Introduction
Lung cancer is the leading cause of
cancer-associated mortality worldwide (1). Epidermal growth factor receptor
(EGFR) mutations have been identified in 10–30% of non-small
cell lung cancers (NSCLCs) (2,3).
EGFR-tyrosine kinase inhibitors (TKIs) have been developed to
target mutated EGFR (4). The
first-generation EGFR-TKIs, namely gefitinib and erlotinib, which
bind reversibly to the adenosine triphosphate (ATP)-binding site of
the receptor, markedly changed the treatment strategy for patients
harboring EGFR-mutated lung cancers (4). The response rates to gefitinib or
erlotinib are 60–80% (3,5). Significant benefits of gefitinib or
erlotinib treatment in patients with NSCLC harboring
EGFR-TKI-sensitizing mutations have been repeatedly demonstrated in
multiple clinical trials (6,7). However, despite the initial favorable
response, lung cancer cells eventually acquire resistance to
gefitinib or erlotinib (4). Studies
from the last few years have identified several EGFR-TKI resistance
mechanisms (8–12). The main mechanism, accounting for ~50%
of the resistance, is a secondary mutation in the EGFR gene
subsequent to the initial TKI-sensitizing mutations, specifically
T790M in exon 20 of EGFR (13,14). The
other mechanisms include amplification of the MET oncogene
(10,15,16),
activation of the hepatocyte growth factor (HGF)-MET signaling
pathway through HGF overexpression (17), epithelial-mesenchymal transition
(18), EGFR amplification
transformation to SCLC (19) and
activation of the fibroblastic growth factor (FGF) 2-FGF receptor
(FGFR) 1 signaling pathway through an autocrine loop (11).
In general, lung cancer cells acquire resistance to
EGFR-TKIs in ~1 year (8). For
patients whose lung cancer acquires resistance to initially
administered EGFR-TKIs, multiple cytotoxic agents are available,
including cisplatin, carboplatin, docetaxel, paclitaxel,
irinotecan, gemcitabine, vinorelbine and pemetrexed (20). Despite the hematological and
non-hematological toxicity of these cytotoxic agents, their
efficacy has been consistently reported in multiple settings
(21–25). However, whether lung cancer cells that
have acquired resistance to EGFR-TKIs also exhibit altered
sensitivity to cytotoxic agents remains to be ascertained. An
alternative mechanism for resistance to EGFR-TKIs may be through
the upregulation of resistance-associated genes, including
ATP-binding cassette (ABC) transporter family genes. ABC proteins
contribute to chemoresistance through the efflux of anticancer
drugs from cancer cells (26). The
association between ABC expression and EGFR-TKI resistance has yet
to be clarified. The present study attempted to explore the
response of EGFR-TKI-resistant lung cancer cells to cytotoxic
agents.
Materials and methods
Cell lines
PC-9 cells were a kind gift from Dr Susumu Kobayashi
(Beth Israel Deaconess Medical Center, Boston, MA, USA).
EGFR-TKI-resistant cell lines were previously established by
long-term (~6 months) exposure to erlotinib and gefitinib in our
previous study (19).
Erlotinib-resistant PC-9 (PC-9ER) cells arose following chronic
exposure to erlotinib through the acquisition of the secondary
EGFR mutation, T790M. Gefitinib-resistant PC-9 (PC-9GR)
cells were obtained by chronic exposure to gefitinib through
activation of the FGF2-FGFR1 signaling pathway (11). The PC-9, PC-9ER and PC-9GR cells were
cultured in RPMI-1640 growth medium supplemented with 10% fetal
bovine serum (Gibco; Thermo Fisher Scientific, Inc., Waltham, MA,
USA) at 37°C in a humidified 5% CO2 incubator. The
present study was approved by the Ethics Committee of Keio
University, School of Medicine (Tokyo, Japan).
Materials
Cisplatin and docetaxel were purchased from Wako
Pure Chemical Industries, Ltd. (Osaka, Japan). Gemcitabine and
vinorelbine were purchased from Sigma-Aldrich (Merck KGaA,
Darmstadt, Germany). Pemetrexed was purchased from LC Laboratories
(Woburn, MA, USA).
Cell proliferation assay
A proliferation assay was performed on PC-9, PC-9ER
and PC-9GR cells seeded on 96-well plates at a density of 2,000
cells/50 µl medium/well, which were incubated as previously
described. The following day, 50 µl of RPMI-1640 medium containing
each anticancer chemotherapy drug: Cisplatin, docetaxel,
pemetrexed, gemcitabine or vinorelbine, dissolved in dimethyl
sulfoxide (DMSO) to a concentration of 0.0001, 0.001, 0.01, 0.1, 1
or 10 µM, was added to each well. Control cells were treated with
DMSO. After 72 h of incubation as previously described, the cells
were treated with the CellTiter 96® AQueous One Solution
Cell Proliferation Assay kit (Promega Corporation, Madison, WI,
USA), according to the manufacturer's protocol. Cell population
density was then measured as 490 nm absorbance using a microplate
reader (Model 680; Bio-Rad Laboratories, Inc., Hercules, CA,
USA).
Apoptosis assay
A total of 3×104 PC-9 and PC-9 ER cells
were seeded into each well of 6-well plates. The cells were treated
with 3 µM gemcitabine or 0.1 µM vinorelbine, for 48 h in the
previously described conditions. Control cells were treated with
the same concentration of DMSO. The apoptotic cells were stained
using the TACS Annexin V-FITC Apoptosis Detection kit (R&D
Systems, Inc., Minneapolis, MN, USA), which included propidium
iodide, according to the manufacturer's protocol. The proportion of
apoptotic cells was evaluated using the Gallios Flow Cytometer
(Beckman Coulter, Inc., Brea, CA, USA) and analyzed with FlowJo
software 7.6.5 (TOMY Digital Biology Co., Ltd., Tokyo, Japan).
