Introduction
Gastric cancer (GC) is one of the most invasive and
aggressive malignancies and remains a major health problem
worldwide due to its high incidence and mortality rate (1). Despite a steady decline in GC incidence,
GC is currently the third highest cause of cancer-associated
mortality worldwide, with 730,000 patients succumbing to the
disease every year (2,3). Patient survival is primarily associated
with disease stage, and the cure rate largely depends upon surgical
resection. However, <5% of patients with advanced GC may survive
≤5 years and the role of surgery as a mainstay treatment is limited
to ~25% of all patients (4).
Chemotherapy has served a major role in the treatment of gastric
cancer over the past twenty years. The 5-fluorouracil (5′-FU) plus
cisplatin (DDP) regimen (FP) of chemotherapy consists of the
continuous infusion of 5-FU with low-dose DDP, which is typically
used to treat GC; however, the success rate of this treatment is
limited due to the development of chemoresistance and toxic side
effects. Therefore, a novel chemotherapy regimen that will improve
clinical outcomes is required for patients with GC.
One strategy to improve anticancer treatment
regimens may be to combine conventional chemotherapeutics with
natural antitumor compounds. Curcumin, also known as 1,7-bis
(4-hydroxy-3methoxyphenol)-1,6-heptadiene-3,5-dione, is obtained
and purified from turmeric (Curcuma longa), which belongs to
the Zingiberaceae plant family indigenous to southern and
southeastern tropical Asia (5). It
has been widely used as a spice, to color cheese and butter, as a
cosmetic and in certain medicinal preparations (6). The safety of Curcuma has been
investigated in various animal models, and it has been established
that turmeric is not toxic even at high doses (7). Previous studies have suggested that
curcumin has a number of pharmacological effects, including
anti-inflammatory, antioxidant and anticancer properties (8–10).
In the present study, the effects and underlying
molecular mechanisms of curcumin combined with the FP regimen of
chemotherapy were investigated in the MGC-803 human gastric cancer
cell line. The results may aid with developing novel treatment
strategies for patients with GC.
Materials and methods
Cell culture and reagents
MGC-803 cells were purchased from the Cell Bank of
Type Culture Collection of Chinese Academy of Sciences (Shanghai,
China). The cells were maintained in RPMI-1640 medium (HyClone; GE
Healthcare Life Sciences, Logan, UT, USA), supplemented with 2
mmol/l L-glutamine, 100 U/ml penicillin, 0.1 µg/ml streptomycin and
10% fetal bovine serum (FBS, Tianhang Biotechnology Co., Ltd.,
Zhejiang, China) at 37°C in a humidified atmosphere containing 5%
CO2. The culture medium was replaced once every two
days. Curcumin was purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). DDP was purchased from Qilu Pharmaceutical
Co., Ltd. (Shandong, China). The 5-FU was purchased from Tianjin
Jinyao Amino Acid Co., Ltd. (Tianjin, China).
Cell groups
There were six treatment groups used in the present
study, as follows: Control (curcumin or FP concentration at 0
µmol/l); CUR (15 µmol/l curcumin); CUR+LD FP [curcumin (15 µmol/l)
combined with low dose FP (25 µmol/l 5-FU + 1 µmol/l DDP)]; CUR+MD
FP [curcumin (15 µmol/l) combined with medium dose FP (50 µmol/l
5-FU + 2 µmol/l DDP)]; MD FP [medium dose FP (50 µmol/l 5-FU + 2
µmol/l DDP)] and HD FP [high dose FP (100 µmol/l 5-FU + 4 µmol/l
DDP)].
Cell viability assay
Cells were seeded in 96-well plates at a
concentration of 4×103 cells/well. Following incubation
for 12 h at 37°C, curcumin and/or low, medium or high dose FP at
the aforementioned concentrations were added. There were 8
duplicate wells for each group with a total volume of 200 µl/well.
Following treatment for 24, 48 and 72 h at 37°C in an atmosphere of
5% CO2, 20 µl MTT solution (Sigma-Aldrich; Merck KGaA)
at a concentration of 5 g/l was added to each well, then the plates
were incubated for 4 h. Dimethyl sulfoxide (DMSO; 150 µl;
Sigma-Aldrich; Merck KGaA) was added to each well prior to
agitation for 10 min at room temperature. A Model 680 microplate
reader (Bio-Rad Laboratories, Inc., Hercules, CA, USA) was used to
measure the absorbance at 570 nm. The inhibitory rate (%) was
calculated using the following equation: Inhibitory rate (%) =
[(1-optical density (OD)E / ODC)] ×100%.
ODE represents the OD value of the experimental group
with drug treatment; ODC represents the OD value of the
control group without drug treatment. The experiment was repeated
≥3 times.
Flow cytometry
Cells were seeded at a density of 4×105
cells/well in 6-well culture plates (Corning Incorporated, Corning,
NY, USA) for 24 h, and treated with/without curcumin and/or FP at
various concentrations for 24 h at 37°C in an atmosphere of 5%
CO2. Apoptosis was then analyzed by flow cytometry using
Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide
(PI; each purchased from Sungene Biotech Co., Ltd., Tianjin, China)
double staining. Prior to flow cytometry analysis, the cells were
collected, washed with cold PBS twice and resuspended gently in 400
µl binding buffer. Annexin V-FITC (5 µl) was added to the cells and
the samples were gently vortexed prior to incubation for 10 min at
4°C in the dark. PI (10 µl) was added and the samples were
incubated for another 5 min at 4°C in the dark. Flow cytometry was
then conducted using a FACSCalibur™ flow cytometer (BD Biosciences,
Franklin Lakes, NJ, USA), and the results were analyzed with BD
CellQuest™ software (version 5.1; BD Biosciences). The experiment
was repeated ≥3 times.
Cell cycle analysis was performed using PI (40
µg/ml) single staining with a flow cytometer. Prior to analysis,
cells were washed in PBS containing 2% FBS and the resulting pellet
was resuspended in DNase-free RNase (200 µg/ml, 0.5 ml) for 2 h at
37°C. Cells were then stained with PI and analyzed by flow
cytometry. The data were analyzed using ModFit LT™ software
(version 3.0; Verity Software House, Topsham, ME, USA) and
expressed as the percentage of cells in each phase of the cell
cycle. The experiment was repeated ≥3 times.
