Introduction
Bladder cancer is the 6th leading cause of new
cancer cases and 9th leading cause of cancer-associated mortality
in males worldwide. An estimated 429,800 new cases of bladder
cancer and 165,100 bladder cancer associated mortalities occurred
in 2012 worldwide (1). The
histological types of bladder cancer are quite diverse, and the
vast majority of cases are urothelial cancer (2). However, urothelial cancer is also known
to have different variants, as determined by histological features.
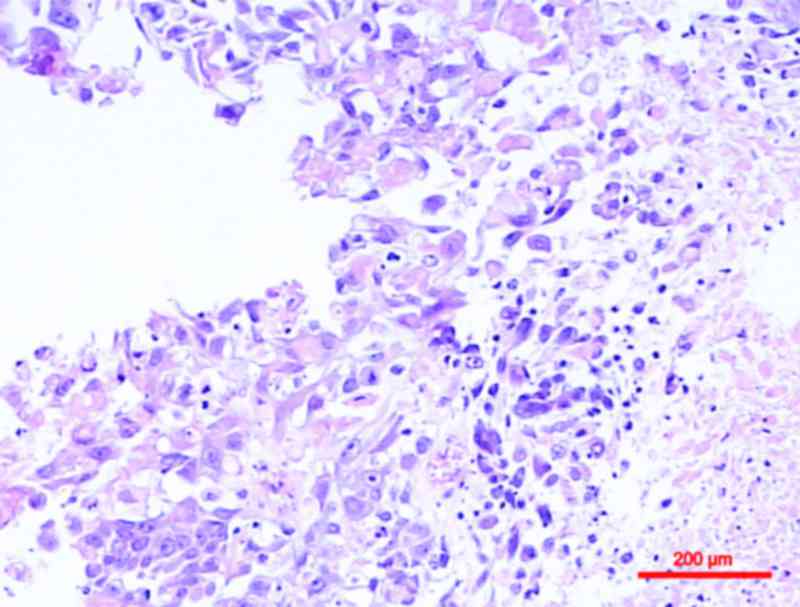
Squamous differentiation, defined by the presence of keratinization
or intercellular bridges, is the most common variant of urothelial
cancer, with previous studies noting its presence in 16.8–22.1% of
cases (3–5) (Fig. 1).
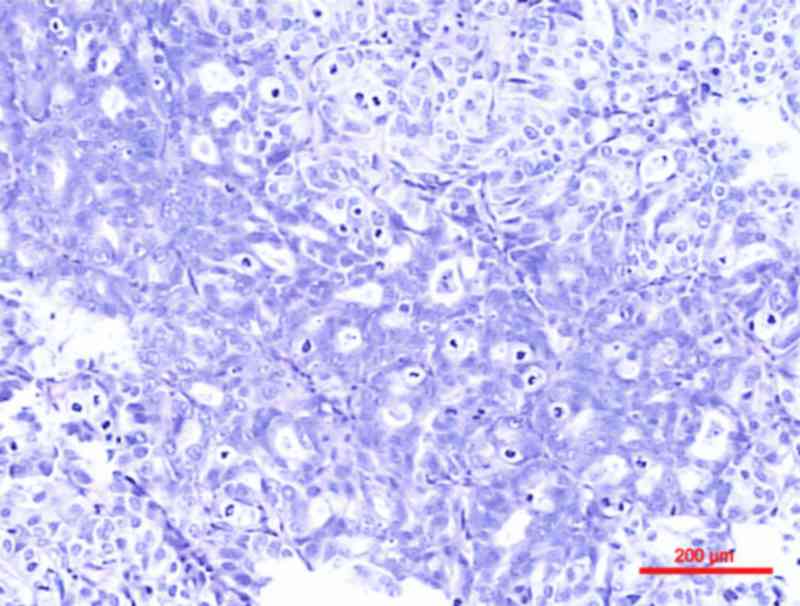
Glandular differentiation, characterized by intratumoral tubular or
enteric gland-like spaces, is less common compared with squamous
differentiation, with an incidence of ≤16% (6) (Fig. 2).
These two histological subtypes often coexist within the same tumor
(7,8).
It is controversial whether urothelial carcinoma of
bladder (UCB) with squamous and/or glandular differentiation behave
more aggressively compared with pure UCBs. Generally, squamous
and/or glandular differentiation has been thought to have little
clinical significance in urothelial carcinomas (9–11).
