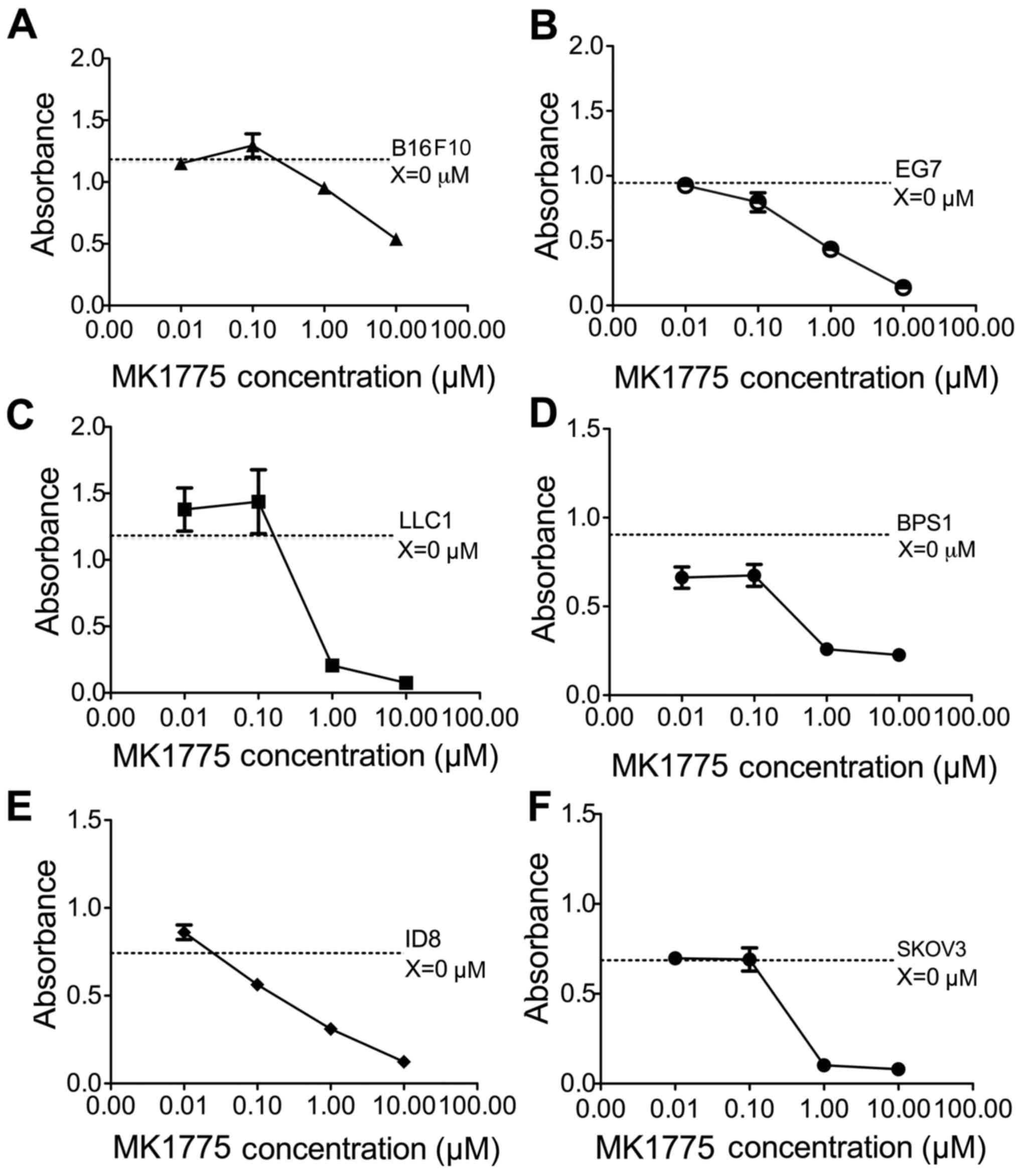
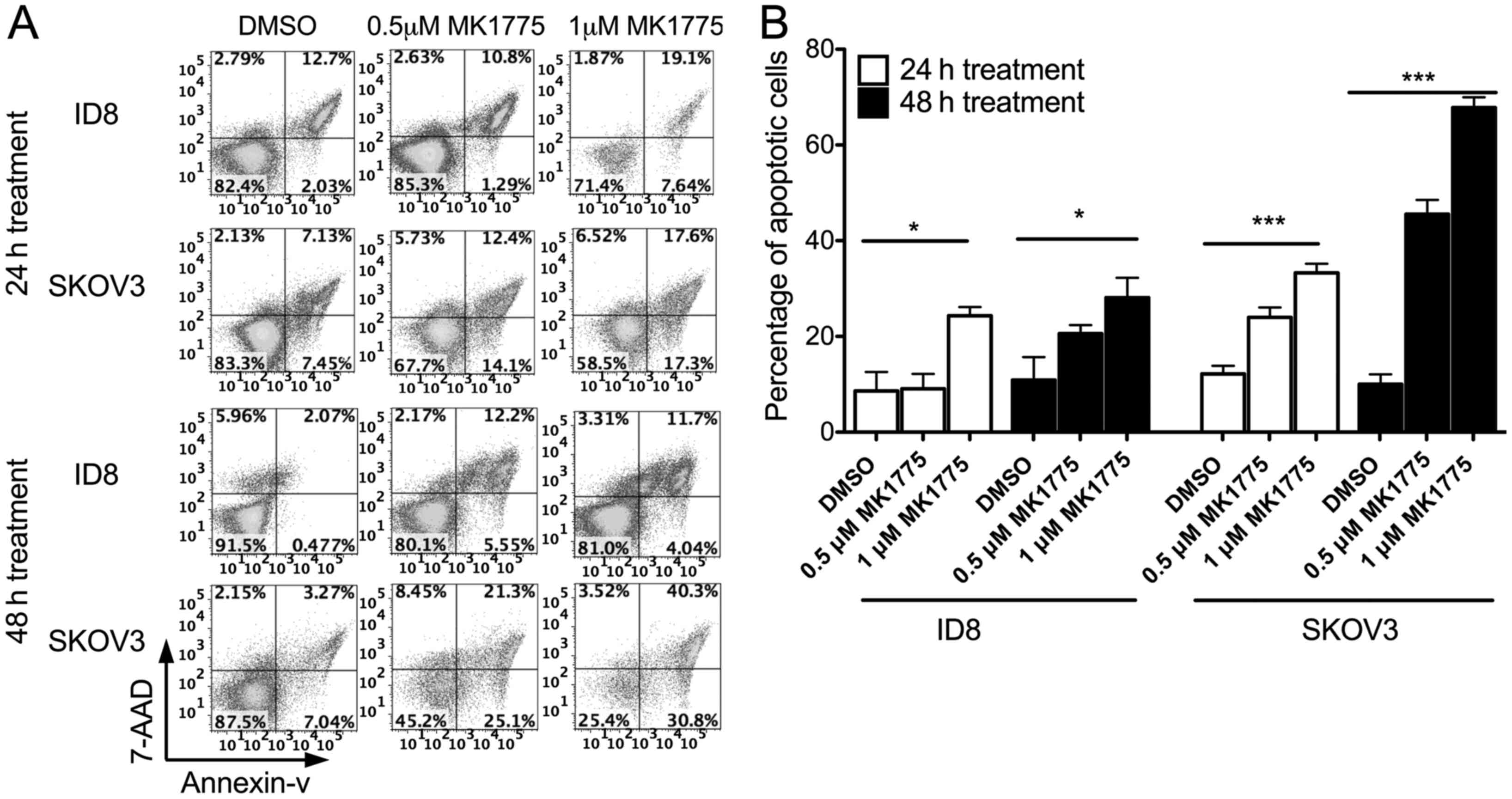
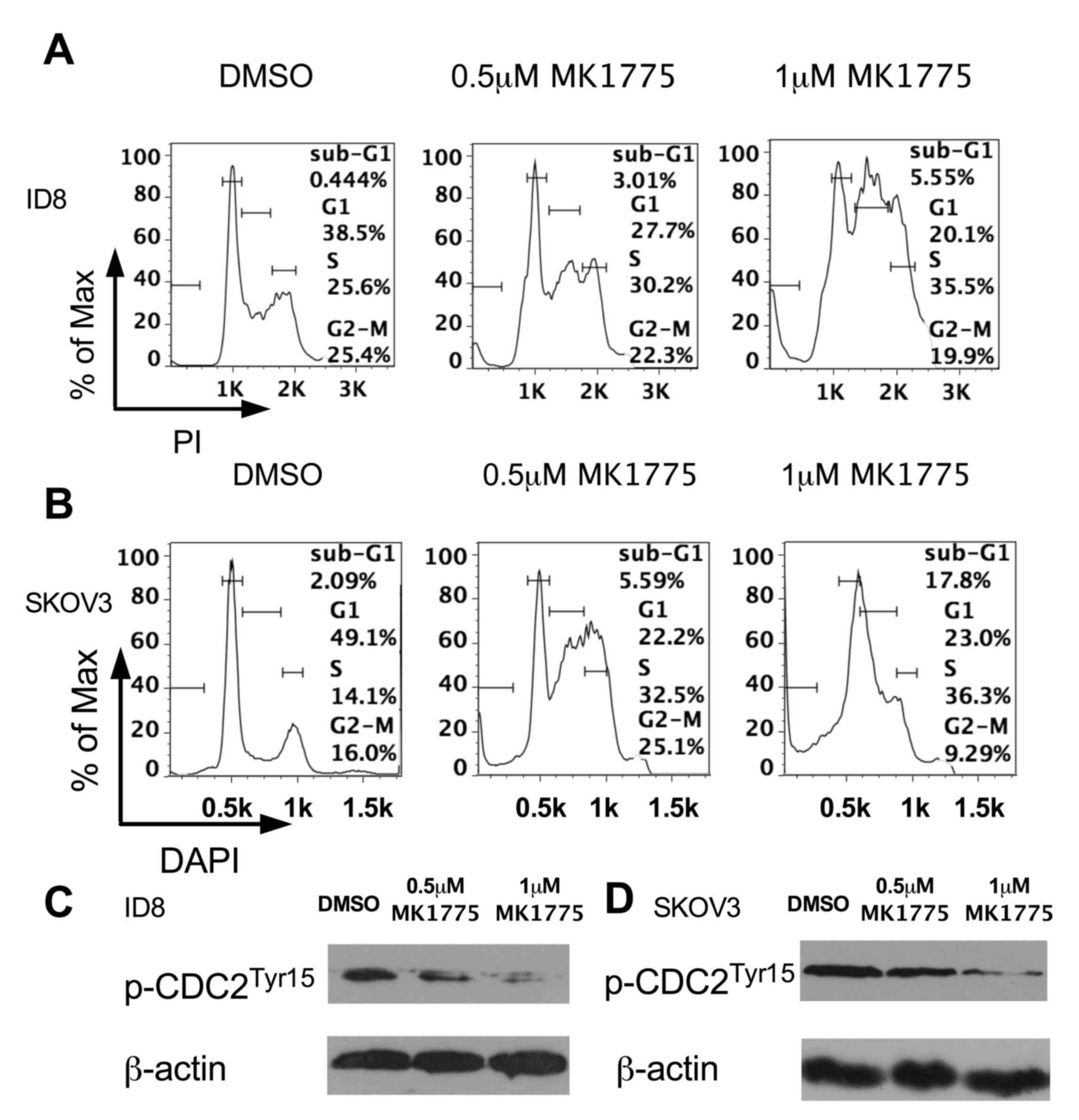
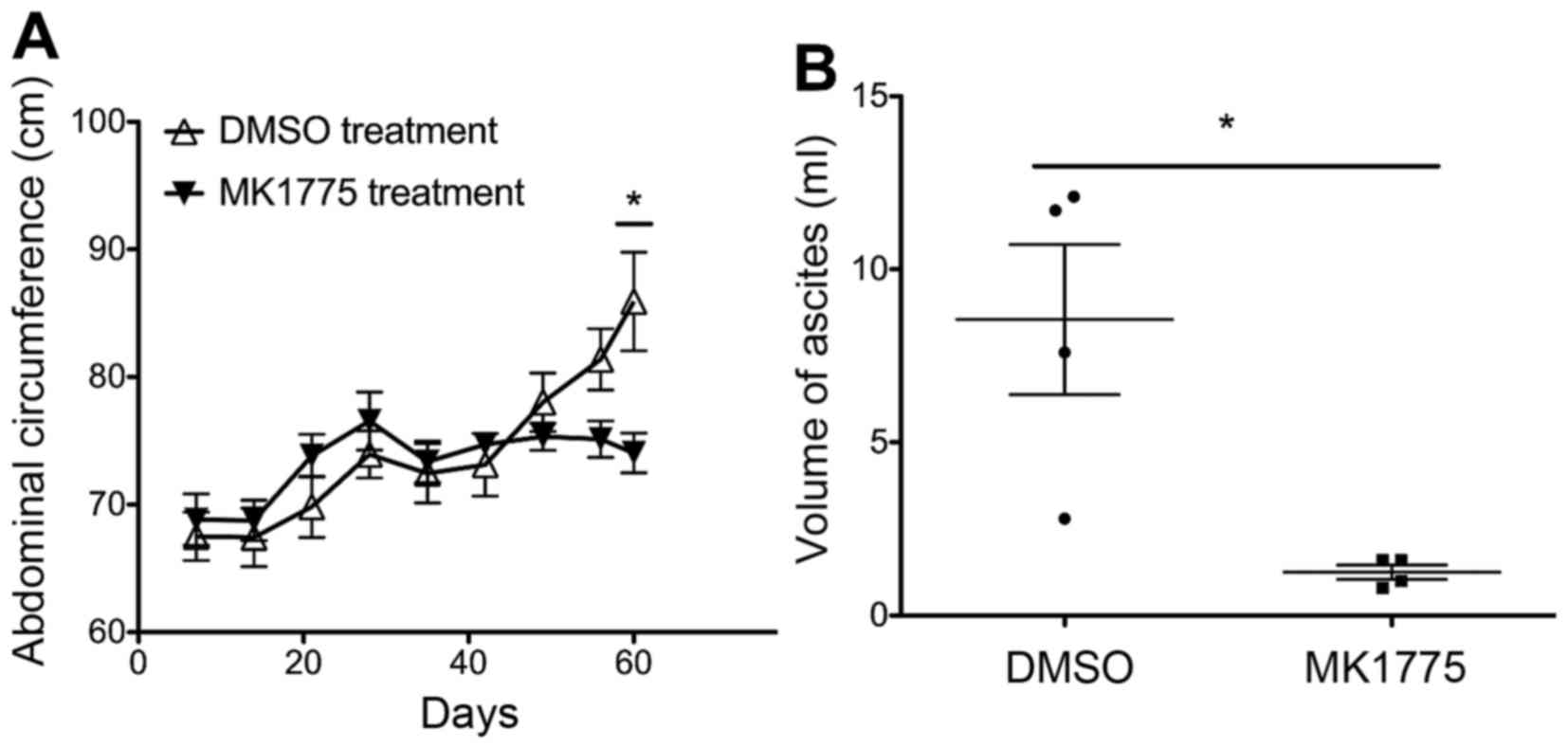
|
1
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2013. CA Cancer J Clin. 63:11–30. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Ferlay J, Shin HR, Bray F, Forman D,
Mathers C and Parkin DM: Estimates of worldwide burden of cancer in
2008: GLOBOCAN 2008. Int J Cancer. 127:2893–2917. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Parker LL and Piwnica-Worms H:
Inactivation of the p34cdc2-cyclin B complex by the human WEE1