Dual acridine orange/ethidium bromide
(AO/EB) fluorescent staining
Cells were seeded at a density of 4×105
cells/well, cultured in 6-well culture plates (Corning
Incorporated) for 24 h and treated with/without curcumin and/or FP
at various concentrations for 24 h. The medium was removed. Trypsin
(0.25%; HyClone; GE Healthcare Life Sciences) was added into each
well. When the cells had detached, the suspensions (25 µl) were
transferred to glass slides. Dual fluorescent staining solution (1
µl) containing 100 µg/ml AO and 100 µg/ml EB (Sigma-Aldrich; Merck
KGaA) was added to each suspension and then covered with a
coverslip. The morphology of apoptotic cells was examined and 1,000
cells were counted within 20 min based on randomly chosen fields of
view using a fluorescent microscope (Nikon Corporation, Tokyo,
Japan). The apoptotic percentage was expressed as a ratio of the
number of apoptotic cells in the experimental groups (curcumin
and/or FP at low, medium or high dose) compared with that in the
untreated control group. The experiment was repeated ≥3 times.
Colony formation assay
The 6-well culture plates were seeded with ~500
viable MGC-803 cells and incubated at 37°C in an atmosphere of 5%
CO2 for 24 h. The cells were then incubated with various
concentrations of curcumin and FP at 37°C in an atmosphere of 5%
CO2 for 24 h. The curcumin and/or FP containing medium
was then removed, and the cells were washed in PBS and incubated
for an additional 10 days in complete medium. The colonies obtained
were washed with PBS and fixed in methanol-acetic acid (3:1)
stationary liquid for 10 min at room temperature, and then washed
with PBS followed by staining with Giemsa (10%; Sigma-Aldrich;
Merck KGaA). The colonies were counted and compared with in the
untreated control group. The inhibitory rate was expressed as a
ratio of the number of colonies in the experimental groups
(curcumin and/or FP at low, medium or high dose) compared with in
the untreated control group.
Transwell migration assay
A Transwell migration assay (Corning Incorporated)
was performed according to the manufacturer's protocol in 24-well
plates. MGC-803 cells were cultured in RPMI-1640 medium for 5 days,
then the cells (1×105) in 100 µl serum-free medium
with/without curcumin and/or FP at various concentrations were
seeded into the upper chamber of an 8-mm pore size Transwell
insert. The lower chambers in the system were filled with
Dulbecco's modified Eagle's medium (HyClone; GE Healthcare Life
Sciences, Logan, UT, USA) supplemented with 10% FBS. After 10 h of
incubation, non-migratory cells in the upper chamber were removed.
MTT solution (100 µl; 5 g/l) was added to each well, then the
24-well plate was incubated at 37°C in an atmosphere of 5%
CO2 for 4 h. DMSO (150 µl) was added to each well prior
to agitation for 10 min at room temperature. The absorbance was
measured using a microplate reader at 570 nm. The migration rate
(%) was calculated using the following equation: Migration rate (%
= (ODE / ODC) ×100%. The experiment was
repeated ≥3 times.
Determination of caspase-3 and
caspase-8 activity
MGC-803 cells were seeded in a 6-well culture plate
at a density of 4×106 cells/well, and treated with or
without curcumin and/or FP at the aforementioned concentrations for
24 h. The medium was removed, and the MGC-803 cells were washed
three times with PBS for 1 min each time, digested with Trypsin
(0.25%; HyClone; GE Healthcare Life Sciences), and collected by
centrifuging at 600 × g for 5 min at 4°C. The activities of
caspase-3 and caspase-8 were measured using a caspase-3 activity
assay kit and caspase-8 activity assay kit (Applygen Technologies
Inc., Beijing, China) according to the manufacturer's protocol.
Each sample was measured in triplicate. Relative caspase-3 activity
was expressed as the absorbance compared with the untreated control
group based on the following equation: Relative activity=OD of
experimental group/OD of control group.
Western blot analysis
Cells were seeded at a density of 4×106
cells/well in a 6-well culture plate and treated with or without
curcumin and/or FP at various concentrations for 24 h. A total of
1×106 cells/well were acquired by cell scraper, washed
with PBS and then suspended in 250 µl lysis buffer (pH 7.5, 1%
Triton-X-100, 40 mmol/l Tris-HCl, 150 mmol/l KCl, 1 mmol/l EDTA,
100 mmol/l NaVO3 and 1 mmol/l phenylmethylsulfonyl fluoride).
Following protein extraction and concentration detection (BCA
Protein Assay Kit;, Beyotime Biotechnology Co., Ltd., Haimen,
China), proteins were separated using SDS-PAGE and then transferred
to polyvinylidene fluoride membranes (EMD Millipore, Billerica, MA,
USA). The membranes were blocked with 5% skimmed milk. The
membranes were then incubated with antibodies against the apoptosis
regulator B-cell lymphoma-2 (Bcl-2; cat. no. 13CM357; dilution
1:1,000; Boster Biological Technology Co., Ltd., Wuhan, China),
Bcl-2-associated X protein (Bax; cat. no. 196841; dilution 1:1000;
Boster Biological Technology Co., Ltd.) and β-actin (cat. no.
E0610; dilution 1:1000; Kerui Biotechnology Co., Ltd., Wuhan,
China) in TBS-Tween containing 5% skimmed milk at 4°C for 12 h.
Following washing, the membranes were incubated with a horseradish
peroxidase immunoglobulin G antibody (cat. no. BST10F01A; dilution
1:10,000; Sungene Biotech Co., Ltd., Tianjin, China) at room
temperature for 1 h. The blots were developed using an enhanced
chemiluminescence kit (Thermo Fisher Scientific, Inc., Waltham, MA,
USA). Quantification of immunoblot intensity was performed using
ImageJ software (version 2.1.4.7; National Institutes of Health,
Bethesda, MD, USA). The experiment was repeated ≥3 times.
Statistical analysis
Data analyses were performed using SPSS software
(version 16.0; SPSS Inc., Chicago, IL, United States). Data are
presented as the mean ± standard deviation. Group comparisons were
evaluated using a one-way analysis of variance. Two-sided tests
were used to evaluate comparisons between two groups. P<0.05 was
considered to indicate a statistically significant difference.
