Introduction
Superficial non-ampullary duodenal tumor (SNADT),
adenoma or carcinoma, is a rare type of gastrointestinal tract
epithelial tumor and can be defined as a lesion limited to the
mucosa, and/or submucosa which is not arising from the papilla of
Vater (1,2). Typically, the likelihood of developing
duodenal adenoma in patients with familial adenomatous polyposis
(FAP) is high and this type of adenoma may progress to carcinoma
(3). In contrast, sporadic NADT may
develop de novo or through the adenoma-carcinoma pathway, as
observed in patients with FAP (4).
The prognosis of patients with advanced stage carcinoma is poor
(5). However, if the duodenal tumor
is diagnosed at an early stage and the patient undergoes complete
resection, using an endoscopic method, the patient may experience a
markedly improved outcome. Therefore, early detection and treatment
of lesions are required (6).
As the incidence of sporadic SNADT is rare,
endoscopic markers suggestive of early stage SNADT have not been
established. In addition, although endoscopic approaches, including
endoscopic mucosa resection (EMR) and endoscopic submucosal
dissection (ESD), are minimally invasive, and localized treatments
in comparison to conventional surgery, there are a number of high
risk complications (including bleeding and perforation) which may
occur during the endoscopic resection (ER) (7–9).
Therefore, the relatively rare incidence, and the presence of a
thin duodenal wall and rich vascularity make it difficult to detect
and treat SNADT lesions.
The present case report describes an elderly patient
presented to the Taizhou People's Hospital (Taizhou, China) for
esophagogastroduodenoscopy and was identified to exhibit a
superficial lesion (~1.2 cm) in the second portion of the duodenum.
Chromoscopy and magnification endoscopy with narrow band imaging
(ME-NBI) indicated an early stage lesion. Subsequently, ESD was
performed to remove the lesion. Histopathology validated the
high-grade intro-epithelial tumor diagnosis with negative margins,
and without lymph or blood vessel involvement.
Case report
In the present case report, a 70-year-old Chinese
male presented to the Taizhou People's Hospital (Taizhou, China)
for a regular health screening and was diagnosed with an early NADT
following a gastroduodenoscopy exam. Pathological examination
validated this diagnosis, following the removal of the lesion by
ESD. On admission, the patient's resting blood pressure was normal,
at 120/80 mmHg, the patient had no significant family medical
history and exhibited no symptoms, including melena, abdominal
pain, weight loss, or dyspepsia. The results of physical
examination were unremarkable. Laboratory results were normal, as
follows: White blood cell count, 7,530 cells/mm3 with
66.5% neutrophils; hemoglobin level, 15.0 g/dl; mean corpuscular
volume, 90.0 fl; mean corpuscular hemoglobin concentration, 33.4
g/dl; and platelet count, 189,000 cells/mm3. Liver and
renal function, and electrolyte levels were all identified to be
normal. Tests for tumor markers, including carcinoembryonic antigen
and carbohydrate antigen 19-9, revealed no abnormalities; however,
the carbohydrate antigen 72-4 was mildly increased. The fecal
occult blood test results were negative. A colonoscopy exam
revealed only a sporadic polyp, which excluded the possibility of
FAP. Abdominal computed tomography did not identify a duodenal mass
or any enlargement of the lymph nodes in the abdomen.