tyrosine kinase. Science. 257:1955–1957. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Watanabe N, Broome M and Hunter T:
Regulation of the human WEE1Hu CDK tyrosine 15-kinase during the
cell cycle. EMBO J. 14:1878–1891. 1995.PubMed/NCBI
|
|
5
|
McGowan CH and Russell P: Cell cycle
regulation of human WEE1. EMBO J. 14:2166–2175. 1995.PubMed/NCBI
|
|
6
|
Nigg EA: Cyclin-dependent protein kinases:
Key regulators of the eukaryotic cell cycle. Bioessays. 17:471–480.
1995. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Edgar BA and Lehner CF: Developmental
control of cell cycle regulators: A flys perspective. Science.
274:1646–1652. 1996. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Magnussen GI, Holm R, Emilsen E, Rosnes
AK, Slipicevic A and Flørenes VA: High expression of Wee1 is
associated with poor disease-free survival in malignant melanoma:
Potential for targeted therapy. PLoS One. 7:e382542012. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Magnussen GI, Hellesylt E, Nesland JM,
Trope CG, Flørenes VA and Holm R: High expression of wee1 is
associated with malignancy in vulvar squamous cell carcinoma
patients. BMC Cancer. 13:2882013. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Mir SE, De Witt Hamer PC, Krawczyk PM,
Balaj L, Claes A, Niers JM, Van Tilborg AA, Zwinderman AH, Geerts
D, Kaspers GJ, et al: In silico analysis of kinase expression
identifies WEE1 as a gatekeeper against mitotic catastrophe in
glioblastoma. Cancer Cell. 18:244–257. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
PosthumaDeBoer J, Wördinger T, Graat HC,
van Beusechem VW, Helder MN, van Royen BJ and Kaspers GJ: WEE1
inhibition sensitizes osteosarcoma to radiotherapy. BMC Cancer.