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA was isolated from cells using an RNeasy
Mini kit (Qiagen, Inc., Valencia, CA, USA) according to the
manufacturer's protocol, and 2 µg of total RNA was subjected to RT
using the High-Capacity RNA-to-cDNA kit (Thermo Fisher Scientific,
Inc., Waltham, MA, USA) according to the manufacturer's protocol.
qPCR was performed using the KAPA SYBR FAST qPCR kit (Kapa
Biosystems, Inc., Wilmington, MA, USA) and an ABI Prism 7000
Sequence Detection System (Applied Biosystems; Thermo Fisher
Scientific, Inc.) according to the manufacturer's protocol. The
thermocycler settings were 40 cycles of 95°C for 5 sec and 60°C for
30 sec. The sequences of the primers used in the present study were
bought from the Roche Life Science Assay Design Center (Roche
Molecular Systems, Inc., Pleasanton, CA, USA), and were as follows:
GAPDH forward, 5′-GAGTCAACGGATTTGGTCGT-3′ and reverse,
5′-TTGATTTTGGAGGGATCTCG-3′; ATP-binding cassette (ABC)B1 forward,
5′-GAAATTTAGAAGATCTGATGTCAAACA-3′ and reverse,
5′-ACTGTAATAATAGGCATACCTGGTCA-3′; ABCC1 forward,
5′-CCATGTGGGAAAACACATCTT-3′ and reverse, 5′-CTGTGCGTGACCAAGATCC-3′;
ABCC2 forward, 5′-AGTGAATGACATCTTCACGTTTG-3′ and reverse,
5′-CTTGCAAAGGAGATCAGCAA-3′; ABCC3 forward,
5′-CCTGGCTGTGCTCTACACCT-3′ and reverse, 5′-GATTCCAGCCGCTTCAGTT-3′;
ABCC5 forward, 5′-GCAGTAAAGCCAGAGGAAGG-3′ and reverse,
5′-CAGCCTGGATGTAGACACCATA-3′; and ABCG2 forward,
5′-TGGCTTAGACTCAAGCACAGC-3′ and reverse,
5′-TCGTCCCTGCTTAGACATCC-3′.
Samples were analyzed in triplicate, and the
experiment was repeated three times. Relative quantification values
were calculated by comparison with GAPDH using the quantification
cycle (Cq) method (27).
Patients and treatments
The patient records of two individuals treated for
EGFR mutation-positive NSCLC at Keio University hospital
