin D: Improving the anti-tumor activity of
5-fluorouracil by increasing its incorporation into RNA via
metabolic modulation. Proc Natl Acad Sci USA. 77:pp. 4966–4970.
1980; View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hunsucker SA, Spychala J and Mitchell BS:
Human cytosolic 5′-nucleotidase I: Characterization and role in
nucleoside analog resistance. J Biol Chem. 276:10498–10504. 2001.
View Article : Google Scholar : PubMed/NCBI
|