Introduction
Superficial cluster of differentiation
(CD)34-positive fibroblastic tumors (SCPFTs), first identified by
Carter et al (1) are rare
mesenchymal neoplasms of intermediate (borderline) malignancy. As
they were only described recently, their characteristics are yet to
be entirely elucidated. They were cured by surgery and had a good
outcome (1). Previous studies of
SCPFTs have lacked imaging data or cytogenetic analyses of this
tumor type (1–4).
The present study describes a patient with SCPFT
located in the subcutaneous adipose tissue of the thigh of an
18-year-old man. The imaging, histopathological and ultrastructural
data, and the cytogenetic study results are discussed. Aside from
histopathology and immunohistochemistry, the examination of this
patient encompassed computed tomography (CT), magnetic resonance
imaging (MRI), and positron emission tomography (PET)-CT, and
cytogenetic analysis to identify chromosomal aberrations. Informed
consent for publication of these data was obtained from the
patient.
Case report
Clinical and imaging data
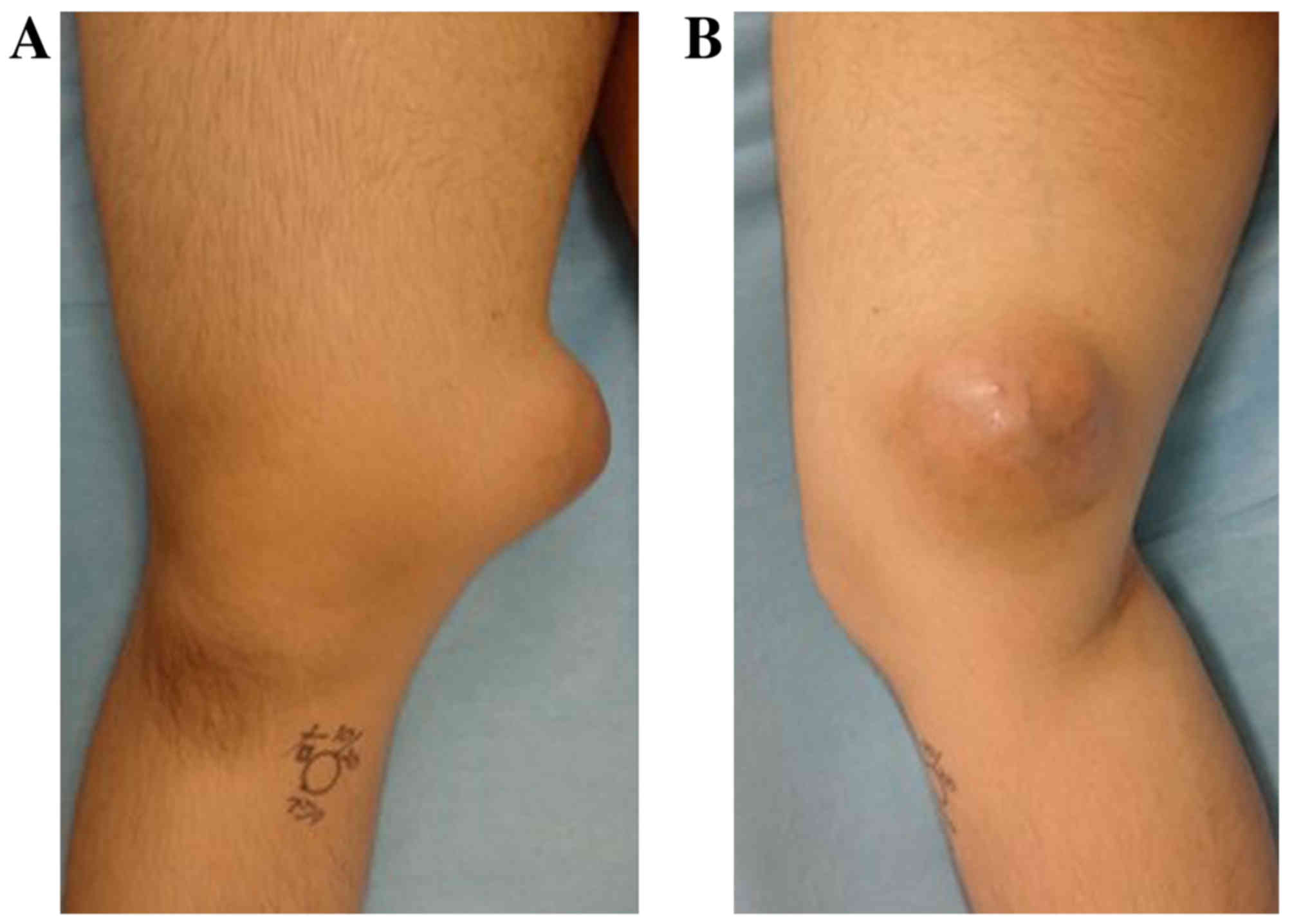
An 18-year-old Japanese male noticed a painless mass
measuring ~3 cm in the medial aspect of his right distal thigh,
without history of trauma. He visited Matsue City Hospital (Matsue,
Japan) in April, 2015, where the mass was initially diagnosed as an
epidermoid cyst. Surgical resection of the mass was planned, but
was cancelled subsequent to the patient developing a cold. The
patient was subsequently lost to follow-up for 18 months, following
which he revisited Matsue City Hospital as the mass had continued
to grow. At that point, a soft tissue sarcoma was suspected. He was
referred to Tottori University Hospital (Yonago, Japan) in October,
2016 for evaluation and treatment. Physical examination revealed a
9-cm, hard, elastic mass in his distal thigh without tenderness or
warm sensation, but with varicosis (Fig.