were selected based on a case review conference, in which patients
with lung cancer who had experienced extraordinary or unusual
patterns of disease presentation or progression, or patients who
received unusual treatment courses, were discussed. Informed
written consent was obtained from these individuals.
Statistical analysis
Data are presented as mean ± standard deviation.
Statistical analysis for the proliferation assay and RT-qPCR was
performed using GraphPad Prism software, version 4.0 (GraphPad
Software, Inc., La Jolla, CA, USA). Two-sided Student's t tests
were used for comparisons. P<0.05 was considered to indicate a
statistically significant difference.
Results
Increased sensitivity of
EGFR-TKI-resistant cells to gemcitabine and vinorelbine
PC-9 cells harboring an EGFR-activating
mutation (EGFR exon 19 deletion) (28) were used in the present study (29). The established EGFR-TKI-resistant
cells used in the present study were designated as PC-9ER and
PC-9GR cells. PC-9ER cells became resistant to erlotinib by
acquiring the EGFR T790M gatekeeper mutation (i.e., a
mutation that sterically hinders inhibitor interaction), whereas
PC-9GR cells became resistant to gefitinib through activation of
the FGF2-FGFR1 pathway (11). First,
the resistance of PC-9ER and PC-9GR cells to EGFR-TKIs was
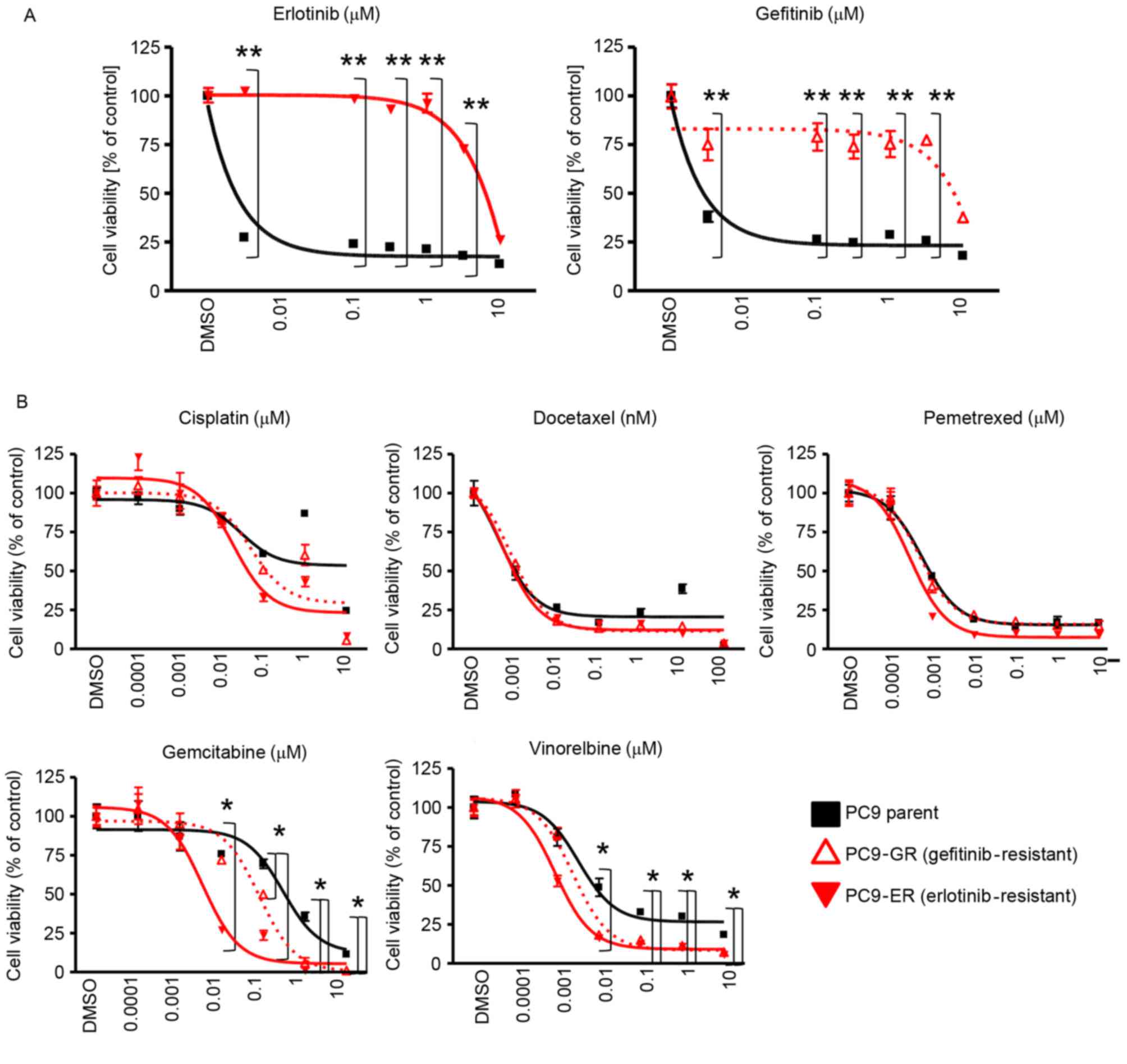
confirmed. The proliferation of PC-9 parent cells was inhibited by
erlotinib and gefitinib at 0.01 µM; however, the proliferation of
PC-9ER and PC-9GR was not significantly inhibited by even 3 mM
erlotinib or gefitinib (Fig. 1A). To
examine whether EGFR-TKI-resistant cells exhibit altered
sensitivity to cytotoxic agents, the sensitivity of PC-9, PC-9ER
and PC-9GR cells was evaluated by MTS assay with or without
cytotoxic agents. The cytotoxic agents included in the present
study were cisplatin, docetaxel, pemetrexed, gemcitabine and