However, previous studies have suggested that the presence of
variant histology is usually associated with more aggressive
behavior and a worse patient outcome (3,12–14). The clinical management for patients
with UCB with divergent histology remains controversial.
However, these aforementioned studies are largely
limited to muscle invasive UCBs treated with radical cystectomy.
The significance of squamous and/or glandular histological
features, characterized at transurethral resection (TURBT), remains
unclear (15,16). The present study therefore aims to
determine the prognostic relevance of squamous and/or glandular
differentiation in patients with nonmuscle invasive urothelial
carcinoma of bladder (NMIUCB) that have been treated with
TURBT.
Materials and methods
Ethics statement
The present study was approved by the Ethics
Committee of the Second Hospital of Tianjin Medical University
(Tianjin, China). The retrospective study conducted did not affect
the clinical course of any patient, and all patient records were
analyzed anonymously. Due to the retrospective nature of the study,
written informed consent for each individual was not necessary.
Patient characteristics
All clinical data were prospectively gathered from
patient charts and electronic medical records. A total of 869
patients with NMIUCB who underwent TURBT between January 2006 and
January 2011 were enrolled into the present study. The inclusion
criteria for the study were: Patients who underwent TURBT as an
initial treatment; a pathological diagnosis of tumors as NMIUCB
[noninvasive papillary urothelial carcinoma (pTa) + tumor invading
into the lamina propria (pT1)]; patients for whom a detailed
assessment of primary tumor histology was available; and patients
who received adjuvant intravesical chemotherapy following TURBT.
Exclusion criteria for the present study include: An absence of
urothelial carcinoma histology in the TURBT specimen; the presence
of urethral or upper tract primaries, or distant metastasis at
diagnosis; and the presence of carcinoma in situ (CIS) and
other histological variants. For each patient, the following
clinical factors were collected: Age, sex, tumor multiplicity,
tumor size, pathological tumor stage and tumor grade. Tumor size
was defined as being the greatest diameter observed during
microscopic analysis of the surgical specimen.
Surgical procedure and pathological
evaluation
All patients were treated with TURBT according to
the following standardized procedure used by all surgeons in the
present study. All visible tumors or suspicious mucosal lesions
were resected with a monopolar loop electrode until the tumor base
reached the deep muscle layer and transparently showed perivesical
fat. Tissue samples were collected and fixed in formalin and
embedded in paraffin. Available hematoxylin and eosin stained
slides (staining for 5 min at 25°C with hematoxylin and 30 s at
25°C with eosin) of tumor tissue samples (thickness 4 µm) were
reassessed using a light microscope (magnification, ×100). The
pathological stage and tumor grade were reassigned by a single
genitourinary pathologist at the Second Hospital of Tianjin Medical
University, according to the 2002 World Health Organization (WHO)
tumor-node-metastasis classification of 6th American Joint
Committee on Cancer and the 1973 WHO grading system, respectively
(17,18). Squamous differentiation was defined by
the presence of intercellular bridges or keratinization and
glandular differentiation was defined as gland formation in the
tumor with or without mucin production (19). According to the published
classification recommendations, tumors with any urothelial
carcinoma (UC) component as well as a second, nonurothelial
malignant component were considered to represent UC with
histological differentiation. Thus, patients with pure non UC were
not included in the present study.
Initial adjuvant intravesical
therapy
Due to the inaccessibility of the drug Bacillus
Calmette-Guerin in China before 2015, all enrolled patients
received the anthracycline antibiotic chemotherapy drugs including
epirubicin or pirarubicin. The dosage and scheme used varied
between patients as this observational study was based on a
retrospective analysis. The schedule for instillation chemotherapy
consisted of weekly instillations for 8 consecutive weeks and
monthly instillations for 12 consecutive months. The
chemotherapeutic drug used was either 40–60 mg epirubicin or 30 mg
pirarubicin.