1). Medical and family history were unremarkable. The white
blood cell count (3.8–8.8×103/µl) and the levels of
C-reactive protein (<0.15 mg/dl), alkaline phosphatase (106–322
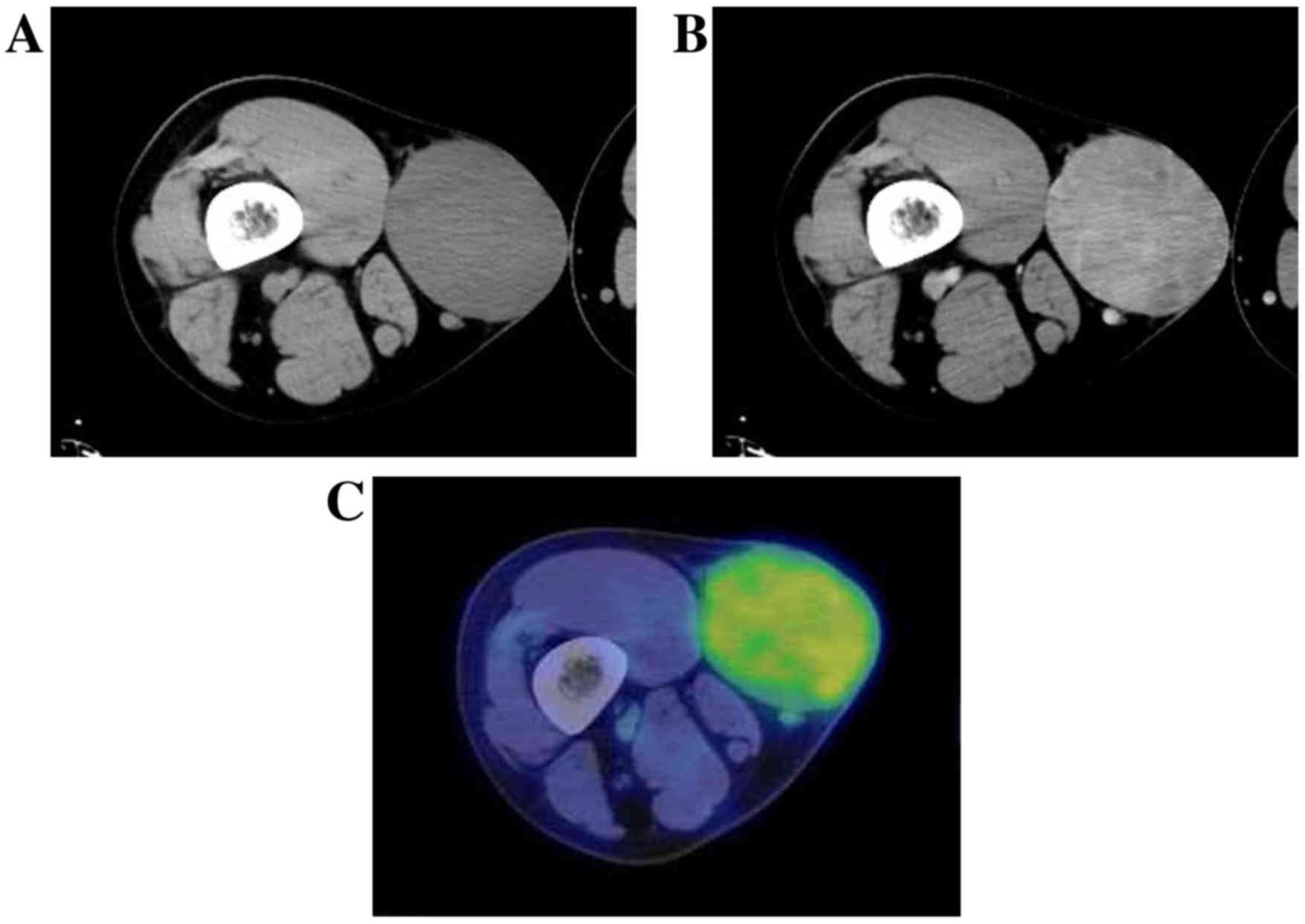
U/l) and lactate dehydrogenase (124–222 U/l) were normal. CT
revealed a well-marginated tumor without calcification in the
subcutaneous adipose tissue, and enhanced CT revealed weak
enhancement within the lesion (Fig. 2A
and B). The tumor demonstrated abnormal uptake on
2-(18F) fluoro-2-deoxy-D-glucose (18F-FDG) PET, with a
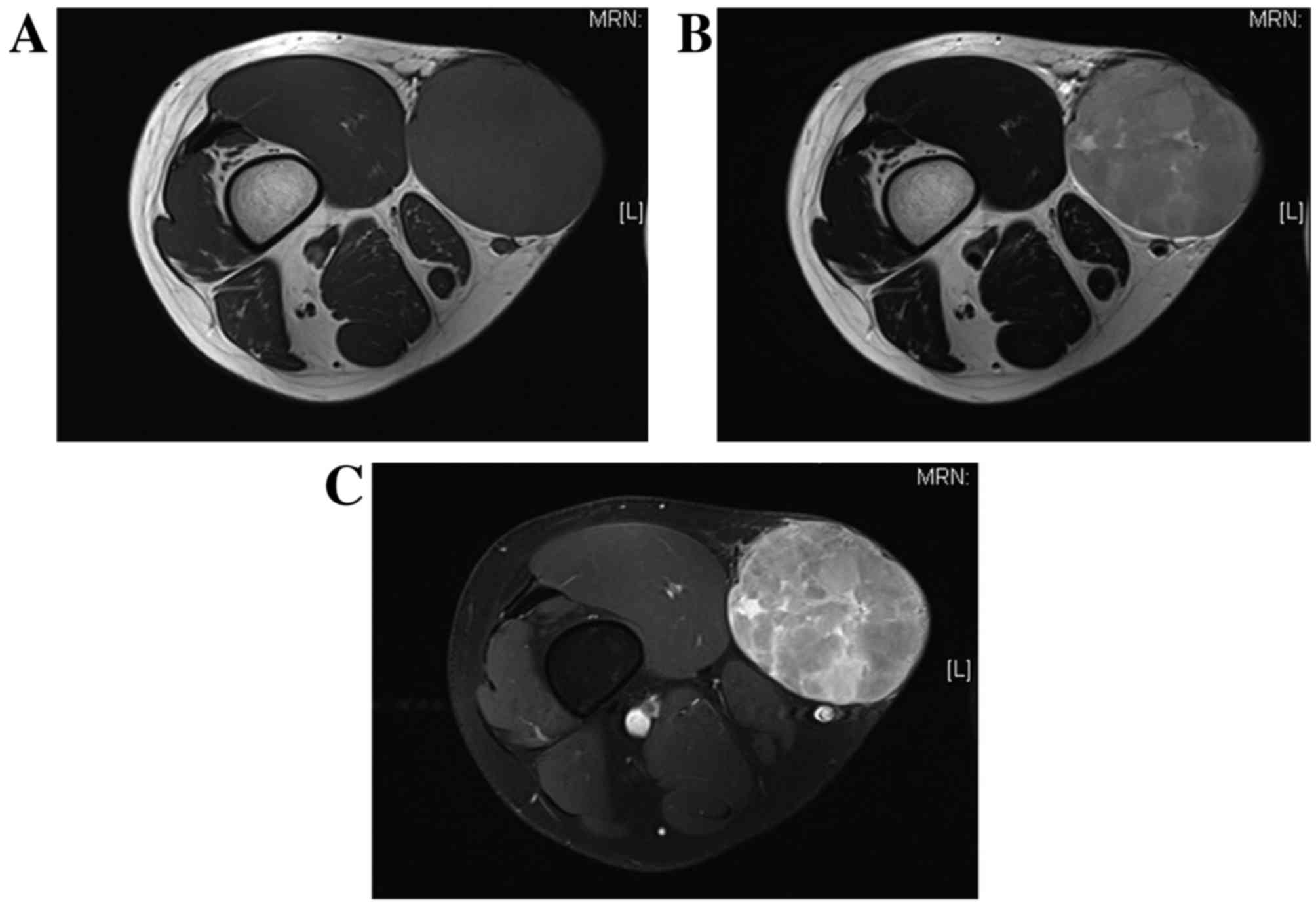
maximum standardized uptake value of 2.57 (Fig. 2C). The MRI scan revealed a clearly
defined tumor measuring 87×70×80 mm and located in the subcutaneous