11:1562011. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Iorns E, Lord CJ, Grigoriadis A, McDonald
S, Fenwick K, Mackay A, Mein CA, Natrajan R, Savage K, Tamber N, et
al: Integrated functional, gene expression and genomic analysis for
the identification of cancer targets. PLoS One. 4:e51202009.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Slipicevic A, Holth A, Hellesylt E, Tropé
CG, Davidson B and Flørenes VA: Wee1 is a novel independent
prognostic marker of poor survival in post-chemotherapy ovarian
carcinoma effusions. Gynecol Oncol. 135:118–124. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Hirai H, Iwasawa Y, Okada M, Arai T,
Nishibata T, Kobayashi M, Kimura T, Kaneko N, Ohtani J, Yamanaka K,
et al: Small-molecule inhibition of Wee1 kinase by MK-1775
selectively sensitizes p53-deficient tumor cells to DNA-damaging
agents. Mol Cancer Ther. 8:2992–3000. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Bridges KA, Hirai H, Buser CA, Brooks C,
Liu H, Buchholz TA, Molkentine JM, Mason KA and Meyn RE: MK-1775, a
novel Wee1 kinase inhibitor, radiosensitizes p53-defective human
tumor cells. Clin Cancer Res. 17:5638–5648. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Rajeshkumar NV, De Oliveira E, Ottenhof N,
Watters J, Brooks D, Demuth T, Shumway SD, Mizuarai S, Hirai H,
Maitra A and Hidalgo M: MK-1775, a potent Wee1 inhibitor,
synergizes with gemcitabine to achieve tumor regressions,
selectively in p53-deficient pancreatic cancer xenografts. Clin
Cancer Res. 17:2799–2806. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Hirai H, Arai T, Okada M, Nishibata T,
Kobayashi M, Sakai N, Imagaki K, Ohtani J, Sakai T, Yoshizumi T, et
al: MK-1775, a small molecule Wee1 inhibitor, enhances anti-tumor
efficacy of various DNA-damaging agents, including 5-fluorouracil.
Cancer Biol Ther. 9:514–522. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
http://clinicaltrials.gov/show/NCT01357161Accessed.
11 23–2013.
|
|
19
|
Mueller S, Hashizume R, Yang X, Kolkowitz
I, Olow AK, Phillips J, Smirnov I, Tom MW, Prados MD, James CD, et
al: Targeting Wee1 for the treatment of pediatric high-grade
gliomas. Neuro Oncol. 16:352–360. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Guertin AD, Li J, Liu Y, Hurd MS, Schuller
AG, Long B, Hirsch HA, Feldman I, Benita Y, Toniatti C, et al:
Preclinical evaluation of the WEE1 inhibitor MK-1775 as
single-agent anticancer therapy. Mol Cancer Ther. 12:1442–1452.
2013. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Harris PS, Venkataraman S, Alimova I,
Birks DK, Balakrishnan I, Cristiano B, Donson AM, Dubuc AM, Taylor
MD, Foreman NK, et al: Integrated genomic analysis identifies the
mitotic checkpoint kinase WEE1 as a novel therapeutic target in
medulloblastoma. Mol Cancer. 13:722014. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Kreahling JM, Gemmer JY, Reed D, Letson D,
Bui M and Altiok S: MK1775, a selective Wee1 inhibitor, shows
single-agent antitumor activity against sarcoma cells. Mol Cancer
Ther. 11:174–182. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Carrassa L, Chilà R, Lupi M, Ricci F,
Celenza C, Mazzoletti M, Broggini M and Damia G: Combined
inhibition of Chk1 and Wee1: In vitro synergistic effect translates
to tumor growth inhibition in vivo. Cell Cycle. 11:2507–2517. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jin D, Fan J, Wang L, Thompson LF, Liu A,
Daniel BJ, Shin T, Curiel TJ and Zhang B: CD73 on tumor cells
impairs antitumor T-cell responses: A novel mechanism of
tumor-induced immune suppression. Cancer Res. 70:2245–2255. 2010.