Results
The effect of curcumin and/or FP on
the viability of MGC-803 cells
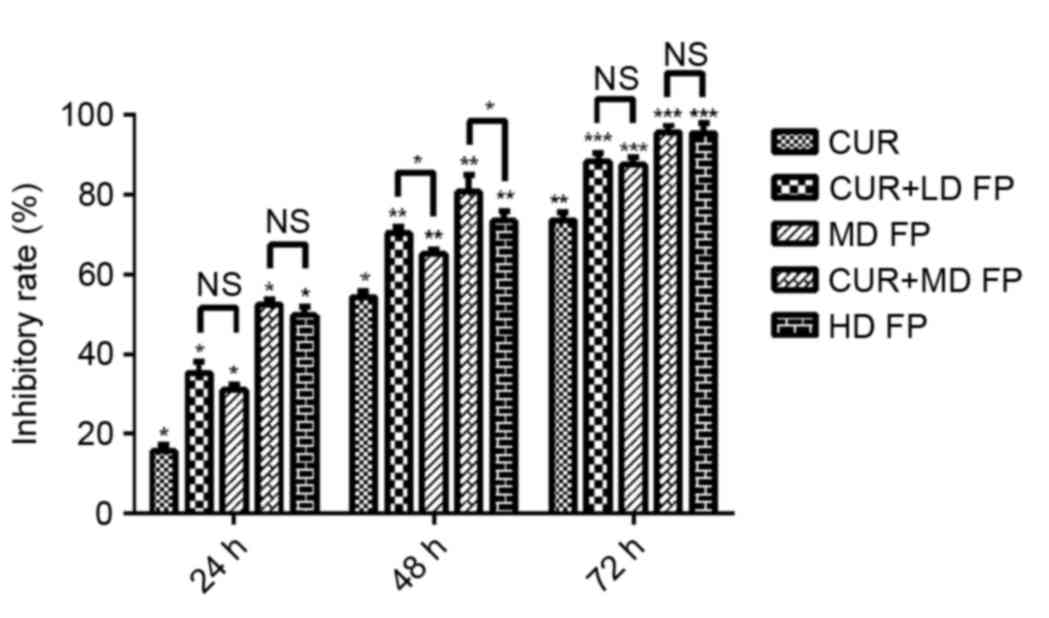
An MTT assay was used to examine the effect of
curcumin and FP on MGC-803 cell viability following drug treatment
for 24, 48 and 72 h. All treatments significantly decreased cell
viability compared with the untreated control (*P<0.05,
**P<0.01, ***P<0.005. Fig. 1).
Following drug treatment for 24 and 72 h, no significant
differences were observed between the MD FP and CUR+LD FP groups,
or between the HD FP and CUR+MD FP groups. However, treatment with
CUR+LD FP for 48 h significantly decreased cell viability compared
with the MD FP treatment group (P<0.05), and treatment with
CUR+MD FP for 48 h significantly decreased cell viability compared
with the HD FP group (P<0.05). Therefore, curcumin enhanced the
effects of FP on MGC-408 cell viability.
Effect of curcumin and/or FP on the
apoptosis and cell cycle of MGC-803 cells
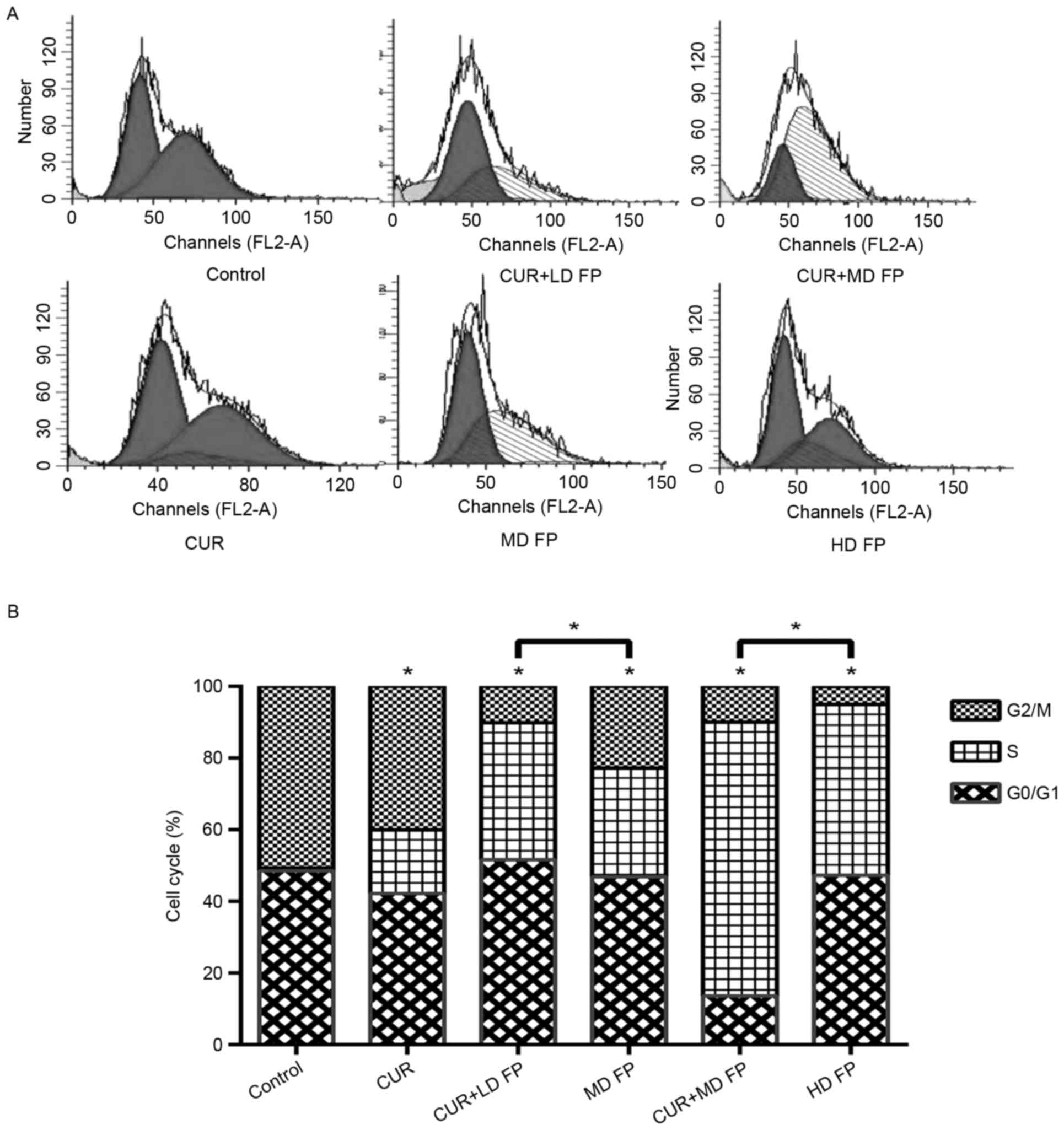
Flow cytometry was performed to investigate the
effect of curcumin and/or FP on the apoptosis and cell cycle of
MGC-803 cells. Compared with the controls, all drug treatments
significantly increased the percentage of apoptotic cells (Fig. 2A and B). The apoptotic percentages of
the combined groups were significantly higher than for the FP
treatment groups 24 h following treatment (P<0.01; CUR+LD FP vs.