adipose tissue; however, part of the tumor margin appeared to
slightly infiltrate the subcutaneous adipose tissue (Fig. 3). Although the tumor appeared to be in
close proximity to the vastus medialis and sartorius muscles, it
did not infiltrate them. The tumor exhibited homogenous low signal
intensity on T1-weighted imaging (Fig.
3A) and high signal intensity on T2-weighted imaging, with
small lobulated structures (Fig. 3B).
Mild enhancement was observed throughout the tumor, while strong
enhancement was evident at the periphery of the small lobulated
structures (Fig. 3C). No distant or
lymph node metastasis was observed.
Based on the aforementioned clinical data and
imaging examinations, the mass was diagnosed as a malignant tumor
of the soft tissue; such tumors include synovial sarcoma, alveolar
soft part sarcoma, and epithelioid sarcoma. Core needle biopsy was
performed for histopathological diagnosis. Although the tumor was
suspected to be an SCPFT, a definite diagnosis was not initially
available. The differential diagnosis was pleomorphic sarcoma.
Thereafter, wide resection was performed.
Pathological data (surgical
specimen)
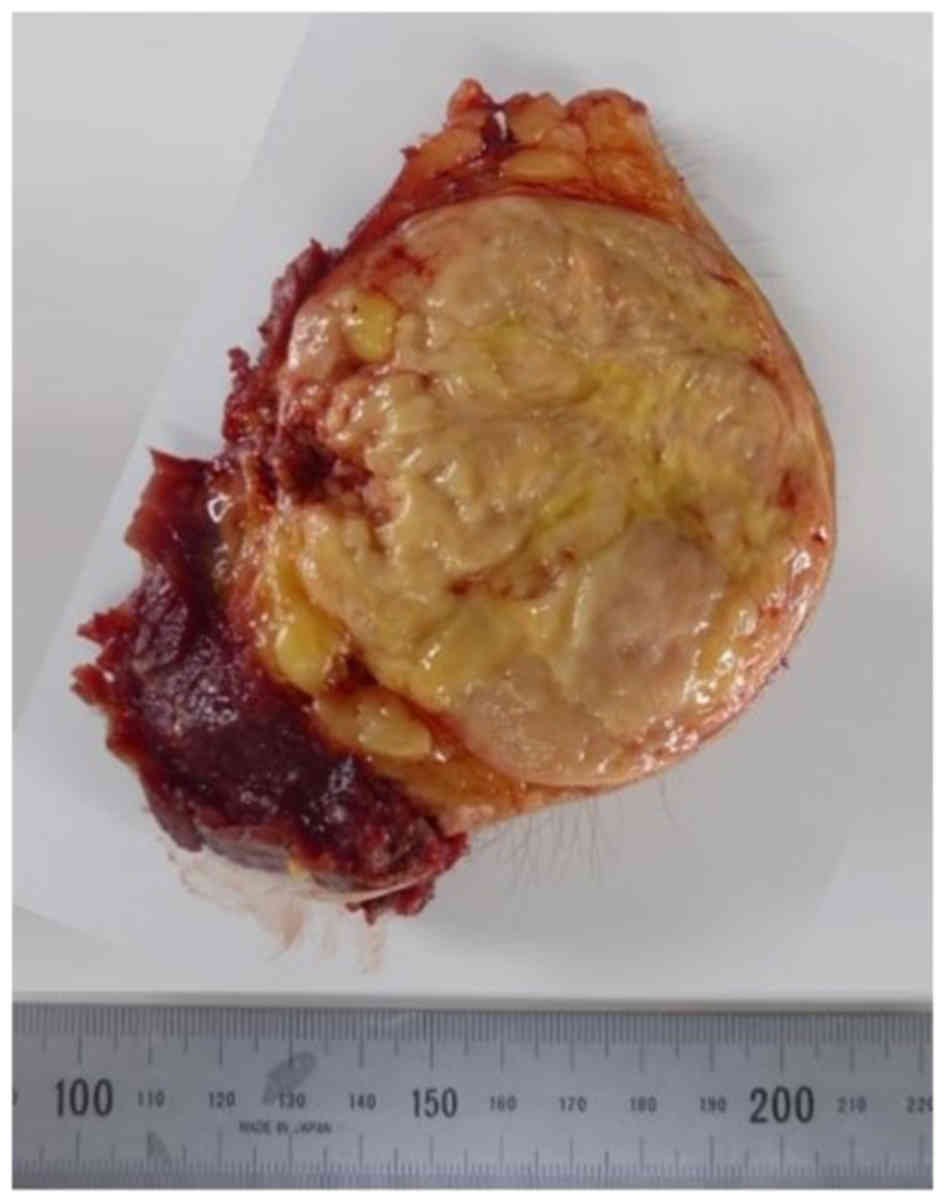
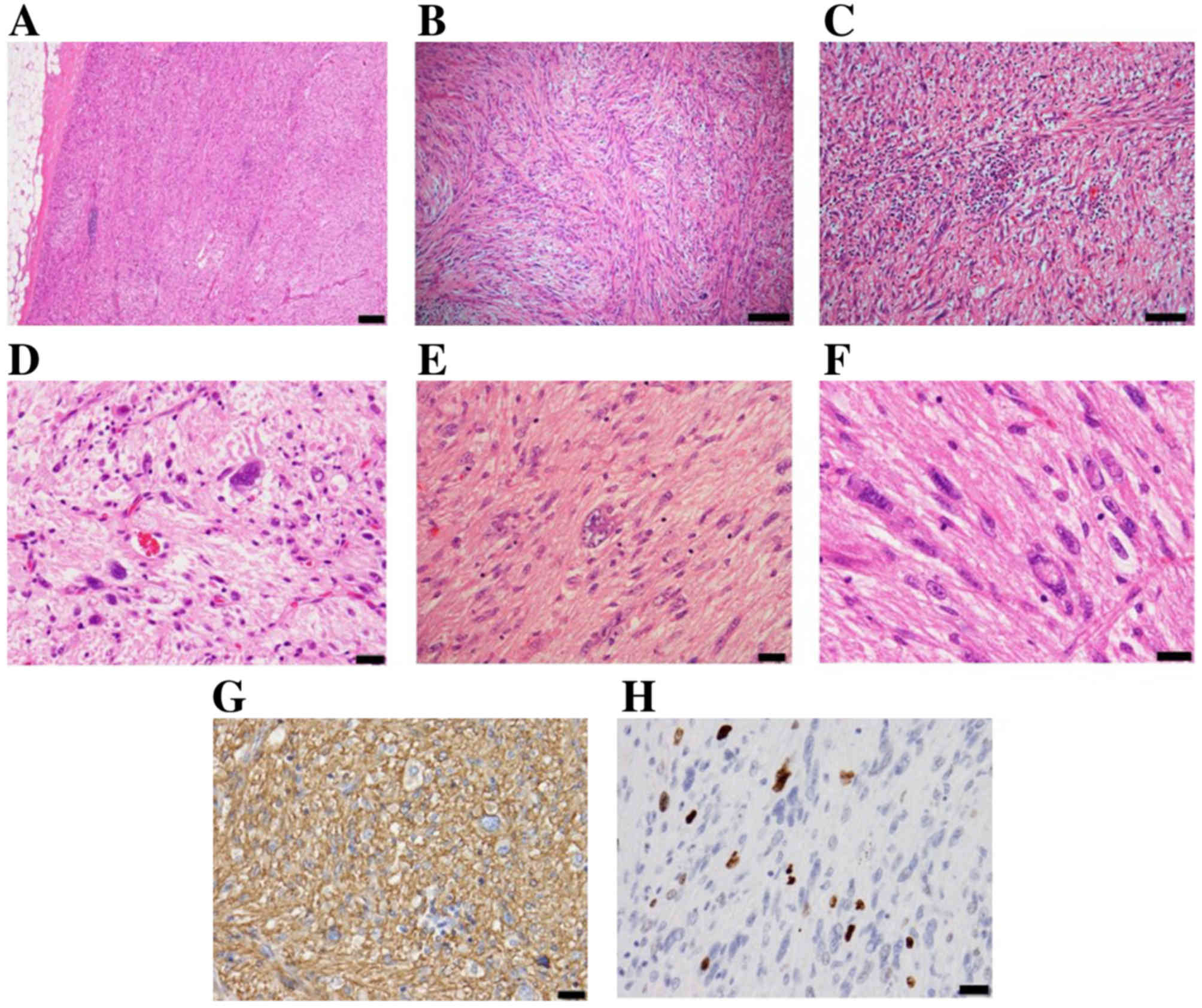
Grossly, the tumor was well-circumscribed and
confined to the superficial fibroadipose tissues. It exhibited a
yellow and fleshy appearance with small lobulated structures
(Fig. 4).