vinorelbine, all of which are widely used in the clinic for the
treatment of patients with NSCLC (22,25,30). The
sensitivity of EGFR-TKI-resistant cells to cisplatin, docetaxel and
pemetrexed was comparable with that of PC-9 parent cells. By
contrast, increased sensitivity of EGFR-TKI-resistant cells to
gemcitabine and vinorelbine was observed (Fig. 1B). These results indicated that
EGFR-TKI-resistant cells had increased sensitivity to gemcitabine
and vinorelbine.
Increased apoptosis of
EGFR-TKI-resistant cells in response to gemcitabine and
vinorelbine
Cytotoxic agents damage the DNA of cancer cells,
which subsequently induces cancer cell apoptosis (31). The aforementioned findings prompted
the authors of the present study to examine whether
EGFR-TKI-resistant cells are more prone to undergo apoptosis with
gemcitabine and vinorelbine treatment. The apoptosis of cancer
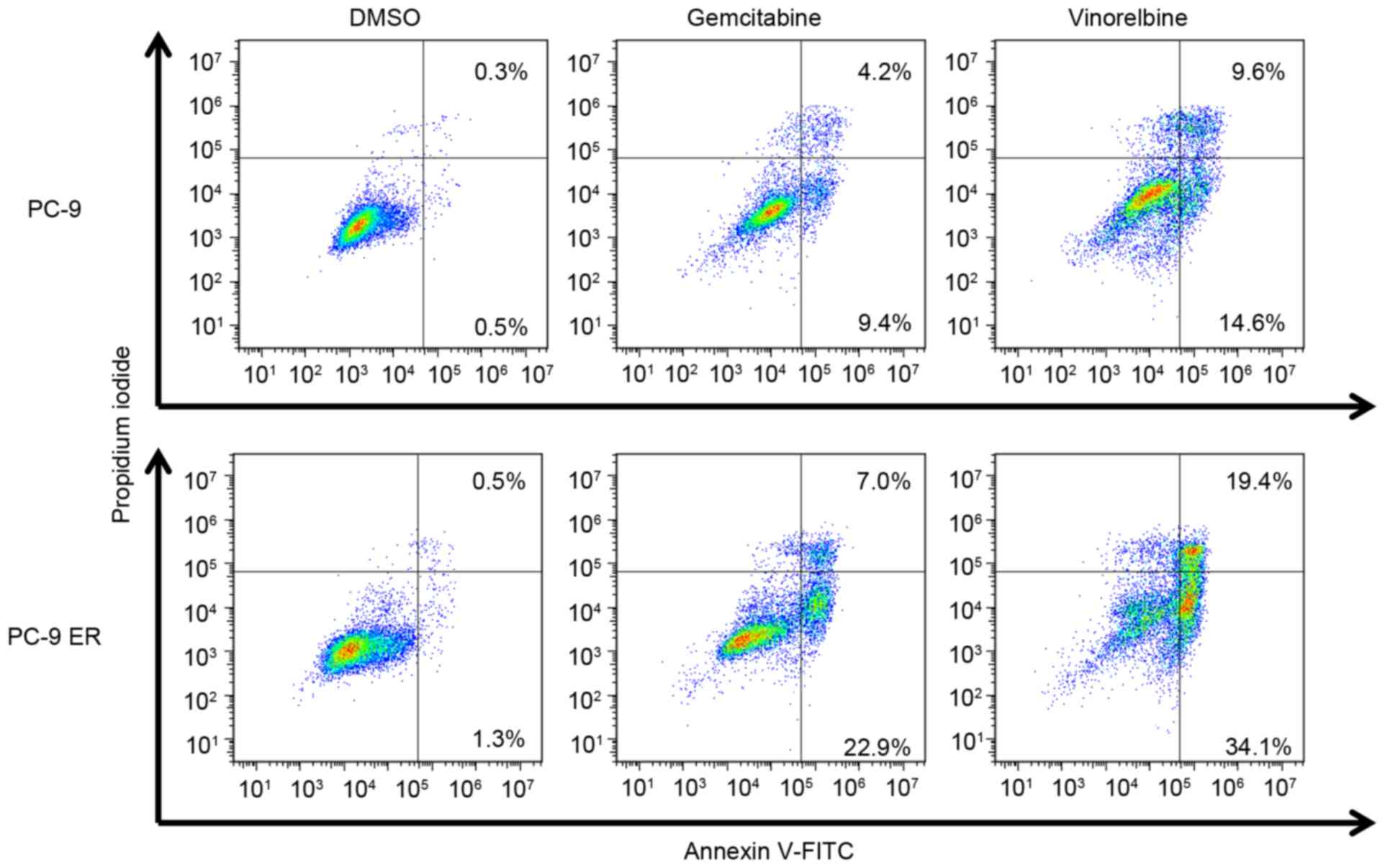
cells was examined using Annexin V and propidium iodide staining.
The proportions of Annexin V-positive PC-9 cells treated with
gemcitabine and vinorelbine were 13.6 and 24.2%, respectively,
while the proportions of Annexin-V-positive PC-9ER cells treated
with gemcitabine and vinorelbine were 29.9 and 53.5%, respectively
(Fig. 2). These data indicated that
EGFR-TKI-resistant cells underwent increased apoptosis upon
gemcitabine and vinorelbine treatment.
Increased or decreased expression of
ABC transporters in EGFR-TKI-resistant cells
ABC transporters efflux cytotoxic agents out from
cancer cells, thereby contributing to the insensitivity of cancer
cells to cytotoxic agents (26,32).
Certain ABC transporter family members have been reported to act as
determinants of cell sensitivity to gemcitabine and vinorelbine
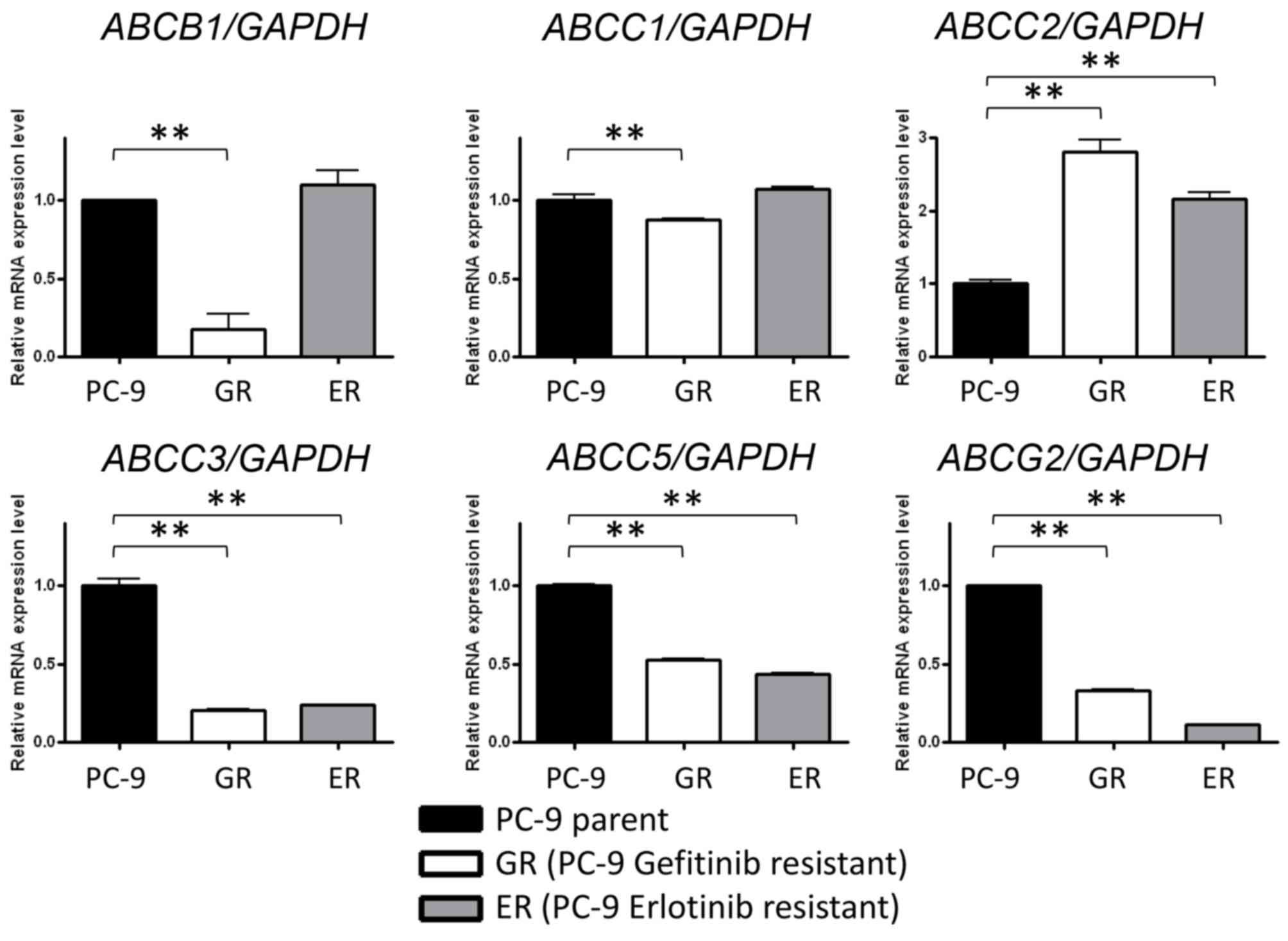
(33). The expression levels of ABC