Follow-up and clinical outcomes
Postoperative follow-up was conducted by rigid
cystoscopy every 3 months for a period of 2 years, every 6 months
thereafter until 5 years and then yearly according to the Chinese
guidelines, which are the same as the US and European guidelines
(20). The end points of the present
study were recurrence and progression. Outcomes of interest in the
present study were recurrence-free survival (RFS) and
progression-free survival (PFS). The RFS period was estimated from
the date of surgery to date of first clinical recurrence (any
grade, any pathological stage of tumor or CIS). The PFS duration
was calculated from the time of surgery to the date when the
disease developed to a higher histological or pathological stage
and/or to metastasis. For patients without recurrence and
progression, the end point was the date of the last available
follow-up cystoscopy.
Statistical analysis
Statistical analysis was performed using the
statistical software SPSS, version 20 (IBM SPSS, Armonk, NY, USA).
Continuous variables according to the presence of the squamous
and/or glandular differentiation were compared using the
independent sample t-test, and categorical variables were compared
using the χ2 test. RFS and PFS curves were calculated by the
Kaplan-Meier method and differences were analyzed by the log-rank
test. Univariate and multivariate Cox proportional hazard analysis
were performed to verify independent predictive parameters of
recurrence and progression. All tests were 2-sided, and P<0.05
was considered to indicate a statistically significant
difference.
Results
The clinicopathological demographics of patients
with pure UCB, and those with UCB and squamous and/or glandular
differentiation are presented in Table
I. The mean age at initial TURBT was 64.89±10.28 years in
patients with pure UCB and 66.16±11.02 years in patients with
squamous and/or glandular differentiation. Among the 869 patients,
195 (22.4%) had UCB with squamous differentiation, 27 (3.1%) had
glandular differentiation, and 10 (1.2%) had squamous and glandular
differentiation, which is similar to the results of a previous
study (2). Age, sex, tumor size,
tumor multiplicity and pathological tumor stage did not differ
depending on the presence of the squamous and/or glandular
differentiation. However, high grade tumors were more common in UCB
with squamous and/or glandular differentiation compared with pure
UCB (58.62 vs. 29.04%, P<0.001).
 | Table I.Clinicopathological patient
demographics stratified by squamous and/or glandular
differentiation in TURBT specimen. |
Table I.
Clinicopathological patient
demographics stratified by squamous and/or glandular
differentiation in TURBT specimen.
| Clinicopathological
features | Pure UCB no. (%) | UCB +
squamous/glandular differentiation (%) | P-value |
|---|
| No. of patients | 637 | 232 |
|
| Mean age at initial
TURBT, years | 64.89±10.28 | 66.16±11.02 |
|
|
<65 | 292 (45.84) | 94 (40.52) | 0.115 |
| ≥65 | 345 (54.16) | 138 (59.48) |
|
| Sex |
|
|
| Male | 525 (82.42) | 182 (78.45) | 0.184 |
|
Female | 112 (17.58) | 50 (21.55) |
|
| Tumor size, cm |
|
|
|
<3 | 437 (68.60) | 149 (64.22) | 0.223 |
| ≥3 | 200 (31.40) | 83 (35.78) |
|
| Multiplicity |
|
|
|
Single | 390 (61.22) | 128 (55.17) | 0.108 |
|
Multiple | 247 (38.78) | 104 (44.83) |
|
| Pathological tumor
stage |
|
|
|
| pTa | 37 (5.81) | 13 (5.60) | 0.909 |
| pT1 | 600 (94.19) | 219 (94.40) |
|
| Tumor grade |
|
|
| Low | 452 (70.96) | 96 (41.38) | <0.001 |
|
High | 185 (29.04) | 136 (58.62) |
|
During the median 76.0 months follow-up, 89/232
patients with squamous and/or glandular differentiation and 149/637
patients with pure UCB experienced disease recurrence (Table II). Similarly, 27/232 patients with
squamous and/or glandular differentiation and 72/637 patients with
pure UCB achieved disease progression. Patients with squamous
and/or glandular differentiation were significantly more likely to
recur compared with those with pure UCB (38.36 vs. 23.39%,
respectively, P<0.001). However, no statistically significant
difference was observed in the rates of progression between
patients with squamous and/or glandular differentiation and those
with pure UCB (11.64 vs. 11.30%, respectively, P=0.891).
 | Table II.Distribution of the two groups in
accordance with recurrence and progression. |
Table II.
Distribution of the two groups in
accordance with recurrence and progression.
| Recurrence and
progression | Pure UCB no.