For light microscopic examination (magnification
×100 to ×400), the tumor was fixed in 10% buffered-formalin for 24
h at room temperature, and embedded in paraffin wax. Serial
sections, 4 µm thick, were stained using hematoxylin (Mayer's
hematoxylin solution) and 0.5% eosin. Histopathologically, the
tumor was well-circumscribed and had not infiltrated into adjacent
tissues. It was composed of irregular spindle-to-oval-shaped cells,
with eosinophilic glassy cytoplasm and hyperchromatic, bizarre and
pleomorphic nuclei that frequently demonstrated intranuclear
pseudoinclusions. Tumor cells were arranged in fascicles and
sheets, with a focal myxoid change in the background. A small
number of inflammatory cells, predominantly lymphocytes, were
identified throughout the lesion. Mitotic figures were rarely
observed (2/50 high-powered fields), and atypical mitoses were not
observed (Fig. 5A-F). No necrosis was
observed.
For immunohistochemical analysis, paraffin-embedded
tumor tissue specimens were cut into 4-µm sections, then dewaxed in
xylene, rehydrated through a graded series of ethanol solutions and
rinsed in distilled water for 5 min. Endogenous peroxidase activity
was blocked by 2% hydrogen peroxide in methanol at room temperature
for 30 min. Subsequent to rinsing with PBS, the sections were
incubated with blocking serum (2% fetal bovine serum) at room
temperature for 20 min and incubated at 4°C overnight with each
primary antibody. Immunohistochemically (by light microscope with
magnification, ×100 to ×200), the tumor cells were diffuse and
strongly positive for CD34 (catalog no. 413111, prediluted, ready
to use; Nichirei, Tokyo, Japan; Fig.
5G) and negative for CD31 (catalog no. M0823, prediluted, ready
to use; Dako; Agilent Technologies, Inc., Santa Clara, CA, USA),
desmin (catalog no. 413651, prediluted, ready to use; Nichirei
Bioscience, Tokyo, Japan), α-smooth muscle actin (catalog no.
M0851, dilution, 1:100; Dako; Agilent Technologies, Inc.), S-100
protein (catalog no. 422091, prediluted, ready to use; Nichirei
Bioscience), cytokeratin (AE1/AE3; catalog no. 412811, prediluted,
ready to use; Nichirei Bioscience), epithelial membrane antigen
(catalog no. M0613, prediluted, ready to use; Dako; Agilent
Technologies, Inc.), signal transducer and activator of
transcription 6 (STAT6; catalog no. sc-29497, dilution, 1:300;
Santa Cruz Biotechnology, Inc., Dallas, TX, USA), B-cell lymphoma-2
(catalog no. 413141, prediluted, ready to use; Nichirei Bioscience)
and c-Kit (catalog no. A4502, dilution 1:100; Dako; Agilent
Technologies, Inc.). Integrase interactor 1 protein (INI1; catalog
no. A301-087A dilution, 1:250; Bethyl Laboratories, Montgomery, TX,
USA) was expressed throughout the lesion. The Mindbomb E3 ubiquitin
protein ligase 1 (MIB1; catalog no. M7240, dilution, 1:100; Dako;
Agilent Technologies, Inc.) labeling index in the tumor was 8.6%
(Fig. 5H).
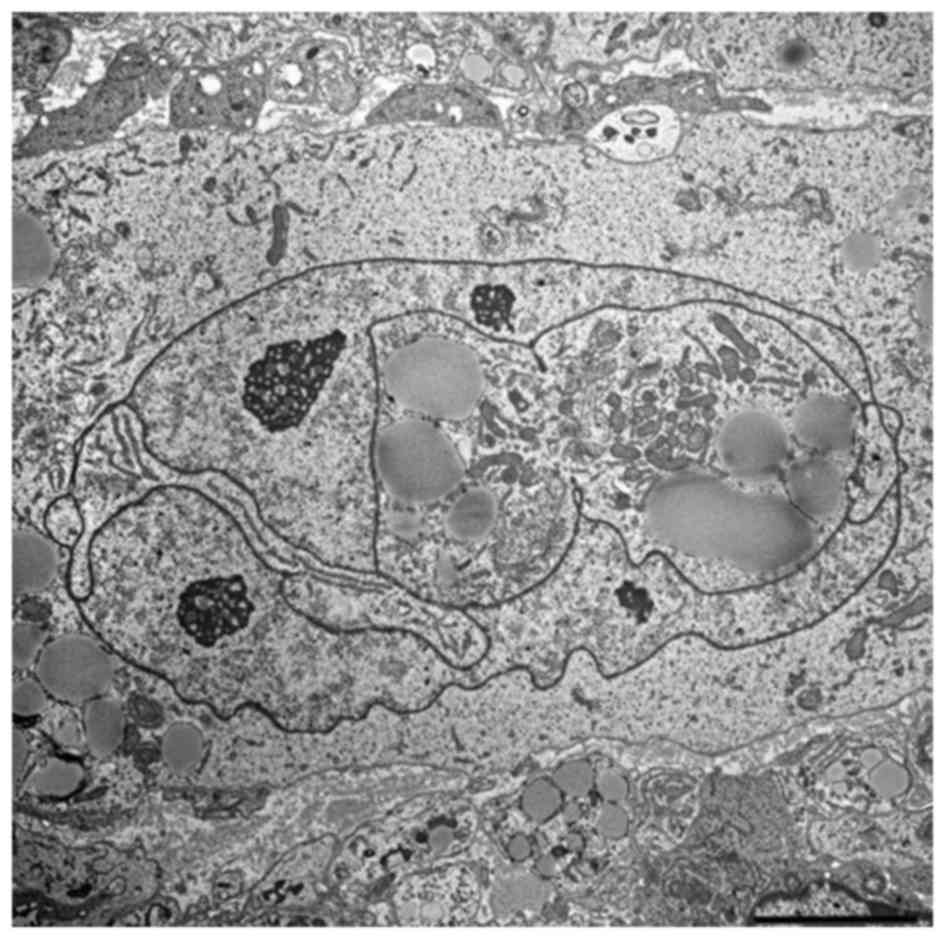
Ultrastructural examination
For transmission electron microscopic study, the
tumor was fixed overnight at 4°C in 2.5% glutaraldehyde in 0.1 M
cacodylate buffer, pH 7.4, followed by post-fixation for 1–2 h in
0.1 M cacodylate-buffered 1% osmium tetraoxide. Subsequent to
dehydration in a series of graded ethanol and n-butyl glycidyl
ether solutions, cells were embedded in epoxy resin and allowed to
set for 48 h at 60°C. Ultrathin sections were stained with uranyl
acetate and lead citrate, and examined with an electron microscope.
