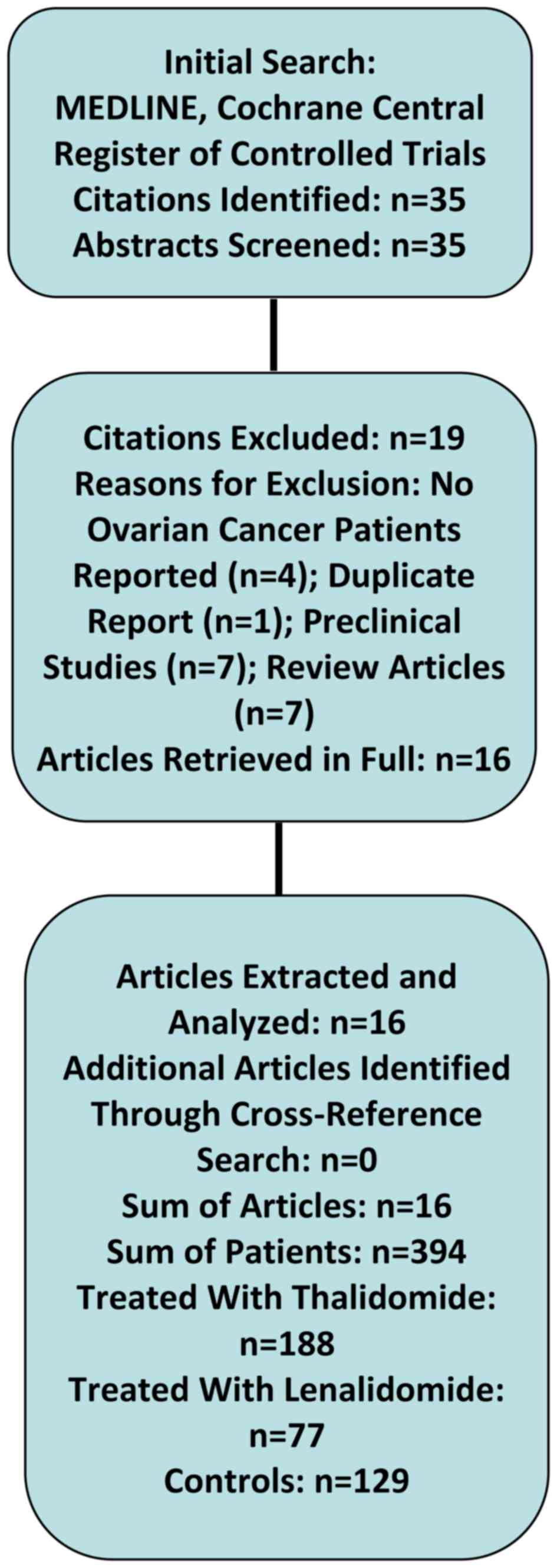
|
1
|
Malvezzi M, Carioli G, Rodriguez T, Negri
E and La Vecchia C: Global trends and predictions in ovarian cancer
mortality. Ann Oncol. 27:2017–2025. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Foley OW, Rauh-Hain JA and del Carmen MG:
Recurrent epithelial ovarian cancer: An update on treatment.
Oncology (Williston Park). 27:288–294, 298. 2003.