(34) transporters, including
ABCB1, ABCC1, ABCC2, ABCC3, ABCC5 and ABCG, were
evaluated. ABCB1 and ABCC1 were only significantly
reduced in PC-9GR cells, whereas ABCC2 expression was
significantly upregulated and ABCC3, ABCC5 and
ABCG2 were significantly repressed in PC-9GR and PC-9ER
cells compared with PC-9 cells (Fig.
3). These data indicated that the decreased expression of
ABCC2, ABCC3, ABCC5 and ABCG2 may be
associated with increased sensitivity of EGFR-TKI-resistant cells
to gemcitabine and vinorelbine.
Clinical cases
It was then examined whether the acquisition of
sensitivity to gemcitabine or vinorelbine, as demonstrated by the
present study in vitro, occurred in a clinical setting. Two
notable cases were identified, which are described below.
Patient 1
The first patient was a 48-year-old non-smoking
woman diagnosed with EGFR mutation-positive (exon 19
deletion) lung adenocarcinoma by bronchoscopy in September 2014 at
Keio University hospital. A subsequent positron emission tomography
scan revealed liver and bone metastatic lesions (cT4N3M1b, stage
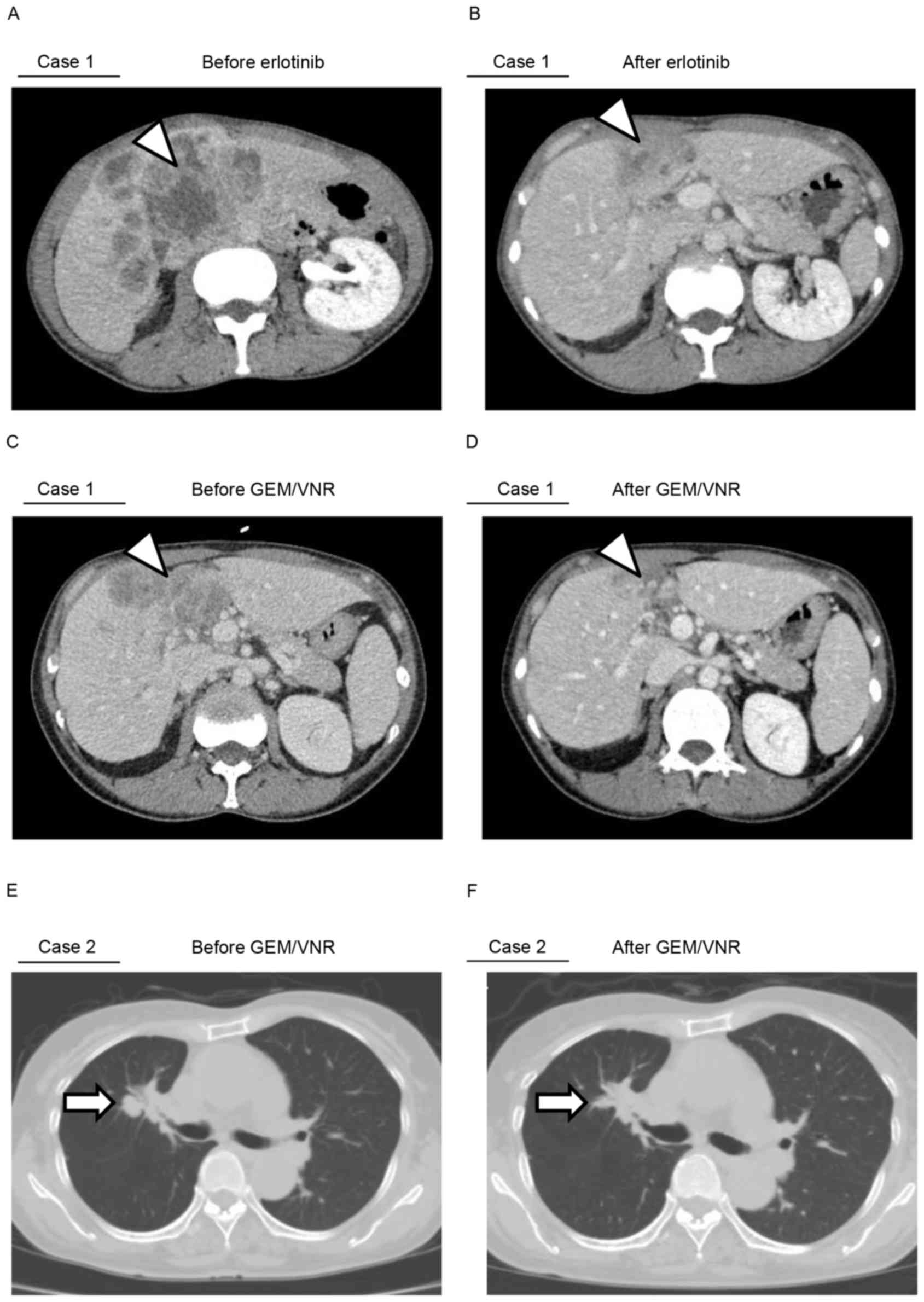
IV). Erlotinib (150 mg) was administered as the first-line
therapeutic against the disease, and it controlled the lesions for
195 days (Fig. 4A and B). Next, 6
courses of carboplatin (area under curve =5), pemetrexed (500
mg/m2) and bevacizumab (15 mg/kg) were administered as
the second-line treatment, which was followed by 3 courses of
pemetrexed as a maintenance therapy until the progression of the
disease, including liver metastatic lesions. The patient is
currently being treated with a regimen of gemcitabine (1,000
mg/m2) plus vinorelbine (25 mg/m2) for 9
courses. Notably, despite the late-line chemotherapy, the regimen
was effective, and it continues to confer sufficient antineoplastic
effect with tolerable side effects (Fig.