The tumor consisted of spindle-to-oval shaped cells without
intercellular junctions. The cells had irregular or convoluted
nuclei with abundant, euchromatin-prominent nucleoli. The
cytoplasmic organelles consisted of scattered, abundant and rough
endoplasmic reticulum, mitochondria, lysosomes, ribosomal rosettes
and aggregated lipid globules (Fig.
6).
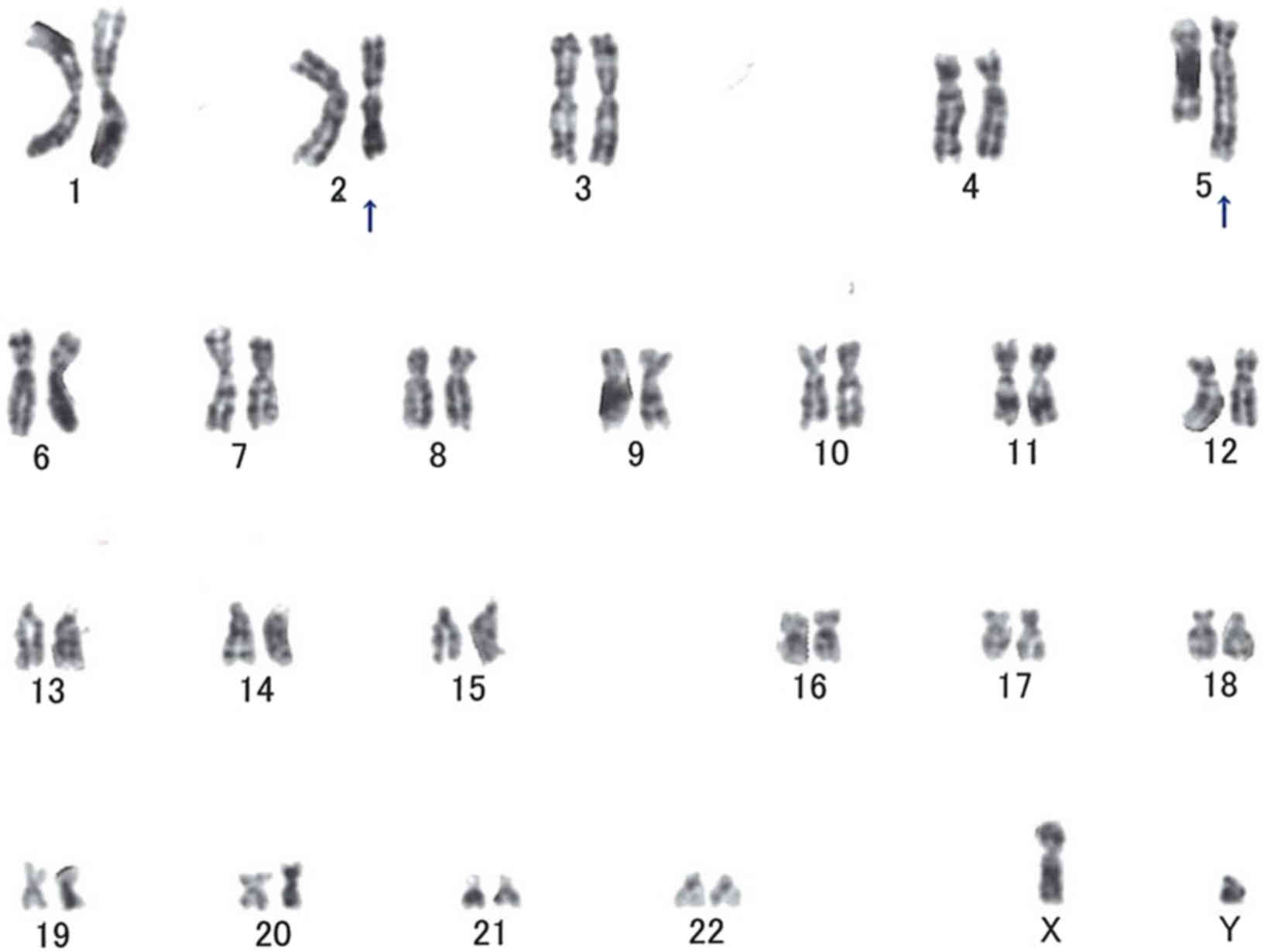
Cytogenetic studies
Cytogenetic analysis was performed on the surgical
specimen. The tissues were cut into pieces and transferred to
culture solution. Cell culture and G-band karyotyping were
performed by SRL Inc. (Tokyo, Japan). Of the 18 metaphase cells
available for analysis, 14 were of normal (46,XY) karyotype, 2
cells had a karyotype of t(2;5)(q31;q31), 1 had a karyotype of
42,Y,-X,-10,-18,-19, add(19)(p13), and 1 had a karyotype of
t(7;14)(q21;q24) (Fig. 7).
The tumor was finally diagnosed definitively as an
SCPFT of the thigh based on clinical, histological and
immunohistochemical data. In particular, the results that CD34 was
strongly positive on immunohistochemistry, and that the tumor site
was superficial, assisted with the diagnosis. The postoperative
course (5 months subsequent to surgery) has been uneventful, with
no evidence of recurrence or metastasis.