|
|
3
|
Wu YS, Shui L, Shen D and Chen X:
Bevacizumab combined with chemotherapy for ovarian cancer: An
updated systematic review and meta-analysis of randomized
controlled trials. Oncotarget. 8:10703–10713. 2017.PubMed/NCBI
|
|
4
|
Oza AM, Cook AD, Pfisterer J, Embleton A,
Ledermann JA, Pujade-Lauraine E, Kristensen G, Carey MS, Beale P,
Cervantes A, et al: Standard chemotherapy with or without
bevacizumab for women with newly diagnosed ovarian cancer (ICON7):
Overall survival results of a phase 3 randomised trial. Lancet
Oncol. 16:928–936. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Burger RA, Brady MF, Bookman MA, Fleming
GF, Monk BJ, Huang H, Mannel RS, Homesley HD, Fowler J, Greer BE,
et al: Incorporation of bevacizumab in the primary treatment of
ovarian cancer. N Engl J Med. 365:2473–2483. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aghajanian C, Blank SV, Goff BA, Judson
PL, Teneriello MG, Husain A, Sovak MA, Yi J and Nycum LR: OCEANS: A
randomized, double-blind, placebo-controlled phase III trial of
chemotherapy with or without bevacizumab in patients with
platinum-sensitive recurrent epithelial ovarian, primary
peritoneal, or fallopian tube cancer. J Clin Oncol. 30:2039–2045.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Pujade-Lauraine E, Hilpert F, Weber B,
Reuss A, Poveda A, Kristensen G, Sorio R, Vergote I, Witteveen P,
Bamias A, et al: Bevacizumab combined with chemotherapy for
platinum resistant recurrent ovarian cancer: The AURELIA open-label
randomized phase III trial. J Clin Oncol. 32:1302–1308. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
du Bois A, Floquet A, Kim JW, Rau J, del
Campo JM, Friedlander M, Pignata S, Fujiwara K, Vergote I, Colombo
N, et al: Incorporation of pazopanib in maintenance therapy of
ovarian cancer. J Clin Oncol. 32:3374–3382. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
du Bois A, Kristensen G, Ray-Coquard I,
Reuss A, Pignata S, Colombo N, Denison U, Vergote I, Del Campo JM,
Ottevanger P, et al: Standard first-line chemotherapy with or
without nintedanib for advanced ovarian cancer (AGO-OVAR 12): A
randomised, double blind, placebo-controlled phase 3 trial. Lancet
Oncol. 17:78–89. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Wang X, Shen Y, Li S, Lv M, Zhang X and
Yang J, Wang F and Yang J: Importance of the interaction between
immune cells and tumor vasculature mediated by thalidomide in
cancer treatment (Review). Int J Mol Med. 38:1021–1029.
2016.PubMed/NCBI
|
|
11
|
Sherbet GV: Therapeutic potential of
thalidomide and its analogues in the treatment of cancer.
Anticancer Res. 35:5767–5772. 2015.PubMed/NCBI
|
|
12
|
Bauer JA, Morrison BH, Grane RW, Jacobs
BS, Borden EC and Lindner DJ: IFN-alpha2b and thalidomide
synergistically inhibit tumor-induced angiogenesis. J Interferon
Cytokine Res. 23:3–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Vargesson N: Thalidomide-induced limb
defects: Resolving a 50-year-old puzzle. Bioessays. 31:1327–1336.
2009. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
McBride W: Thalidomide and congenital
malformations. Lancet. 1:3581961.PubMed/NCBI
|
|
15
|
Connors T: Anticancer drug development:
The way forward. Oncologist. 1:180–181. 1996.PubMed/NCBI
|
|
16
|
Bramuzzo M, Ventura A, Martelossi S and
Lazzerini M: Thalidomide for inflammatory bowel disease: Systematic
review. Medicine (Baltimore). 95:e42392016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Milanovic D, Sticht C, Röhrich M, Maier P,
Grosu AL and Herskind C: Inhibition of 13-cis retinoic acid-induced
gene expression of reactive-resistance genes by thalidomide in
glioblastoma tumours in vivo. Oncotarget. 6:28938–28948. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Liang PC, Ch'ang HJ, Hsu C, Chen LT, Shih
TT and Liu TW: Perfusion parameters of dynamic contrast-enhanced
magnetic resonance imaging predict outcomes of hepatocellular
carcinoma receiving radiotherapy with or without thalidomide.
Hepatol Int. 9:258–268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Gao M, Kong Y, Wang H, Xie B, Yang G, Gao
L, Zhang Y, Zhan F, Dai B, Tao Y and Shi J: Thalidomide treatment
for patients with previously untreated multiple myeloma: A
meta-analysis of randomized controlled trials. Tumour Biol.
37:11081–11098. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guirguis AA and Ebert BL: Lenalidomide:
Deciphering mechanisms of action in myeloma, myelodysplastic
syndrome and beyond. Curr Opin Cell Biol. 37:61–67. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Galustian C and Dalgleish A: Lenalidomide:
A novel anticancer drug with multiple modalities. Expert Opin
Pharmacother. 10:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kanwar VS, Heath J, Krasner CN and Pearce
JM: Advanced small cell carcinoma of the ovary in a
seventeen-year-old female, successfully treated with surgery and
multi-agent chemotherapy. Pediatr Blood Cancer. 50:1060–1062. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Phippen NT and Leath CA III: Weekly
topotecan and daily thalidomide in patients with recurrent
epithelial ovarian cancer: A report of 2 cases. J Reprod Med.