MD FP; CUR+MD FP vs. HD FP). The apoptotic percentage was examined
using two staining methods (Annexin V-FITC/PI and dual AO/EB
staining). The results of dual AO/EB staining also demonstrated
that curcumin significantly increased FP-induced cell apoptosis
(P<0.05) and no significant differences were observed between
the apoptotic percentage measured by Annexin V-FITC/PI flow
cytometry and by AO/EB (P>0.05; Fig.
1A). These results demonstrated that curcumin increases the
level of FP-induced cell apoptosis.
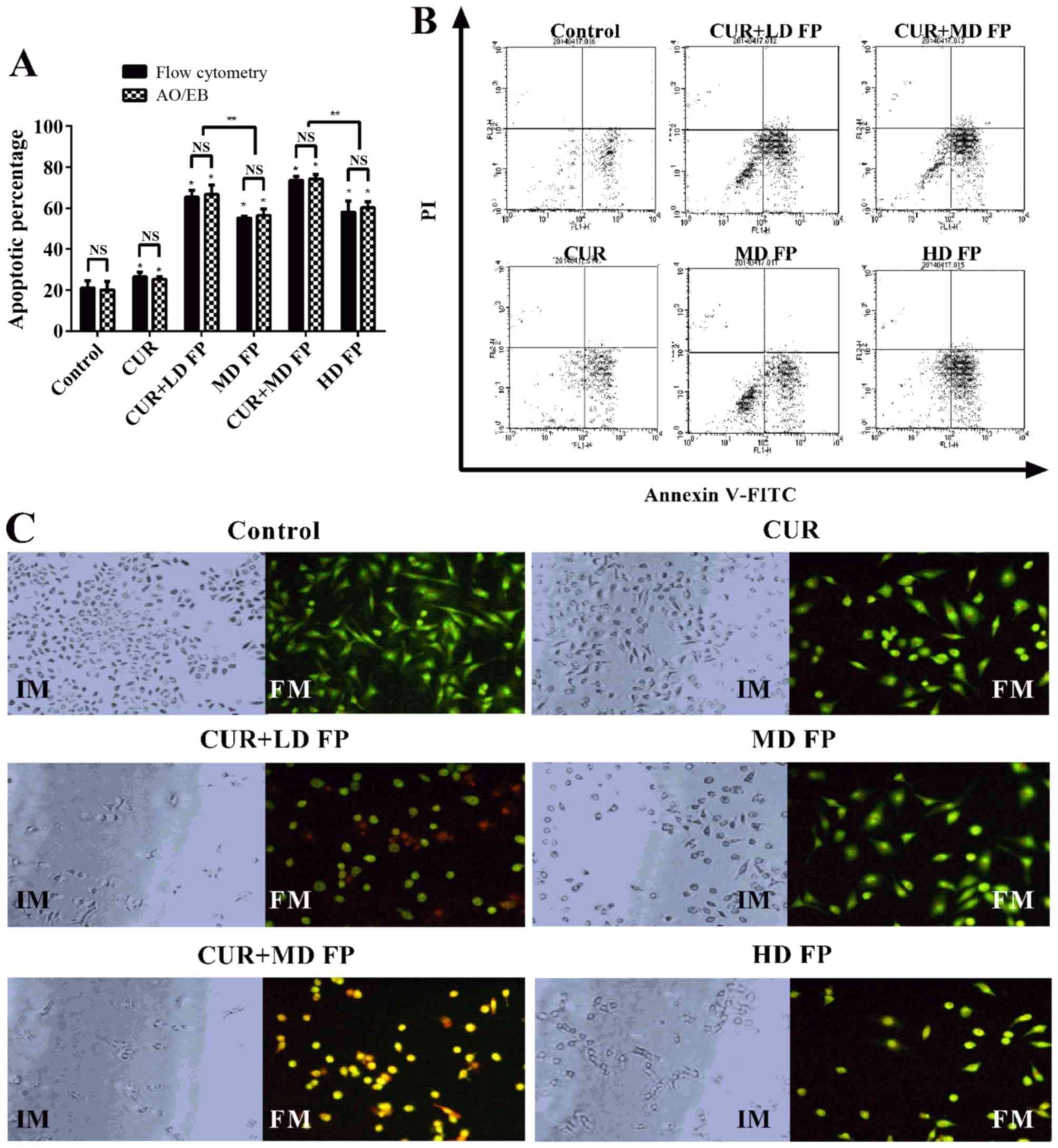 | Figure 2.Effect of curcumin and/or FP treatment
on MGC-803 cell apoptosis. (A) The percentage of apoptotic MGC-803
cells following treatment with curcumin and/or FP for 24 h,
measured using Annexin V-FITC/PI staining and dual AO/EB staining.
(B) Representative flow cytometry charts of Annexin V-FITC/PI
staining in untreated (control) MGC-803 cells and cells treated
with curcumin and/or FP. (C) Morphological changes, imaged with an
IM, and dual AO/EB staining, imaged with a FM, of MGC-803 cells
following treatment with curcumin and/or FP. Magnification, ×200.
Data are representative of ≥3 independent experiments. *P<0.05,
**P<0.01. FP, 5′-fluorouracil plus cisplatin; FITC, fluorescein
isothiocyanate; PI, propidium iodide; AO, acridine orange; EB,
ethidium bromide; CUR, curcumin; LD, low-dose; MD, low-dose; HD,
high-dose; IM, inverted microscope; FM, fluorescence
microscope. |
Following treatment with curcumin and/or FP for 24
h, there were marked changes in cellular morphology, including cell
shrinkage and nuclear fragmentation in the drug treatment groups,
compared with in the control group (Fig.
2C). For the non-apoptotic cells, the nucleus was circular and
uniformly distributed in the center of the cell. For early
apoptotic cells, the nucleus exhibited yellow-green fluorescence by
AO staining and concentrated into a crescent or granular nucleus
that was located on one side of the cell. For late apoptotic cells,
the nucleus exhibited orange fluorescence by EB staining, and, the
chromatin condensed and distributed around the nuclear membrane.
For necrotic cells, the cell volume was increased, and the nucleus
exhibited uneven orange-red fluorescence and an unapparent outline,
indicating it was disintegrating.
Cell cycle analysis demonstrated that there was a
change in S phase arrest in response to treatment with curcumin
and/or FP compared with the control group (Fig. 3). Treatment with FP alone resulted in
cell arrest at S phase (MD FP 30.32%, HD FP 47.77%), whereas
combined treatment resulted in a significant increase in the number
of cells arrested at the S phase, compared with the control group
and the FP treatment groups (CUR+LD FP 38.23%, CUR+MD FP 76.38%;
P<0.05; Fig. 3B).