4C and D). The patient remains alive at the time of
publishing.
Patient 2
The second patient was a 75-year-old woman, who was
diagnosed with EGFR mutation-positive (exon 21 L858R) lung
adenocarcinoma by bronchoscopy in May 2007 at Keio University
hospital. Subsequent computed tomography and magnetic resonance
imaging scans revealed the staging as cT4N2M0, stage IIIB.
Gefitinib (250 mg) was the first-line regimen administered to the
patient, and this EGFR-TKI stabilized the primary lesions for 14
months. Subsequent to confirming the enlargement of the primary
lesion, 4 courses of carboplatin plus docetaxel (60
mg/m2) were administered as second-line chemotherapy.
Brain and hilar mediastinal lymph node metastases was observed
after 11 months. The third-line option was to re-challenge the
brain lesions with gefitinib (250 mg) and γ-knife radiosurgery.
After 6 months of gefitinib treatment, the primary lesion again
progressed; therefore, fourth-line chemotherapy was administered,
using gemcitabine (1,000 mg/m2) plus vinorelbine (25
mg/m2) for 7 courses. The late-line non-platinum doublet
chemotherapy regimen markedly led to complete remission of advanced
lung adenocarcinoma in this case (Fig. 4E
and F). In addition, following the re-growth of the primary
lesion 1 year after being treatment-free, the re-challenge of 4
courses of gemcitabine plus vinorelbine controlled the lesions. The
patient remains alive at the time of publishing.
Clinical cases conclusion
These results indicated the possibility of using
gemcitabine and vinorelbine as effective agents for patients whose
lung cancer acquires resistance to EGFR-TKIs.
Discussion
In the present study, the sensitivity of
EGFR-TKI-resistant cells to five cytotoxic agents commonly used in
the treatment of patients with NSCLC was evaluated. Notably, an
increased sensitivity of EGFR-TKI-resistant cells to gemcitabine
and vinorelbine was observed.
Several factors are reported to affect cell
sensitivity to gemcitabine and vinorelbine. Thus, overexpression of
breast cancer gene 1 (30), class
III β-tubulin (35), the
ABC transporter family genes ABCB1/multidrug resistance
protein 1 (MDR1) and ABCC10/multidrug
resistance-associated protein 71 (33), and the non-ABC transporter protein Ral
interacting protein 76 (36), has
been reported to lead to resistance to vinorelbine. Overexpression
of ABCC5 (34), human
equilibrative nucleoside transporter 1 (37) and ribonucleotide reductase
(38) has been reported to lead to
resistance to gemcitabine. Several members of the ABC transporter
superfamily, including MDR1 (also known as ABCC1),
confer drug resistance to drug-sensitive cells by effluxing
anticancer or antiviral agents or their metabolites from cells when
expressed at high levels (32).
Therefore, the gene expression levels of the ABC transporter family
in relation to gemcitabine/vinorelbine treatment in the three
tested cell lines were examined. It was observed that the
expression levels of ABCC3, ABCC5 and ABCG2 were
commonly repressed in EGFR-TKI-resistant cell lines, similar to an
earlier study demonstrating the association between ABCC5
expression levels and acquired resistance to gemcitabine (34). However, whether the repressed
expression of ABCC3, ABCC5 and ABCG2 affects the
sensitivity to gemcitabine and vinorelbine of EGFR-TKI-resistant
cells is not known. To understand the mechanism of increased
sensitivity to gemcitabine and vinorelbine of EGFR-TKI-resistant
cells, additional in vitro and in vivo experiments
are necessary.
In the two cases reported in the present study,
gemcitabine and vinorelbine exerted a marked response towards lung
cancers that had acquired resistance to EGFR-TKIs, even with
late-line treatment. However, it remains unclear whether previous
EGFR-TKI treatment increased the sensitivity to subsequent
gemcitabine and vinorelbine treatment. This aspect requires further
investigation in future studies.
The present findings indicated a new treatment
option for EGFR-TKI-resistant NSCLC chemotherapy. However,
additional evaluation through randomized trials remains
necessary.