Discussion
When this tumor type was first described in 2014 in
a series of 18 cases, Carter et al (1) proposed the descriptive term SCPFT based
on clinical, morphological and immunochemical data. To date, only
five case series of SCPFT have been described (1–5), in which
almost all tumors were described histopathologically and
immunohistochemically. Only one study, that of Li et al
(5), described imaging data, namely
T1-weighted MRI findings. To the best of our knowledge, CT and
PET-CT features of SCPFT have not been described prior to the
present study.
The available studies on SCPFT described certain
shared characteristics: A fibroblastic tumor of the superficial
soft tissues with cellular pleomorphism, an extremely low mitotic
rate, and strong diffuse CD34 positivity (1–5). In their
18-case series, Carter et al (1) described SCPFTs as slow-growing masses
that are present for at least 1 year prior to diagnosis, that range
in size from 1.5 to 10 cm (mean, 4.1 cm), and that most commonly
afflict adults (age range, 20–76 years; median, 38 years) of any
sex (10 males and 8 females). In two-thirds of the cases, the
tumors occurred in the lower limbs, including the thighs. All of
the tumors showed confinement to the superficial fibroadipose
tissues, and absent or minimal involvement of the subjacent muscle
was observed. There was no history of a previous cutaneous neoplasm
at the same location in any of the patients. Clinical follow-up
data (median duration, 24 months; range, 1–104 months) were
available for 13 of the 18 patients (72%). Of these 13 patients, 12
were alive with no evidence of disease when the study was
published, and 1 patient with a tumor in the thigh experienced
metastasis to an external iliac lymph node 7 years after marginal
excision of the primary tumor, but was alive at the end of the
follow-up. Table I describes the
clinical outcomes reported in previous studies.
 | Table I.Clinical outcomes reported in previous
studies of superficial CD34-positive fibroblastic tumors. |
Table I.
Clinical outcomes reported in previous
studies of superficial CD34-positive fibroblastic tumors.
| Author, year | No. | No. with available
follow-up data | Median follow-up
duration (months) | No. with local
recurrences and distant metastases | Outcome | (Refs.) |
|---|
| Carter et al,
2014 | 18 | 13 | 24 | 1 (regional lymph
node metastasis) | 1 ANED 12 CDF | (1) |
| Hendry et al,
2015 | 2 | 2 | 3 | None | All CDF | (4) |
| Wada et al,
2016 | 1 | 1 | 3 | None | CDF | (2) |
| Lao et al,
2017 | 11 | 11 | 6 | None | All CDF | (3) |
| Li et al,
2016 | 1 | 1 | 24 | None | CDF | (5) |
| Present study | 1 | 1 | 5 | None | CDF | – |
On microscopic examination, the tumors in all
patients described by Carter et al (1) were confined to the superficial
fibroadipose tissues and were composed of spindle-to-epithelioid
cells with an abundant granular or glassy cytoplasm arranged in
fascicles and sheets. Marked pleomorphisms and a low incidence of
mitotic figures were observed in all patients. On
immunohistochemical examination, all tumors exhibited strong,
diffuse CD34 positivity. Limited cytokeratin expression was
observed in the neoplastic cells of 11 out of 16 assessed cases.
Lao et al (3) suggested that
it is not uncommon to misdiagnose SCPFT as a pleomorphic dermal
neoplasm (particularly atypical fibroxanthoma, pleomorphic dermal
sarcoma, or undifferentiated pleomorphic sarcoma/malignant fibrous
histiocytoma) due to this marked pleomorphism.
The present study is a typical case of SCPFT based
on the histopathological data, which are consistent with previous
studies. The specific findings that CD34 was strongly positive on
immunohistochemistry, and that the tumor site was superficial,
assisted with the diagnosis. Although PET imaging findings for this
type of tumor have not been discussed previously, the maximum
standard update value on 18F-FDG PET was 2.57 in the patient of the
present study; similar values are frequently observed in soft
tissue tumors of intermediate malignancy (6–8). The PET
findings in the current patient are considered consistent with an
intermediate malignancy as described by Carter et al
(1). On ultrastructural examination,
the tumor cells in the present case exhibited a tendency towards
fibroblast differentiation, consistent with the findings described
by Hendry et al (4). It is
unclear whether this feature is specific to all SCPFTs, as Hendry
et al (4) were the only group
aside from ours to perform ultrastructural examination. Cytogenetic
studies in the present case revealed a chromosomal translocation of
t(2;5)(q31;q31), according to the International System for Human
Cytogenetic Nomenclature criteria (9). Juvenile-onset soft tissue sarcomas are
often characterized by disease-specific fusion genes resulting from
translocations, such as t(X;18) in synovial sarcomas, t(12;22) or
t(2;22) in clear cell sarcomas, t(2;13) in alveolar
rhabdomyosarcoma, and t(X;17) in alveolar soft part sarcomas
(10–13). Additionally, Carter et al
(1) suggested that SCPFT most
commonly affects young to middle-aged adults. Therefore, we
hypothesized that the t(2;5)(q31;q31) translocations observed in
the present case suggest the existence of a chromosomal aberration
specific to SCPFT. In previous studies, 3 patients with
translocation-associated soft tissue tumors involving 2q31,
including 2 patients with synovial sarcoma and 1 with lipoblastoma,
and 1 patient with translocation-associated embryonal
rhabdomyosarcoma involving 5q31, were documented (14–17).
Clinically, surgeons who are not orthopedic
oncologists may perform unplanned excisions for such tumors owing
to their small sizes and superficial locations; they may not
accurately diagnose SCPFT owing to its rarity. As mentioned, the
tumor of the present patient was initially diagnosed as an
epidermoid cyst. Surgical resection was planned, and the patient
almost underwent surgery at a nearby hospital 18 months prior to
visiting Tottori University Hospital.