Impact of curcumin and/or FP on the
colony formation and migration ability of MGC-803 cells
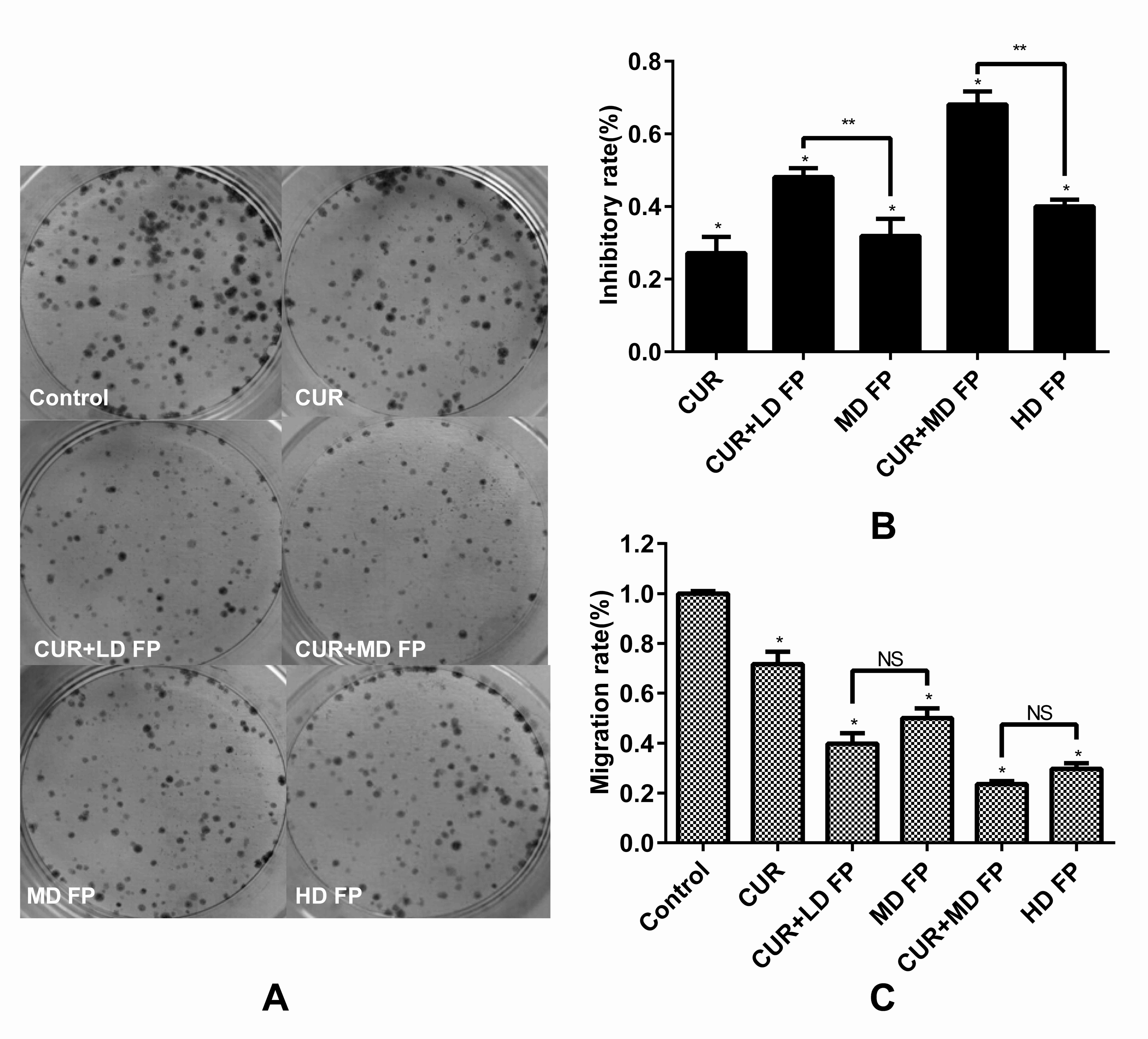
A colony formation assay demonstrated that the
proliferation rate and colony numbers of the MGC-803 cells treated
with curcumin and/or FP were significantly decreased compared with
the control group (P<0.05; Fig. 4A and
B). The inhibitory rate of the combined treatment group was
significantly higher compared with that of the FP treatment group
(CUR+LD FP 38.1% vs. MD FP 31.9%, CUR+MD FP 68.1% vs. HD FP 40%;
P<0.01; Fig. 4B). In addition, a
significantly lower number of cells migrated through the Transwell
filter when curcumin and/or FP were added into the migration
chamber, as compared with the untreated control group without
curcumin and FP (P<0.05; Fig. 4C).
No significant differences were observed in the migration rate of
the combined treatment group compared with that of the FP treatment
group (P>0.05; Fig. 4C). Overall,
curcumin enhanced the effects of FP treatment on cell viability,
and the colony formation and migration abilities of MGC-803
cells.