(%) | UCB +
squamous/glandular differentiation | P-value |
|---|
| Recurrence |
|
|
|
|
Yes | 149 (23.39) | 89 (38.36) | <0.001 |
| No | 488 (76.61) | 143 (61.64) |
|
| Mean RFS, mo
(range) | 93.01 (2–114) | 80.54 (3–113) | <0.001 |
| Progression |
|
|
|
|
Yes | 72 (11.30) | 27 (11.64) | 0.891 |
| No | 565 (88.70) | 205 (88.36) |
|
| Mean PFS, mo
(range) | 104.74 (3–114) | 103.65 (5–114) | 0.813 |
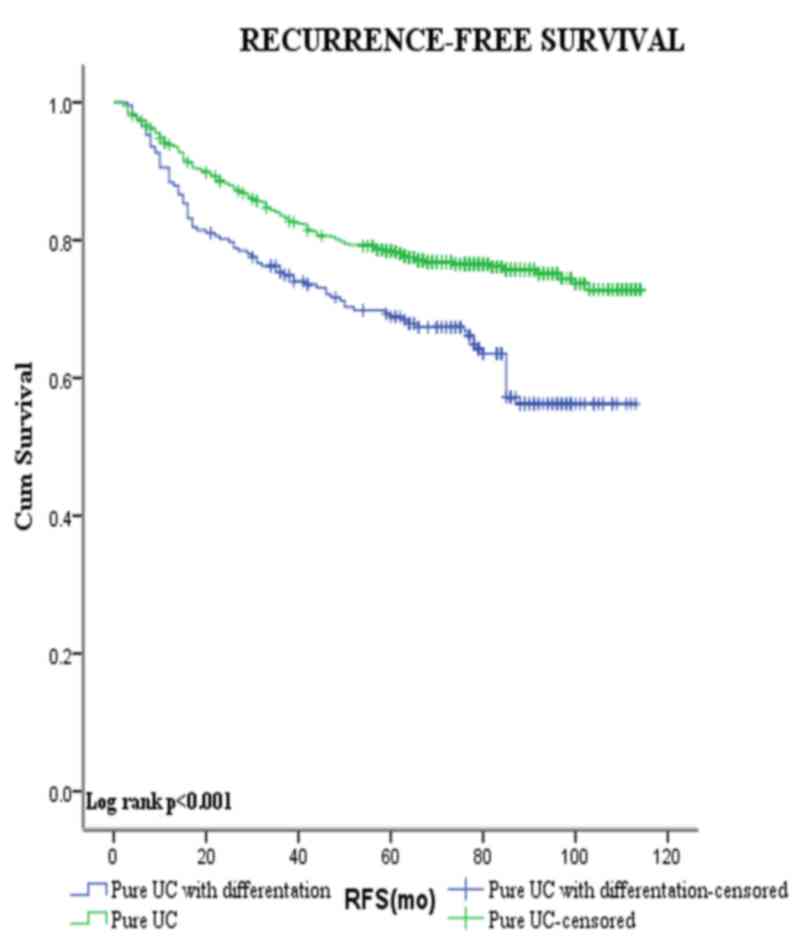
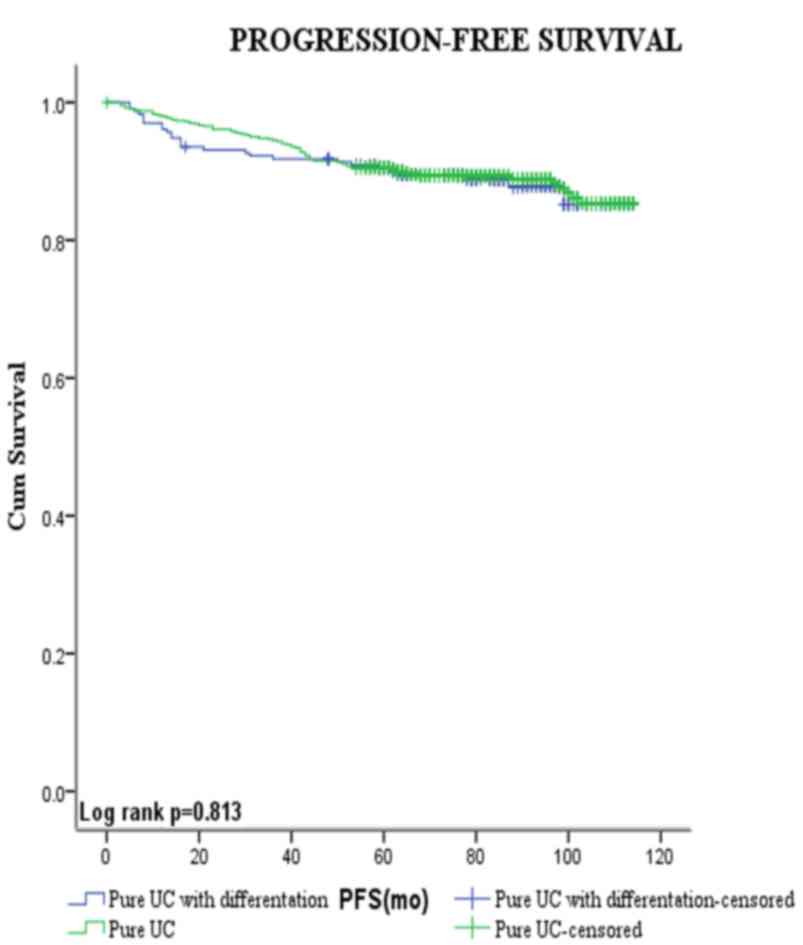
Figs. 3 and 4 show the Kaplan-Meier plots for
recurrence-free and progression-free survival estimates stratified
by pure UCB vs. those with squamous and/or glandular
differentiation. Patients with squamous and/or glandular cell
differentiated UCB had shorter mean RFS duration compared with
those with pure UCB (80.5 vs. 93.0 months, respectively,
P<0.001). By contrast, no significant difference was observed
between the PFS of the two groups (104.7 vs. 103.6 months,
respectively, P=0.813).
The present study conducted additional analysis
using Cox's proportional hazard regression analysis to evaluate the
role of each variable in recurrence (Table III). The results of univariate
analysis revealed that tumor multiplicity [hazard ratio (HR) 1.86,
95% confidence interval (CI) 1.44–2.40, P<0.001], tumor size (HR
1.82, 95% CI 1.41–2.35, P<0.001), tumor grade (HR 1.78, 95% CI
1.38–2.30, P<0.001), pathological tumor stage (HR 2.44, 95% CI
1.08–5.49, P=0.031) and the presence of squamous and/or glandular