Acknowledgements
The authors would like to thank Ms. Mikiko Shibuya
(Division of Pulmonary Medicine, Department of Medicine, Keio
University School of Medicine) for her technical assistance. The
present study was supported in part by Grants-in-Aid for Scientific
Research on Priority Areas from the Ministry of Education, Culture,
Sports, Science and Technology of Japan awarded to K. Soejima
(grant no. 22590870) and H. Yasuda (grant nos. 25860656, 15H05666
and 15K14398).
Glossary
Abbreviations
Abbreviations:
|
EGFR
|
epidermal growth factor receptor
|
|
TKIs
|
tyrosine kinase inhibitors
|
|
NSCLC
|
non-small cell lung cancer
|
|
ER
|
erlotinib-resistant
|
|
GR
|
gefinitib-resistant
|
|
ABC
|
adenosine triphosphate-binding
cassette
|
References
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Cancer Genome Atlas Research Network:
Comprehensive molecular profiling of lung adenocarcinoma. Nature.
511:543–550. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Jänne PA and Johnson BE: Effect of
epidermal growth factor receptor tyrosine kinase domain mutations
on the outcome of patients with non-small cell lung cancer treated
with epidermal growth factor receptor tyrosine kinase inhibitors.
Clin Cancer Res. 12:4416s–4420s. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Zhou C and Yao LD: Strategies to improve
outcomes of patients with Egrf-Mutant non-small cell lung cancer:
Review of the literature. J Thorac Oncol. 11:174–186. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Mitsudomi T and Yatabe Y: Mutations of the
epidermal growth factor receptor gene and related genes as
determinants of epidermal growth factor receptor tyrosine kinase
inhibitors sensitivity in lung cancer. Cancer Sci. 98:1817–1824.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Shepherd FA, Rodrigues Pereira J, Ciuleanu
T, Tan EH, Hirsh V, Thongprasert S, Campos D, Maoleekoonpiroj S,
Smylie M, Martins R, et al: Erlotinib in previously treated
non-small-cell lung cancer. N Engl J Med. 353:123–132. 2005.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Mok TS, Wu YL, Thongprasert S, Yang CH,
Chu DT, Saijo N, Sunpaweravong P, Han B, Margono B, Ichinose Y, et
al: Gefitinib or carboplatin-paclitaxel in pulmonary
adenocarcinoma. N Engl J Med. 361:947–957. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Morgillo F, Della Corte CM, Fasano M and
Ciardiello F: Mechanisms of resistance to EGFR-targeted drugs: Lung
cancer. ESMO Open. 1:e0000602016. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Kobayashi S, Ji H, Yuza Y, Meyerson M,
Wong KK, Tenen DG and Halmos B: An alternative inhibitor overcomes
resistance caused by a mutation of the epidermal growth factor
receptor. Cancer Res. 65:7096–7101. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Engelman JA, Zejnullahu K, Mitsudomi T,
Song Y, Hyland C, Park JO, Lindeman N, Gale CM, Zhao X, Christensen
J, et al: MET amplification leads to gefitinib resistance in lung
cancer by activating ERBB3 signaling. Science. 316:1039–1043. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Terai H, Soejima K, Yasuda H, Nakayama S,
Hamamoto J, Arai D, Ishioka K, Ohgino K, Ikemura S, Sato T, et al:
Activation of the FGF2-FGFR1 autocrine pathway: A novel mechanism
of acquired resistance to gefitinib in NSCLC. Mol Cancer Res.
11:759–767. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Nukaga S, Yasuda H, Tsuchihara K, Hamamoto
J, Masuzawa K, Kawada I, Naoki K, Matsumoto S, Mimaki S, Ikemura S,
et al: Amplification of EGFR wild type alleles in non-small cell
lung cancer cells confers acquired resistance to mutation-selective
EGFR tyrosine kinase inhibitors. Cancer Res. 15:2078–2089. 2017.
View Article : Google Scholar
|
|
13
|
Kobayashi S, Boggon TJ, Dayaram T, Jänne
PA, Kocher O, Meyerson M, Johnson BE, Eck MJ, Tenen DG and Halmos
B: EGFR mutation and resistance of non-small-cell lung cancer to
gefitinib. N Engl J Med. 352:786–792. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Pao W, Miller VA, Politi KA, Riely GJ,
Somwar R, Zakowski MF, Kris MG and Varmus H: Acquired resistance of
lung adenocarcinomas to gefitinib or erlotinib is associated with a
second mutation in the EGFR kinase domain. PLoS Med. 2:e732005.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bean J, Brennan C, Shih JY, Riely G, Viale
A, Wang L, Chitale D, Motoi N, Szoke J, Broderick S, et al: MET
amplification occurs with or without T790M mutations in EGFR mutant
lung tumors with acquired resistance to gefitinib or erlotinib.
Proc Natl Acad Sci USA. 104:20932–20937. 2007. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Nakachi I, Naoki K, Soejima K, Kawada I,
Watanabe H, Yasuda H, Nakayama S, Yoda S, Satomi R, Ikemura S, et
al: The combination of multiple receptor tyrosine kinase inhibitor
and mammalian target of rapamycin inhibitor overcomes erlotinib
resistance in lung cancer cell lines through c-Met inhibition. Mol
Cancer Res. 8:1142–1151. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Yano S, Wang W, Li Q, Matsumoto K,
Sakurama H, Nakamura T, Ogino H, Kakiuchi S, Hanibuchi M, Nishioka
Y, et al: Hepatocyte growth factor induces gefitinib resistance of
lung adenocarcinoma with epidermal growth factor
receptor-activating mutations. Cancer Res. 68:9479–9487. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Thomson S, Petti F, Sujka-Kwok I, Epstein
D and Haley JD: Kinase switching in mesenchymal-like non-small cell
lung cancer lines contributes to EGFR inhibitor resistance through
pathway redundancy. Clin Exp Metastasis. 25:843–854. 2008.