54:583–586. 2009.PubMed/NCBI
|
|
24
|
Buttin BM and Moore MJ:
Thalidomide-induced reversible interstitial pneumonitis in a
patient with recurrent ovarian cancer. Gynecol Oncol. 111:546–548.
2008. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Braun AG and Weinreb SL: Teratogen
metabolism: Spontaneous decay products of thalidomide and
thalidomide analogues are not bioactivated by liver microsomes.
Teratog Carcinog Mutagen. 5:149–158. 1985. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Braun AG and Dailey JP: Thalidomide
metabolite inhibits tumor cell attachment to concanavalin A coated
surfaces. Biochem Biophys Res Commun. 98:1029–1034. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Bauer JA, Morrison BH, Grane RW, Jacobs
BS, Borden EC and Lindner DJ: IFN-alpha2b and thalidomide
synergistically inhibit tumor-induced angiogenesis. J Interferon
Cytokine Res. 23:3–10. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Kobayashi H, Yagyu T, Kondo T, Kurita N,
Inagaki K, Haruta S, Kawaguchi R, Kitanaka T, Sakamoto Y, Yamada Y,
et al: Suppression of urokinase receptor expression by thalidomide
is associated with inhibition of nuclear factor kappaB activation
and subsequently suppressed ovarian cancer dissemination. Cancer
Res. 65:10464–10471. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Li W, Peng ZL and Cao ZY: Study of
thalidomide on the growth and angiogenesis of ovary cancer SKOV3
transplanted subcutaneously in nude mice. Zhonghua Fu Chan Ke Za
Zhi. 40:186–189. 2005.(In Chinese). PubMed/NCBI
|
|
30
|
Strese S, Fryknäs M, Larsson R and Gullbo
J: Effects of hypoxia on human cancer cell line chemosensitivity.
BMC Cancer. 13:3312013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Piura B, Medina L, Rabinovich A, Dyomin V
and Huleihel M: Thalidomide distinctly affected TNF-8.5, IL-6 and
MMP secretion by an ovarian cancer cell line (SKOV-3) and primary
ovarian cancer cells. Eur Cytokine Netw. 24:122–129.
2013.PubMed/NCBI
|
|
32
|
Eisen TG: Thalidomide in solid tumors: The
London experience. Oncology (Williston Park). 14:(12 Suppl 13).
S17–S20. 2000.
|
|
33
|
Eleutherakis-Papaiakovou V, Bamias A and
Dimopoulos MA: Thalidomide in cancer medicine. Ann Oncol.
15:1151–1160. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Ma WW and Jimeno A: Strategies for
suppressing angiogenesis in gynecological cancers. Drugs Today
(Barc). 43:259–273. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Galustian C and Dalgleish A: Lenalidomide:
A novel anticancer drug with multiple modalities. Expert Opin
Pharmacother. 10:125–133. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Malayev Y, Levene R and Gonzalez F:
Palliative chemotherapy for malignant ascites secondary to ovarian
cancer. Am J Hosp Palliat Care. 29:515–521. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Gadducci A, Sergiampietri C and Guiggi I:
Antiangiogenic agents in advanced, persistent or recurrent
endometrial cancer: A novel treatment option. Gynecol Endocrinol.
29:811–816. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Benesch M, Lackner H, Pilhatsch A,
Gürtl-Lackner B, Schwinger W and Urban C: Long-term remission in a
female with multiple relapsed juvenile granulosa cell tumor. J
Pediatr Hematol Oncol. 37:e486–e489. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Jeyakumar A, Chalas E and Hindenburg A:
Sustained complete remission in a patient with platinum-resistant
ovarian yolk sac tumor. Gynecol Oncol. 82:578–580. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Eisen T, Boshoff C, Mak I, Sapunar F,
Vaughan MM, Pyle L, Johnston SR, Ahern R, Smith IE and Gore ME:
Continuous low dose Thalidomide: A phase II study in advanced
melanoma, renal cell, ovarian and breast cancer. Br J Cancer.
82:812–817. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Abramson N, Stokes PK, Luke M, Marks AR
and Harris JM: Ovarian and papillary-serous peritoneal carcinoma:
Pilot study with thalidomide. J Clin Oncol. 20:1147–1149. 2002.
View Article : Google Scholar : PubMed/NCBI
|
|
42
|
Gordinier ME, Dizon DS, Weitzen S,
Disilvestro PA, Moore RG and Granai CO: Oral thalidomide as
palliative chemotherapy in women with advanced ovarian cancer. J
Palliat Med. 10:61–66. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
43
|
Zhang MM, Chan JK, Husain A, Guo HY and
Teng NN: Safety and efficacy of lenalidomide (Revlimid) in
recurrent ovarian and primary peritoneal carcinoma. Gynecol Oncol.