differentiation (HR 1.72, 95% CI 1.33–2.24, P<0.001) were
significant factors associated with disease recurrence.
 | Table III.Univariate and multivariate analyses
according to recurrence. |
Table III.
Univariate and multivariate analyses
according to recurrence.
|
| Univariate | Multivariate |
|---|
|
|
|
|
|---|
| Factor | HR (95% CI) | P-value | HR (95% CI) | P-value |
|---|
| Age, years |
|
|
|
|
|
<65 | 1.16
(0.90–1.49) | 0.265 | – | – |
|
≥65 |
|
|
|
|
| Sex |
|
|
|
|
|
Male | 1.08
(0.77–1.50) | 0.657 | – | – |
|
Female |
|
|
|
|
| Multiplicity |
|
|
|
|
|
Single | 1.86
(1.44–2.40) | <0.001 | 1.73
(1.34–2.24) | <0.001 |
|
Multiple |
|
|
|
|
| Tumor size, cm |
|
|
|
|
|
<3 | 1.82
(1.41–2.35) | <0.001 | 1.72
(1.33–2.22) | <0.001 |
| ≥3 |
|
|
|
|
| Tumor grade |
|
|
|
|
|
Low | 1.78
(1.38–2.30) | <0.001 | 1.42
(1.08–1.86) | 0.012 |
|
High |
|
|
|
|
| Pathological tumor
stage |
|
|
|
|
|
pTa | 2.44
(1.08–5.49) | 0.031 | 2.04
(0.90–4.60) | 0.088 |
|
pT1 |
|
|
|
|
| Squamous and/or
glandular differentiation |
|
|
|
|
|
Absent | 1.72
(1.33–2.24) | <0.001 | 1.46
(1.10–1.92) | 0.008 |
|
Present |
|
|
|
|
However, in multivariate Cox's proportional hazard
regression analysis, only tumor multiplicity (HR 1.73, 95% CI
1.34–2.24, P<0.001), tumor size (HR 1.72, 95% CI 1.33–2.22,
P<0.001), tumor grade (HR 1.42, 95% CI 1.08–1.86, P=0.012) and
the presence of squamous and/or glandular differentiation (HR 1.46,
95% CI 1.10–1.92, P=0.008) were demonstrated to be significant
independent predictors of recurrence.