In summary, the present study described a typical
case of SCPFT in the thigh of an 18-year-old man. To the best of
our knowledge, this is the first study of an SCPFT in which imaging
data (CT, MRI, and PET-CT) and chromosomal aberrations are
described in detail for this type of tumor. It was identified that
t(2;5)(q31;q31) may be a disease-specific chromosomal aberration in
SCPFT. As additional characteristics of SCPFTs remain to be
identified, it is important to accumulate more patients and review
long-term follow-up data. This will assist in improving recognition
of these tumors, and avoid misdiagnosing them as high-grade
sarcomas.
Acknowledgements
The authors would like to thank Mr. T. Horie of the
Technical Department, Division of Medical Science, Tottori
University (Tottori, Japan), for his excellent technical
assistance, and Dr S. Oda of the Department of Pathology and
Experimental Medicine, Okayama University (Okayama, Japan) for
performing STAT6 immunostaining.
References
|
1
|
Carter JM, Weiss SW, Linos K, DiCaudo DJ
and Folpe AL: Superficial CD34-positive fibroblastic tumor: Report
of 18 cases of a distinctive low-grade mesenchymal neoplasm of
intermediate (borderline) malignancy. Mod Pathol. 27:294–302. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Wada N, Ito T, Uchi H, Nakahara T, Tsuji
G, Yamada Y, Oda Y and Furue M: Superficial CD34-positive
fibroblastic tumor: A new case from Japan. J Dermatol. 43:934–936.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Lao IW, Yu L and Wang J: Superficial
CD34-positive fibroblastic tumor: A clinicopathological and
immunohistochemical study of an additional series. Histopathology.
70:394–401. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Hendry SA, Wong DD, Papadimitriou J,
Robbins P and Wood BA: Superficial CD34-positive fibroblastic
tumour: Report of two new cases. Pathology. 47:479–482. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Li W, Molnar SL, Mott M, White E and De
Las Casas LE: Superficial CD34-positive fibroblastic tumor:
Cytologic features, tissue correlation, ancillary studies, and
differential diagnosis of a recently described soft tissue
neoplasm. Diagn Cytopathol. 44:926–930. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Ioannidis JP and Lau J: 18F-FDG PET for
the diagnosis and grading of soft-tissue sarcoma: A meta-analysis.
J Nucl Med. 44:717–724. 2003.PubMed/NCBI
|
|
7
|
Tian R, Su M, Tian Y, Li F, Li L, Kuang A
and Zeng J: Dual-time point PET/CT with F-18 FDG for the
differentiation of malignant and benign bone lesions. Skeletal
Radiol. 38:451–458. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Aoki J, Watanabe H, Shinozaki T, Takagishi
K, Tokunaga M, Koyama Y, Sato N and Endo K: FDG-PET for
preoperative differential diagnosis between benign and malignant
soft tissue masses. Skeletal Radiol. 32:133–138. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mitelman F: International Standing
Committee on Human Cytogenetic Nomenclature: ISCN 1995: an
international system for human cytogenetic nomenclature (1995):
recommendations of the International Standing Committee on Human
Cytogenetic Nomenclature. Memphis, Tennessee, USA. October 9–13,
1994. Karger, Basel: 1995
|
|
10
|
Suurmeijer AJH, de Bruijn D, van Geurts
Kessel A and Miettinen MM: Synovial sarcoma. In: World Health
Organization Classification of TumoursSoft Tissue and Bone. 4th.
Fletcher CDM, Bridge JA, Hogendoorn PCW and Mertens F: IARC Press;
Lyon: pp. 213–215. 2013
|
|
11
|
Antonescu CR: Clear cell sarcoma of soft
tissue. In: World Health Organization Classification of TumoursSoft
Tissue and Bone. 4th. Fletcher CDM, Bridge JA, Hogendoorn PCW and
Mertens F: IARC Press; Lyon: pp. 221–222. 2013
|
|
12
|
Parham DM and Barr FG: Alveolar
rhabdomyosarcoma. In: World Health Organization Classification of
TumoursSoft Tissue and Bone. 4th. Fletcher CDM, Bridge JA,
Hogendoorn PCW and Mertens F: IARC Press; Lyon: pp. 130–132.
2013
|
|
13
|
Ordonez NG and Ladanyi M: Alveolar soft
part sarcoma. In: World Health Organization Classification of
TumoursSoft Tissue and Bone. 4th. Fletcher CDM, Bridge JA,
Hogendoorn PCW and Mertens F: IARC Press; Lyon: pp. 218–220.
2013
|
|
14
|
Panagopoulos I, Mertens F, Isaksson M,
Limon J, Gustafson P, Skytting B, Akerman M, Sciot R, Dal Cin P,
Samson I, et al: Clinical impact of molecular and cytogenetic
findings in synovial sarcoma. Genes Chromosomes Cancer. 31:362–372.
2001. View
Article : Google Scholar : PubMed/NCBI
|
|
15
|
Przybyl J, Sciot R, Rutkowski P, Siedlecki
JA, Vanspauwen V, Samson I and Debiec-Rychter M: Recurrent and
novel SS18-SSX fusion transcripts in synovial sarcoma: Description
of three new cases. Tumour Biol. 33:2245–2253. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshida H, Miyachi M, Ouchi K, Kuwahara Y,
Tsuchiya K, Iehara T, Konishi E, Yanagisawa A and Hosoi H:
Identification of COL3A1 and RAB2A as novel translocation partner
genes of PLAG1 in lipoblastoma. Genes Chromosomes Cancer.
53:606–611. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Roberts I, Foster N, Nacheva E and Coleman
N: Paint-assisted microdissection-FISH: Rapid and simple mapping of
translocation breakpoints in the embryonal rhabdomyosarcoma cell
line RD. Cytometry A. 58:177–184. 2004. View Article : Google Scholar : PubMed/NCBI
|