Effect of curcumin and/or FP on the
expression and activity of apoptosis-associated proteins in MGC-803
cells
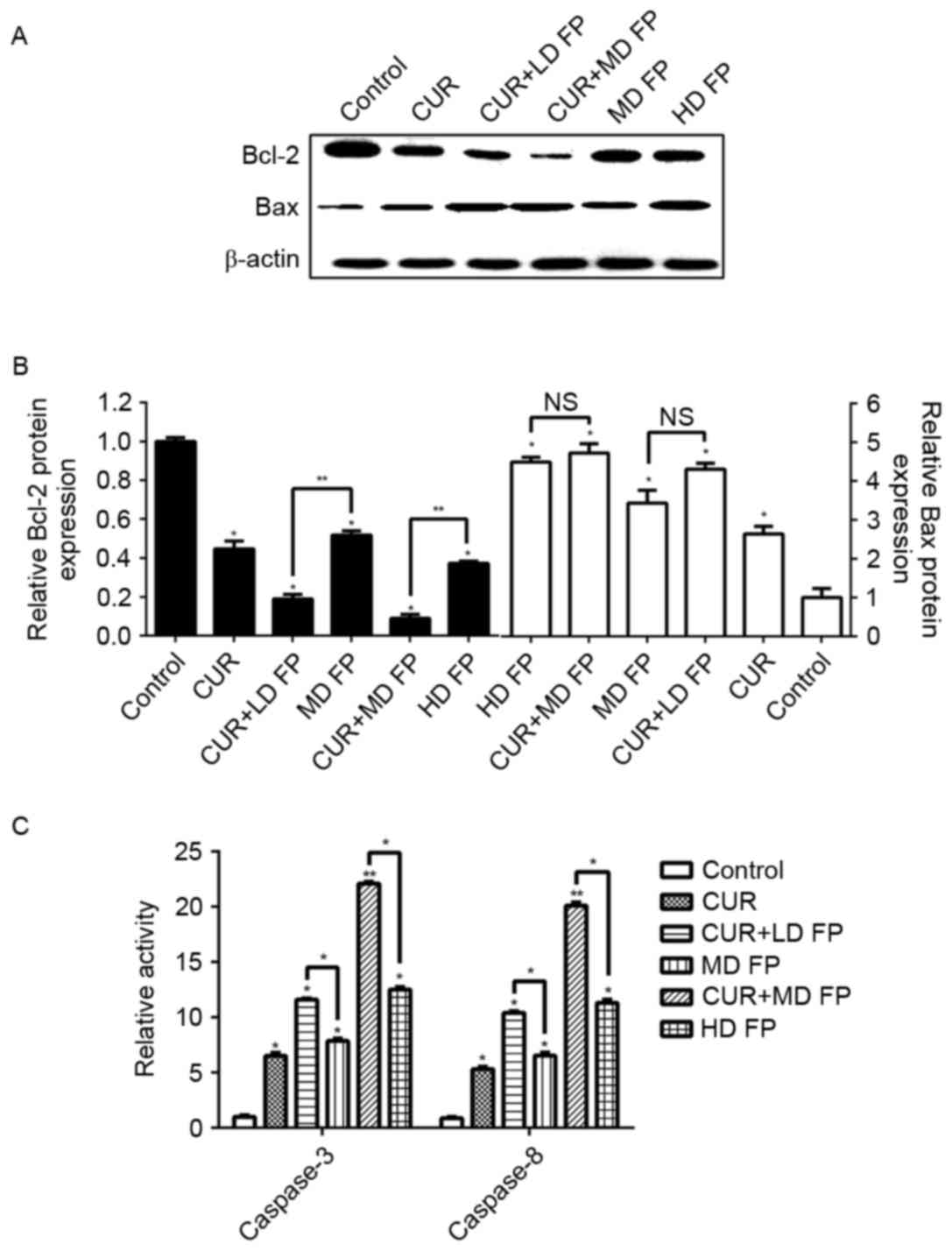
To investigate the underlying molecular mechanisms
of the effects of combined curcumin and FP treatment on MGC-803
apoptosis the expression and activity of apoptosis-associated
proteins was investigated using western blot analysis and activity
assay kits, respectively, including Bcl-2, Bax, caspase-3 and
caspase-8. Treatment with curcumin and/or FP significantly
decreased the expression of Bcl-2 and increased the expression of
Bax, compared with in the untreated control group (P<0.05;
Fig. 5A and B). Bcl-2 expression was
significantly higher in the combined treatment groups compared with
in the FP treatment groups (CUR+LD FP 0.19 vs. MD FP 0.52, CUR+MD
FP 0.09 vs. HD FP 0.37; P<0.01; Fig.
5B). However, no significant differences were observed in Bax
expression between the combined treatment groups and the FP
treatment groups.
The relative caspase-3 and caspase-8 activities of
the drug treatment groups with curcumin and/or FP were
significantly elevated compared with the untreated control group
without curcumin and FP (P<0.05, or P<0.01 for the CUR+MD FP
group; Fig. 5C). The relative
caspase-3 and caspase-8 activities of the CUR+MD FP group were
significantly higher compared with those of the HD FP group
(caspase-3: 22.1 vs. 12.5; caspase-8: 20.1 vs. 11.3; P<0.05;
Fig. 5C), whereas no significant
differences were observed between the caspase-3/caspase-8
activities of the CUR+LD FP group and the MD FP group (caspase-3:
11.6 vs. 7.86; caspase-8: 10.4 vs. 6.54; P>0.05; Fig. 5C). These data suggest that curcumin
enhances the apoptotic effects of FP treatment in MGC-803 cells via
the promotion of Bcl-2 and the inhibition of Bax, followed by
elevating the activation of caspase-3 and caspase-8.
Discussion
GC is one of the most common types of malignant
tumor (1). It has previously been
reported that, although >50% of patients diagnosed with GC
successfully undergo surgical tumor resection, 60% of those
patients subsequently present with local recurrence or distant
metastasis (11). Chemotherapy
remains an indispensable form of treatment, particularly for
patients with advanced stage GC. Regimens based on 5-FU and DDP
treatments have been a typical approach for patients with GC. The
synergistic effect between 5-FU and DDP was reported as early as
the end of the 1970s (12). FP as a
combination chemotherapy regimen was established for the treatment
of cancer, particularly for patients with advanced-stage cancer
(13,14). FP primarily exerts its cytotoxic
effects by inhibiting enzyme activity, preventing the synthesis of
DNA, blocking cell cycle progression and promoting apoptosis
(15). The effect of 5-FU is enhanced
by low-dose DDP. However, chemotherapy resistance and side effects
have become challenges for the treatment of cancer using this
method. Thus, it is essential to explore alternative effective
anticancer chemotherapy regimens.
Curcumin is a plant polyphenol extracted from the
spice turmeric (Curcuma longa), which is used as an herbal
remedy in traditional Chinese and Indian medicine (5). The efficacy, pharmacological safety and
cost effectiveness of curcumin and no observed toxicity make it an
ideal compound to investigate for its anticancer properties
(16,17). Curcumin has previously been reported
to possess anti-inflammatory, antioxidant, anticarcinogenic,
antimutagenic, anticoagulant, antiarthritic, antibacterial,
antifungal, antiprotozoal, antiviral, anti-Alzheimer,
anti-psoriatic and neuroprotective activities (18–20).
Curcumin exerts anti-proliferative and apoptotic effects in various
types of cancer, including lung, ovarian, esophageal and liver
cancer, in addition to glioma (21–26). The
molecular mechanisms underlying the anticancer effects of curcumin
are complex. Previous studies have demonstrated that curcumin can
mediate apoptosis through the upregulation of caspase-8 and
caspase-3 (27). Curcumin may also
able to attenuate the incidence of cancer via the reduction of
phospho-IκBα and 8-OHdG expression during tumor initiation
(28). In addition, curcumin may
reduce the invasive ability of A431 cells by inhibiting the
activation of the STAT3 signaling pathway and the expression of
STAT3 as a target gene in the pathway (29).