Discussion
Bladder cancer comprises a wide range of
histological types. UC is derived from uroepithelium, and is the
most common type of bladder cancer accounting for >90% of
bladder cancer cases (2). UC is known
to demonstrate variant histologic features, otherwise known as
divergent differentiation. Squamous and glandular elements
represent the most common types of divergent histology in primary
UCB, and these features may coexist within a single tumor (2). However, the clinical significance of
squamous and/or glandular differentiation remains uncertain
(11,12,14,21).
Generally, it has been considered that there are no
prognostic differences between patients with UCB with and without
differentiation (9–11). Kim et al (11) conducted a retrospective review of
1,013 patients who had undergone radical cystectomy, and reported
that patients with urothelial carcinoma, and squamous and/or
glandular differentiation were more likely to possess extravesical
tumors and a node positive disease. Multivariate analysis
controlling for clinicopathological variables revealed that
squamous and/or glandular differentiation was not significantly
associated with the risk of mortality from bladder cancer (11). Similarly, in a single referral center
study of 2,444 patients who had undergone radical cystectomy with
extended lymph node dissection, the outcomes of patients with UCB
with squamous and/or glandular differentiation were similar to
those of patients with pure UCB, considering comparable
demographic, clinicopathological and management characteristics
(22).
However, other studies contradict these findings,
indicating that squamous and/or glandular differentiation appears
to be an unfavorable prognostic feature in such patients undergoing
radical cystectomy, which is possibly due to its association with
high grade tumors (3,4,13,21,23,24).
Squamous and/or glandular differentiation is usually found in
moderate to high grade tumors, and often has deeply invasive
behavior. Antunes et al (3)
identified that squamous differentiation was an independent
prognostic factor for cancer specific survival in patients with
bladder cancer that had been treated with radical cystectomy. In
addition, Honma et al (12)
reported that the existence of a squamous cell carcinoma component
in the specimen may result in a strong impact on the development of
local recurrence following radical cystectomy.
Treatment options for patients with UCB with
squamous and/or glandular differentiation is currently debated.
Previous studies have suggested that these variants may be more
resistant to chemotherapy and radiation therapy compared with pure
UCB (13,19,25).
However, Scosyrev et al (10)
reported that patients with squamous or glandular histological UCB
variants exhibited an improved response to neoadjuvant chemotherapy
[methotrexate, vinblastine, doxorubicin (Adriamycin) and cisplatin]
in a post hoc analysis of a prospective trial. Additional studies
are required in order to evaluate the role of chemotherapy and
radiation therapy in UCBs with squamous and/or glandular
differentiation.
The effect of squamous and/or glandular
differentiation in transurethral resections of the bladder on
prognosis also remains unclear. Billis et al (15) reported that urothelial bladder
carcinomas with squamous and/or glandular differentiation were more
aggressive neoplasms compared with pure UCBs. A statistically
significant association was observed between higher stage and
differentiation in two groups of urothelial carcinoma with and
without squamous and/or glandular differentiation (15). Erdemir et al (23) reported that squamous and/or glandular
differentiation was significantly associated with higher
pathological stage and histological grade. Patients with variant
UCB histology had a higher risk of disease recurrence and
progression, and lower survival rates following TURBT compared with
patients with pure UCB.
A limited number of studies have investigated the
impact of squamous and/or glandular differentiation on oncologic
outcomes in non-invasive UCB. In a study by Miller and
Epstein(25), the presence of
glandular differentiation in noninvasive UCB was associated with an
increased risk of developing to high-grade prognostically poor
invasive bladder carcinomas including poorly-differentiated
UCB.