View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Dominguez D, Ye C, Geng Z, Chen S, Fan J,
Qin L, Long A, Wang L, Zhang Z, Zhang Y, et al: Exogenous IL-33
restores dendritic cell activation and maturation in established
cancer. J Immunol. 198:1365–1375. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Dankort D, Curley DP, Cartlidge RA, Nelson
B, Karnezis AN, Damsky WE Jr, You MJ, DePinho RA, McMahon M and
Bosenberg M: Braf(V600E) cooperates with Pten loss to induce
metastatic melanoma. Nat Genet. 41:544–552. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Hooijkaas AI, Gadiot J, van der Valk M,
Mooi WJ and Blank CU: Targeting BRAFV600E in an inducible murine
model of melanoma. Am J Pathol. 181:785–794. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Van Linden AA, Baturin D, Ford JB, Fosmire
SP, Gardner L, Korch C, Reigan P and Porter CC: Inhibition of Wee1
sensitizes cancer cells to antimetabolite chemotherapeutics in
vitro and in vivo, independent of p53 functionality. Mol Cancer
Ther. 12:2675–2684. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Lim ST, Miller NL, Nam JO, Chen XL, Lim Y
and Schlaepfer DD: Pyk2 inhibition of p53 as an adaptive and
intrinsic mechanism facilitating cell proliferation and survival. J
Biol Chem. 285:1743–1753. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Anglesio MS, Wiegand KC, Melnyk N, Chow C,
Salamanca C, Prentice LM, Senz J, Yang W, Spillman MA, Cochrane DR,
et al: Type-specific cell line models for type-specific ovarian
cancer research. PLoS One. 8:e721622013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Do K, Doroshow JH and Kummar S: Wee1
kinase as a target for cancer therapy. Cell Cycle. 12:3159–3164.
2013. View
Article : Google Scholar : PubMed/NCBI
|
|
32
|
Gu L, Zheng H, Murray SA, Ying H and Jim
Xiao ZX: Deregulation of Cdc2 kinase induces caspase-3 activation
and apoptosis. Biochem Biophys Res Commun. 302:384–391. 2003.
View Article : Google Scholar : PubMed/NCBI
|
|
33
|
Targeting p53 mutant ovarian cancer: Phase
I result of the WEE1 inhibitor MK-1775 with carboplatin plus
paclitaxel in patients (pts) with platinum-sensitive, p53-mutant
ovarian cancer (OC). 45th Annual Meeting of the American Society of
Clinical Oncology, Meeting Library. http://meeting library.asco.org2013.
|
|
34
|
http://clinicaltrials.gov/show/NCT01164995Accessed.
November 23–2013.
|
|
35
|
Bucher N and Britten CD: G2 checkpoint
abrogation and checkpoint kinase-1 targeting in the treatment of
cancer. Br J Cancer. 98:523–528. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Kawabe T: G2 checkpoint abrogators as
anticancer drugs. Mol Cancer Ther. 3:513–519. 2004.PubMed/NCBI
|
|
37
|
Vance S, Liu E, Zhao L, Parsels JD,
Parsels LA, Brown JL, Maybaum J, Lawrence TS and Morgan MA:
Selective radiosensitization of p53 mutant pancreatic cancer cells
by combined inhibition of Chk1 and PARP1. Cell Cycle. 10:4321–4329.
2011. View Article : Google Scholar : PubMed/NCBI
|
|
38
|
Chilà R, Basana A, Lupi M, Guffanti F,
Gaudio E, Rinaldi A, Cascione L, Restelli V, Tarantelli C, Bertoni
F, et al: Combined inhibition of Chk1 and Wee1 as a new therapeutic
strategy for mantle cell lymphoma. Oncotarget. 6:3394–3408. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
39
|
Magnussen GI, Emilsen E, Giller Fleten K,
Engesæter B, Nähse-Kumpf V, Fjær R, Slipicevic A and Flørenes VA:
Combined inhibition of the cell cycle related proteins Wee1 and
Chk1/2 induces synergistic anti-cancer effect in melanoma. BMC
Cancer. 15:4622015. View Article : Google Scholar : PubMed/NCBI
|
|
40
|
Huang J, Wang L, Cong Z, Amoozgar Z, Kiner
E, Xing D, Orsulic S, Matulonis U and Goldberg MS: The PARP1
inhibitor BMN 673 exhibits immunoregulatory effects in a Brca1(−/−)
murine model of ovarian cancer. Biochem Biophys Res Commun.
463:551–556. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
41
|
Quinn JE, Carser JE, James CR, Kennedy RD
and Harkin DP: BRCA1 and implications for response to chemotherapy
in ovarian cancer. Gynecol Oncol. 113:134–142. 2009. View Article : Google Scholar : PubMed/NCBI
|