Curcumin has been reported to inhibit the activation
of myeloid-derived suppressor cells (MDSCs), promote the
differentiation of MDSCs, and interfere with the interaction
between MDSCs and cancer cells and to suppress tumor growth
(30). In addition, curcumin has been
demonstrated to protect against chemoresistance in human gastric
cancer cells by downregulating nuclear factor κ-light chain
enhancer of activated B cells (NF-κB) and subsequent NF-κB-mediated
anti-apoptotic genes, including Bcl-2 and Bcl-extra large in the
SGC 7901 human gastric cancer cell line (31). Curcumin suppresses proliferation and
invasion in human gastric cancer cells by downregulating
serine/threonine-protein kinase PAK1 activity and cyclin D1
expression (32). A previous study
identified that the anti-metastatic effect of curcumin on
endometrial carcinoma is associated with inhibition of the
expression and activity of MMP-2 and −9 via downregulation of the
ERK signaling pathway (33).
Previous studies have investigated the effects of
combination chemotherapy with curcumin. Curcumin and its analogues
(PGV-0 and PGV-1) enhance the cytotoxicity of doxorubicin in MCF-7
cells via the inhibition of human epidermal growth factor activity
and NF-κB activation (34). Curcumin
may enhance the antitumor activity of docetaxel in ATC cells by
interfering with NF-κB and cyclooxygenase-2 (35). However, the anticancer effects of
curcumin combined with FP in gastric cancer cells have not yet been
reported, to the best of our knowledge. The present study
investigated the synergistic effects of curcumin and FP
chemotherapy on the MGC-803 human gastric cancer cell line using an
MTT assay, flow cytometry, double AO/EB fluorescent staining, a
colony formation assay and a Transwell migration assay. The results
demonstrated that curcumin combined with low-dose FP or medium-dose
FP enhances the effects of FP alone on cell viability and
apoptosis. Therefore, the side effects of FP may be reduced via the
co-administration of curcumin and low-dose FP chemotherapy, rather
than the administration of FP chemotherapy alone.
The present study investigated the molecular
mechanisms underlying the anticancer effects of curcumin combined
with FP using western blot analyses, and the results suggested that
the combination of curcumin and FP can effectively increase Bax
expression whilst also decreasing Bcl-2 expression. The Bcl-2, Bax,
caspase-3 and caspase-8-associated signaling pathways have been
reported to be associated with GC (36,37). In
addition, the results from the caspase-3 and caspase-8 activity
assay kits demonstrated that the combination of curcumin and FP
could significantly promote the activity of caspase-3 and
caspase-8.
In conclusion, curcumin enhances the anticancer
effects of FP in MGC-803 cells by decreasing cell viability,
inhibiting colony formation, inhibiting cell migration and inducing
apoptosis via the activation of caspase-3/−8, downregulation of
Bcl-2 and upregulation of Bax. These results suggest that curcumin
may be used in synergy with chemotherapy regimen FP to treat
patients with GC. Further studies are required in order to evaluate
the efficacy of this combined treatment in vivo.
Acknowledgements
The present study was supported by the Nature
Science Foundation of Hubei Province (grant no. 2013CFB067).
References
|
1
|
Chen Y, Li Y, Wang H, Lu J, Jin M and
Zhang Z: Maternal gastric carcinoma with metastasis to the
placenta: A case report. Oncol Lett. 8:2509–2510. 2014.PubMed/NCBI
|
|
2
|
Nagini S: Carcinoma of the stomach: A
review of epidemiology, pathogenesis, molecular genetics and
chemoprevention. World J Gastrointest Oncol. 4:156–169. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Ferlay J, Soerjomataram I, Dikshit R, Eser
S, Mathers C, Rebelo M, Parkin DM, Forman D and Bray F: Cancer
incidence and mortality worldwide: Sources, methods and major
patterns in GLOBOCAN 2012. Int J Cancer. 136:E359–E386. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang D, Hendifar A, Lenz C, Togawa K, Lenz
F, Lurje G, Pohl A, Winder T, Ning Y, Groshen S and Lenz HJ:
Survival of metastatic gastric cancer: Significance of age, sex and
race/ethnicity. J Gastrointest Oncol. 2:77–84. 2011.PubMed/NCBI
|
|
5
|
Pulido-Moran M, Moreno-Fernandez J,
Ramirez-Tortosa C and Ramirez-Tortosa M: Curcumin and health.
Molecules. 21:2642016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Aggarwal BB, Kumar A, Aggarwal MS and
Shishodia S: Curcumin derived from turmeric (Curcuma longa): A
spice for all seasons. Phytopharm Cancer Chemo Prev. 349–387.
2005.
|
|
7
|
Lao CD, Ruffin MT IV, Normolle D, Heath
DD, Murray SI, Bailey JM, Boggs ME, Crowell J, Rock CL and Brenner
DE: Dose escalation of a curcuminoid formulation. BMC Complement
Altern Med. 6:102006. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Kamat AM, Tharakan ST, Sung B and Aggarwal
BB: Curcumin potentiates the anticancer effects of Bacillus
Calmette-Guerin against bladder cancer through the downregulation
of NF-kappaB and upregulation of TRAIL receptors. Cancer Res.
69:8958–8966. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kunnumakkara AB, Anand P and Aggarwal BB:
Curcumin inhibits proliferation, invasion, angiogenesis and
metastasis of different cancers through interaction with multiple
cell signaling proteins. Cancer Lett. 269:199–225. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mahajanakatti AB, Murthy G, Sharma N and
Skariyachan S: Exploring inhibitory potential of Curcumin against
various cancer targets by in silico virtual screening. Interdiscip
Sci. 6:13–24. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Van Cutsem E: The treatment of advanced
gastric cancer: New findings on the activity of the taxanes.
Oncologist. 9:(Suppl). S9–S15. 2004. View Article : Google Scholar
|
|
12
|
LoRusso P, Pazdur R, Redman BG, Kinzie J
and Vaitkevicius V: Low-dose continuous infusion 5-fluorouracil and
cisplatin: Phase II evaluation in advanced colorectal carcinoma. Am
J Clin Oncol. 12:486–490. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Bouché O, Ychou M, Burtin P, Bedenne L,
Ducreux M, Lebreton G, Baulieux J, Nordlinger B, Martin C, Seitz
JF, et al: Adjuvant chemotherapy with 5-fluorouracil and cisplatin
compared with surgery alone for gastric cancer: 7-year results of
the FFCD randomized phase III trial (8801). Ann Oncol.