Unlike previous studies, the emphasis of the present
study was to investigate the impact of squamous and/or glandular
differentiation on recurrence and progression rates in patients
with NMIUCB following TURBT. All patients enrolled in the present
study were pathologically diagnosed as having NMIUCB (pTa + pT1)
and TURBT was performed as the initial treatment. The results
demonstrated that patients with squamous and/or glandular
differentiation had a higher recurrence rate and shorter mean RFS
duration compared with patients with pure UC. However, no
statistically significant difference was observed between the
incidence of progression and mean PFS duration.
Multivariate Cox regression analysis revealed that
squamous and/or glandular differentiation, tumor size, tumor count
and tumor grade were independent prognostic factors of RFS. The
most notable finding was the grade distribution of the tumors with
differentiation. Patients with differentiation were significantly
more likely to possess high grade tumors compared with those with
pure UCB. These findings suggested that the presence of squamous
and/or glandular differentiation in patients with NMUCB is
associated with more aggressive behavior. Follow-up should
therefore be closer for patients with squamous and/or glandular
differentiation due to a higher risk of recurrence.
The present study was not devoid of limitations.
Firstly, limitations occurred due to the retrospective nature of
the present study, with there being nonrandomized samples from a
single institution. In addition, a re-review of all specimens was
not performed and therefore the present study relied on the
pathologist to identify and report the variant histologies.
Furthermore, the present study did not investigate the impact of
squamous and glandular differentiation on NMUCB individually which
may have a disparate prognostic importance.
In summary, the presence of squamous and/or
glandular differentiation in NMIUCB identified at TURBT indicates a
locally aggressive and advance disease. As an independent
prognostic factor of recurrence, patients with the variant form of
NMIUCB present a shorter RFS duration, and should therefore be
followed up closely in case of recurrence.
Acknowledgements
The present study was funded by grants from The
Natural Science Foundation of Tianjin (grant no. 14JCYBJC26300) and
National Key Specialty Construction of Clinical Projects, The
Natural Science Foundation of Tianjin (grant no. 15JCYBJC24600),
and Tianjin Major Scientific and Technological special Project
(grant no. 12ZCDZSY16900). The authors would like to thank all the
study participants, urologists and study coordinators for their
participation in the present study.
References
|
1
|
Torre LA, Bray F, Siegel RL, Ferlay J,
Lortet-Tieulent J and Jemal A: Global cancer statistics, 2012. CA
Cancer J Clin. 65:87–108. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Chalasani V, Chin JL and Izawa JI:
Histologic variants of urothelial bladder cancer and nonurothelial
histology in bladder cancer. Can Urol Assoc J. 3:(6 Suppl 4).
S193–S198. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Antunes AA, Nesrallah LJ, Dall'Oglio MF,
Maluf CE, Camara C, Leite KR and Srougi M: The role of squamous
differentiation in patients with transitional cell carcinoma of the
bladder treated with radical cystectomy. Int Braz J Urol.
33:339–346. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Yang MH, Yen CC, Chen PM, Wang WS, Chang
YH, Huang WJ, Fan FS, Chiou TJ, Liu JH and Chen KK:
Prognostic-factors-based risk-stratification model for invasive
urothelial carcinoma of the urinary bladder in Taiwan. Urology.
59:232–239. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Lopez-Beltran A, Requena MJ,
Alvarez-Kindelan J, Quintero A, Blanca A and Montironi R: Squamous
differentiation in primary urothelial carcinoma of the urinary
tract as seen by MAC387 immunohistochemistry. J Clin Pathol.
60:332–335. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Domanowska E, Jozwicki W, Domaniewski J,
Golda R, Skok Z, Wiśniewska H, Sujkowska R, Wolski Z and Jozwicka
G: Muscle-invasive urothelial cell carcinoma of the human bladder:
Multidirectional differentiation and ability to metastasize. Hum
Pathol. 38:741–746. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Grace DA and Winter CC: Mixed
differentiation of primary carcinoma of the urinary bladder.
Cancer. 21:1239–1243. 1968. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Fegen JP, Albert DJ and Persky L:
Adenocarcinoma and transitional cell carcinoma occurring
simultaneously in the urinary bladder (mixed tumor). J Surg Oncol.