105:194–198. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
44
|
Chan JK, Manuel MR, Ciaravino G, Cheung
MK, Husain A and Teng NN: Safety and efficacy of thalidomide in
recurrent epithelial ovarian and peritoneal carcinoma. Gynecol
Oncol. 103:919–923. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
45
|
Downs LS Jr, Judson PL, Argenta PA, Ghebre
R, Geller MA, Bliss RL, Boente MP, Nahhas WA, Abu-Ghazaleh SZ, Chen
MD and Carson LF: A prospective randomized trial of thalidomide
with topotecan compared with topotecan alone in women with
recurrent epithelial ovarian carcinoma. Cancer. 112:331–339. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
46
|
Hurteau JA, Brady MF, Darcy KM, McGuire
WP, Edmonds P, Pearl ML, Ivanov I, Tewari KS, Mannel RS, Zanotti K
and Benbrook DM: Randomized phase III trial of tamoxifen versus
thalidomide in women with biochemical-recurrent-only epithelial
ovarian, fallopian tube or primary peritoneal carcinoma after a
complete response to first-line platinum/taxane chemotherapy with
an evaluation of serum vascular endothelial growth factor (VEGF): A
gynecologic oncology group study. Gynecol Oncol. 119:444–450. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
47
|
Carter JS and Downs LS Jr: A prospective
clinical trial of lenalidomide with topotecan in women with
advanced epithelial ovarian carcinoma. Int J Clin Oncol.
16:666–670. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
48
|
Muthuramalingam SR, Braybrooke JP, Blann
AD, Madhusudan S, Wilner S, Jenkins A, Han C, Kaur K, Perren T and
Ganesan TS: A prospective randomised phase II trial of thalidomide
with carboplatin compared with carboplatin alone as a first-line
therapy in women with ovarian cancer, with evaluation of potential
surrogate markers of angiogenesis. Eur J Gynaecol Oncol.
32:253–258. 2011.PubMed/NCBI
|
|
49
|
Ganesan P, Piha-Paul S, Naing A, Falchook
G, Wheler J, Fu S, Hong DS, Kurzrock R, Janku F, Laday S, et al:
Phase I clinical trial of lenalidomide in combination with
sorafenib in patients with advanced cancer. Invest New Drugs.
32:279–286. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
50
|
Selle F, Sevin E, Ray-Coquard I, Mari V,
Berton-Rigaud D, Favier L, Fabbro M, Lesoin A, Lortholary A and
Pujade-Lauraine E: A phase II study of lenalidomide in
platinum-sensitive recurrent ovarian carcinoma. Ann Oncol.
25:2191–2196. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
51
|
Tilluckdharry L, Dean R, Farver C and
Ahmad M: Thalidomide-related eosinophilic pneumonia: A case report
and brief literature review. Cases J. 1:1432008. View Article : Google Scholar : PubMed/NCBI
|
|
52
|
Gordinier ME and Dizon DS: Dyspnea during
thalidomide treatment for advanced ovarian cancer. Ann
Pharmacother. 39:962–965. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
53
|
Santi RM, Ceccarelli M, Catania G,
Monagheddu C, Evangelista A, Bernocco E, Monaco F, Federico M,
Vitolo U, Cortelazzo S, et al: PO-03 - Khorana score and histotype
predict the incidence of early venous thromboembolism (VTE) in non
hodgkin lymphoma (NHL). A pooled data analysis of twelve clinical
trials of fondazione italiana linfomi (FIL). Thromb Res. 140:(Suppl
1). S1772016. View Article : Google Scholar : PubMed/NCBI
|
|
54
|
Joerger M, Schaer-Thuer C, Koeberle D,
Matter-Walstra K, Gibbons-Marsico J, Diem S, Thuerlimann B and
Cerny T: Off-label use of anticancer drugs in eastern Switzerland:
A population-based prospective cohort study. Eur J Clin Pharmacol.
70:719–725. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
55
|
Dasanu CA, Mewawalla P and Grabska J:
Multiple myeloma and its therapies: to what extent do they
contribute to the increased incidence of second malignant
neoplasms? Curr Med Res Opin. 28:1129–1140. 2012. View Article : Google Scholar : PubMed/NCBI
|