16:1488–1497. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Nakata B, Sowa M, Tsuji A, Kamano T,
Sasaki K, Fukunaga Y, Takahashi M, Tsujitani S, Mikami Y, Mitachi
Y, et al: Continuous infusion of 5-fluorouracil with versus without
low-dose, consecutive administration of cisplatin in advanced
colorectal cancer. A prospective randomized phase II study. J Exp
Clin Cancer Res. 26:51–60. 2007.PubMed/NCBI
|
|
15
|
Kim R, Nishimoto N, Inoue H, Yoshida K and
Toge T: An analysis of the therapeutic efficacy of protracted
infusion of low-dose 5-fluorouracil and cisplatin in advanced
gastric cancer. J Infect Chemother. 6:222–228. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Xingde W, Xingqiu H, Chengxian G, et al:
Long-period virulent test of Curcumin. J Zhejiang College TCM.
24:61–65. 2000.
|
|
17
|
Perkins S, Verschoyle RD, Hill K, Parveen
I, Threadgill MD, Sharma RA, Williams ML, Steward WP and Gescher
AJ: Chemopreventive efficacy and pharmacokinetics of curcumin in
the min/+ mouse, a model of familial adenomatous polyposis. Cancer
Epidemiol Biomarkers Prev. 11:535–540. 2002.PubMed/NCBI
|
|
18
|
Aggarwal BB, Sundaram C, Malani N and
Ichikawa H: Curcumin: The Indian solid gold. Adv Exp Med Biol.
595:1–75. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Esatbeyoglu T, Huebbe P, Ernst IM, Chin D,
Wagner AE and Rimbach G: Curcumin-from molecule to biological
function. Angew Chem Int Ed Engl. 51:5308–5332. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Calaf GM, Echiburú-Chau C, Roy D, Chai Y,
Wen G and Balajee AS: Protective role of curcumin in oxidative
stress of breast cells. Oncol Rep. 26:1029–1035. 2011.PubMed/NCBI
|
|
21
|
Li PM, Li YL, Liu B, Wang WJ, Wang YZ and
Li Z: Curcumin induces MHCC97H liver cancer cell apoptosis by
activating ROS/TLR-4/caspase signaling pathway. Asian Pac J Cancer
Prev. 15:2329–2334. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Wu SH, Hang LW, Yang JS, Chen HY, Lin HY,
Chiang JH, Lu CC, Yang JL, Lai TY, Ko YC and Chung JG: Curcumin
induces apoptosis in human non-small cell lung cancer NCI-H460
cells through ER stress and caspase cascade and
mitochondria-dependent pathways. Anticancer Res. 30:2125–2133.
2010.PubMed/NCBI
|
|
23
|
Goel A and Aggarwal BB: Curcumin, the
golden spice from Indian saffron, is a chemosensitizer and
radiosensitizer for tumors and chemoprotector and radioprotector
for normal organs. Nut Cancer. 62:919–930. 2010. View Article : Google Scholar
|
|
24
|
Subramaniam D, Ponnurangam S, Ramamoorthy
P, Standing D, Battafarano RJ, Anant S and Sharma P: Curcumin
induces cell death in esophageal cancer cells through modulating
notch signaling. PLoS One. 7:e305902012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Gupta SC, Patchva S and Aggarwal BB:
Therapeutic roles of curcumin: Lessons learned from clinical
trials. AAPS J. 15:195–218. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Su CC, Wang MJ and Chiu TL: The
anti-cancer efficacy of curcumin scrutinized through core signaling
pathways in glioblastoma. Int J Mol Med. 26:217–224.
2010.PubMed/NCBI
|
|
27
|
Zhu L, Han MB, Gao Y, Wang H, Dai L, Wen Y
and Na LX: Curcumin triggers apoptosis via upregulation of
Bax/Bcl-2 ratio and caspase activation in SW872 human adipocytes.
Mol Med Rep. 12:1151–1156. 2015.PubMed/NCBI
|
|
28
|
Sintara K, Thong-Ngam D, Patumraj S and
Klaikeaw N: Curcumin attenuates gastric cancer induced by
N-Methyl-N-nitrosourea and saturated sodium chloride in rats. J
Biomed Biotechnol. 2012:9153802012. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Wu J, Lu WY and Cui LL: Inhibitory effect
of curcumin on invasion of skin squamous cell carcinoma A431 cells.
Asian Pac J Cancer Prev. 16:2813–2818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Tu SP, Jin H, Shi JD, Zhu LM, Suo Y, Lu G,
Liu A, Wang TC and Yang CS: Curcumin induces the differentiation of
myeloid-derived suppressor cells and inhibits their interaction
with cancer cells and related tumor growth. Cancer Prev Res
(Phila). 5:205–215. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Yu LL, Wu JG, Dai N, Yu HG and Si JM:
Curcumin reverses chemoresistance of human gastric cancer cells by
downregulating the NF-κB transcription factor. Oncol Rep.
26:1197–1203. 2011.PubMed/NCBI
|
|
32
|
Cai XZ, Wang J, Li XD, Wang GL, Liu FN,
Cheng MS and Li F: Curcumin suppresses proliferation and invasion
in human gastric cancer cells by downregulation of PAK1 activity
and cyclin D1 expression. Cancer Biol Ther. 8:1360–1368. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Chen Q, Gao Q, Chen K, Wang Y, Chen L and
Li XU: Curcumin suppresses migration and invasion of human
endometrial carcinoma cells. Oncol Lett. 10:1297–1302.
2015.PubMed/NCBI
|
|
34
|
Meiyanto E, Putri DD, Susidarti RA,
Murwanti R, Sardjiman Fitriasari A, Husnaa U, Purnomo H and
Kawaichi M: Curcumin and its analogues (PGV-0 and PGV-1) enhance
sensitivity of resistant MCF-7 cells to doxorubicin through
inhibition of HER2 and NF-κB activation Asian Pac. J Cancer Prev.
15:179–184. 2014.
|
|
35
|
Hong JM, Park CS, Nam-Goong IS, Kim YS,
Lee JC, Han MW, Choi JI, Kim YI and Kim ES: Curcumin enhances
docetaxel-induced apoptosis of 8505C anaplastic thyroid carcinoma
cells. Endocrinol Metab (Seoul). 29:54–61. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Xu JD, Cao XX, Long ZW, Liu XP, Furuya T,
Xu JW, Liu XL, De Xu Z, Sasaki K and Li QQ: BCL2L10 protein
regulates apoptosis/proliferation through differential pathways in
gastric cancer cells. J Pathol. 223:400–409. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
37
|
Barrezueta LF, Oshima CT, Lima FO, De
Oliveira Costa H, Gomes TS, Neto RA and De Franco MF: The intrinsic
apoptotic signaling pathway in gastric adenocarcinomas of Brazilian
patients: Immunoexpression of the Bcl-2 family (Bcl-2, Bcl-x, Bak,
Bax, Bad) determined by tissue microarray analysis. Mol Med Rep.
3:261–267. 2010. View Article : Google Scholar : PubMed/NCBI
|