View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Sequist LV, Waltman BA, Dias-Santagata D,
Digumarthy S, Turke AB, Fidias P, Bergethon K, Shaw AT, Gettinger
S, Cosper AK, et al: Genotypic and histological evolution of lung
cancers acquiring resistance to EGFR inhibitors. Sci Transl Med.
3:75ra262011. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Gentzler RD and Johnson ML: Complex
decisions for first-line and maintenance treatment of advanced
wild-type non-small cell lung cancer. Oncologist. 20:299–306. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Butts CA, Ding K, Seymour L,
Twumasi-Ankrah P, Graham B, Gandara D, Johnson DH, Kesler KA, Green
M, Vincent M, et al: Randomized phase III trial of vinorelbine plus
cisplatin compared with observation in completely resected stage IB
and II non-small-cell lung cancer: Updated survival analysis of
JBR-10. J Clin Oncol. 28:29–34. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Scagliotti GV, Parikh P, von Pawel J,
Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U,
Digumarti R, Zukin M, et al: Phase III study comparing cisplatin
plus gemcitabine with cisplatin plus pemetrexed in
chemotherapy-naive patients with advanced-stage non-small-cell lung
cancer. J Clin Oncol. 26:3543–3551. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Socinski MA, Bondarenko I, Karaseva NA,
Makhson AM, Vynnychenko I, Okamoto I, Hon JK, Hirsh V, Bhar P,
Zhang H, et al: Weekly nab-paclitaxel in combination with
carboplatin versus solvent-based paclitaxel plus carboplatin as
first-line therapy in patients with advanced non-small-cell lung
cancer: Final results of a phase III trial. J Clin Oncol.
30:2055–2062. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Langer CJ: The emerging world role of
irinotecan in lung cancer. Oncology (Williston Park). 15:(7 Suppl
8). S15–S21. 2001.
|
|
25
|
Fossella FV: Docetaxel in second-line
treatment of non-small-cell lung cancer. Clin Lung Cancer. 3:(Suppl
2). S23–S28. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Fletcher JI, Haber M, Henderson MJ and
Norris MD: ABC transporters in cancer: More than just drug efflux
pumps. Nat Rev Cancer. 10:147–156. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Arao T, Fukumoto H, Takeda M, Tamura T,
Saijo N and Nishio K: Small in-frame deletion in the epidermal
growth factor receptor as a target for ZD6474. Cancer Res.
64:9101–9104. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yasuda H, Park E, Yun CH, Sng NJ,
Lucena-Araujo AR, Yeo WL, Huberman MS, Cohen DW, Nakayama S,
Ishioka K, et al: Structural, biochemical, and clinical
characterization of epidermal growth factor receptor (EGFR) exon 20
insertion mutations in lung cancer. Sci Transl Med. 5:216ra1772013.
View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Busacca S, Sheaff M, Arthur K, Gray SG,
O'Byrne KJ, Richard DJ, Soltermann A, Opitz I, Pass H, Harkin DP,
et al: BRCA1 is an essential mediator of vinorelbine-induced
apoptosis in mesothelioma. J Pathol. 227:200–208. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Cheung-Ong K, Giaever G and Nislow C:
DNA-damaging agents in cancer chemotherapy: Serendipity and
chemical biology. Chem Biol. 20:648–659. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
32
|
Haimeur A, Conseil G, Deeley RG and Cole
SP: The MRP-related and BCRP/ABCG2 multidrug resistance proteins:
Biology, substrate specificity and regulation. Curr Drug Metab.
5:21–53. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Bessho Y, Oguri T, Ozasa H, Uemura T,
Sakamoto H, Miyazaki M, Maeno K, Sato S and Ueda R: ABCC10/MRP7 is
associated with vinorelbine resistance in non-small cell lung
cancer. Oncol Rep. 21:263–268. 2009.PubMed/NCBI
|
|
34
|
Oguri T, Achiwa H, Sato S, Bessho Y,
Takano Y, Miyazaki M, Muramatsu H, Maeda H, Niimi T and Ueda R: The
determinants of sensitivity and acquired resistance to gemcitabine
differ in non-small cell lung cancer: A role of ABCC5 in
gemcitabine sensitivity. Mol Cancer Ther. 5:1800–1806. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Gan PP, Pasquier E and Kavallaris M: Class
III beta-tubulin mediates sensitivity to chemotherapeutic drugs in
non small cell lung cancer. Cancer Res. 67:9356–9363. 2007.
View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Stuckler D, Singhal J, Singhal SS, Yadav
S, Awasthi YC and Awasthi S: RLIP76 transports vinorelbine and
mediates drug resistance in non-small cell lung cancer. Cancer Res.
65:991–998. 2005.PubMed/NCBI
|
|
37
|
Mackey JR, Mani RS, Selner M, Mowles D,
Young JD, Belt JA, Crawford CR and Cass CE: Functional nucleoside
transporters are required for gemcitabine influx and manifestation
of toxicity in cancer cell lines. Cancer Res. 58:4349–4357.
1998.PubMed/NCBI
|
|
38
|
Goan YG, Zhou B, Hu E, Mi S and Yen Y:
Overexpression of ribonucleotide reductase as a mechanism of
resistance to 2,2-difluorodeoxycytidine in the human KB cancer cell
line. Cancer Res. 59:4204–4207. 1999.PubMed/NCBI
|