3:387–392. 1971. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kastritis E, Dimopoulos MA, Antoniou N,
Deliveliotis C, Chrisofos M, Skolarikos A, Gika D and Bamias A: The
outcome of patients with advanced pure squamous or mixed squamous
and transitional urothelial carcinomas following platinum-based
chemotherapy. Anticancer Res. 26:3865–3869. 2006.PubMed/NCBI
|
|
10
|
Scosyrev E, Ely BW, Messing EM, Speights
VO, Grossman HB, Wood DP, de Vere White RW, Vogelzang NJ, Trump DL,
Natale RB, et al: Do mixed histological features affect survival
benefit from neoadjuvant platinum-based combination chemotherapy in
patients with locally advanced bladder cancer? A secondary analysis
of southwest oncology group-directed intergroup study (S8710). BJU
Int. 108:693–699. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Kim SP, Frank I, Cheville JC, Thompson RH,
Weight CJ, Thapa P and Boorjian SA: The impact of squamous and
glandular differentiation on survival after radical cystectomy for
urothelial carcinoma. J Urol. 188:405–409. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Honma I, Masumori N, Sato E, Takayanagi A,
Takahashi A, Itoh N, Tamagawa M, Sato MA and Tsukamoto T: Local
recurrence after radical cystectomy for invasive bladder cancer: An
analysis of predictive factors. Urology. 64:744–748. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Frazier HA, Robertson JE, Dodge RK and
Paulson DF: The value of pathologic factors in predicting
cancer-specific survival among patients treated with radical
cystectomy for transitional cell carcinoma of the bladder and
prostate. Cancer. 71:3993–4001. 1993. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Jozwicki W, Domaniewski J, Skok Z, Wolski
Z, Domanowska E and Jozwicka G: Usefulness of histologic
homogeneity estimation of muscle-invasive urinary bladder cancer in
an individual prognosis: A mapping study. Urology. 66:1122–1126.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Billis A, Schenka AA, Ramos CC, Carneiro
LT and Araujo V: Squamous and/or glandular differentiation in
urothelial carcinoma: Prevalence and significance in transurethral
resections of the bladder. Int Urol Nephrol. 33:631–633. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mazzucchelli R, Morichetti D,
Lopez-Beltran A, Cheng L, Scarpelli M, Kirkali Z and Montironi R:
Neuroendocrine tumours of the urinary system and male genital
organs: Clinical significance. BJU Int. 103:1464–1470. 2009.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Sobin LH and Wittekind C: TNM
Classification of Malignant Tumours. Wiley-Liss; 2002
|
|
18
|
Mostofi FK, Sobin LH and Torlini H:
Histologic typing of urinary bladder tumours. World Health
Organization; Geneva: pp. 1–20. 1973
|
|
19
|
Coulson WF: Clinical importance of
squamous metaplasia in invasive transitional cell carcinoma of the
bladder. J Clin Pathol. 42:1227–1228. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Babjuk M, Burger M, Zigeuner R, Shariat
SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A,
Palou Redorta J, et al: EAU guidelines on non-muscle-invasive
urothelial carcinoma of the bladder: Update 2013. Eur Urol.
64:639–653. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Mazzucchelli L, Bacchi M, Studer UE,
Markwalder R, Sonntag RW and Kraft R: Invasion depth is the most
important prognostic factor for transitional-cell carcinoma in a
prospective trial of radical cystectomy and adjuvant chemotherapy.
Int J Cancer. 57:15–20. 1994. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Shapur NK, Katz R, Pode D, Shapiro A,
Yutkin V, Pizov G, Appelbaum L, Zorn KC and Duvdevani M: Is radical
cystectomy mandatory in every patient with variant histology of
bladder cancer. Rare Tumors. 3:e222011. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Erdemir F, Tunc M, Ozcan F, Parlaktas BS,
Uluocak N, Kilicaslan I and Gokce O: The effect of squamous and/or
glandular differentiation on recurrence, progression and survival
in urothelial carcinoma of bladder. Int Urol Nephrol. 39:803–807.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Logothetis CJ, Dexeus FH, Chong C, Sella
A, Ayala AG, Ro JY and Pilat S: Cisplatin, cyclophosphamide and
doxorubicin chemotherapy for unresectable urothelial tumors: The
M.D. Anderson experience. J Urol. 141:33–37. 1989. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Miller JS and Epstein JI: Noninvasive
urothelial carcinoma of the bladder with glandular differentiation:
Report of 24 cases. Am J Surg Pathol. 33:1241–1248. 2009.
View Article : Google Scholar : PubMed/NCBI
|