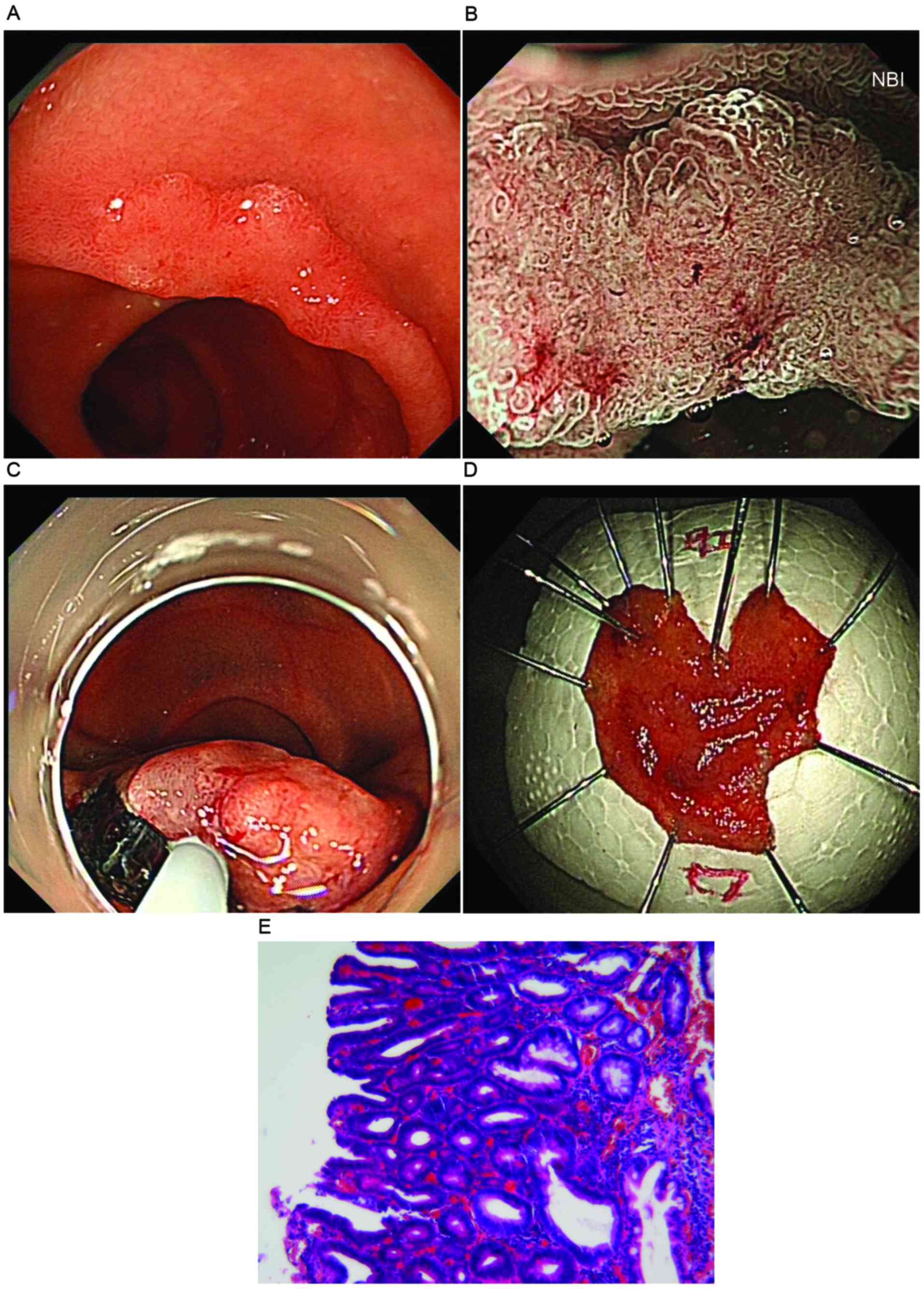
Following an upper endoscopy exam, a red flat lesion
was identified (~1.2 cm), which was elevated with a slightly
depressed area and located on the second portion of the duodenum.
The mass was edematous and extended laterally along the side
opposite the ampulla. The tumor was soft and it bled easily at the
touch (Fig. 1A). In order to overcome
the limitation of traditional white-light endoscopy, ME-NBI was
used to identify distinctions between potentially malignant
lesions. The surface pattern of the mass was preserved in one
region and was absent in another region, which may indicate a mixed
type of tumor (10). In addition,
irregular vascular patterns with clear demarcation were observed
(Fig. 1B), which indicated a category
4/5 tumor associated with high-grade dysplasia (HGD) or
intramucosal carcinoma, according to the revised Vienna
classification (11).
Considering the age of the patient and the
characteristic of the identified lesion, the patient's family
members were consulted and written informed consent was obtained to
remove the lesion through endoscopy. Forcep biopsy was not
performed to avoid associated fibrosis, which may affect additional
endoscopic treatment. ESD as a total biopsy was initially
performed. If histology from the ESD had revealed the tumor was
confined to the mucosal layer, and the horizontal and vertical
margin was negative, the patient may have achieved remission and
subsequently subjected to follow-up. However, if histology had
identified that the tumor exhibited submucosal invasion or
lymphovascular involvement, or the margin was positive, additional
treatments, including a partial resection or
pancreaticoduodenectomy with lymphadenectomy, may have been
considered.
Briefly, a soft transparent hood (D-201-13404) was
attached to the tip of the gastroscope with water jet functions
(GIF-Q260J) and a CO2 insufflation system (UCR) (all
from Olympus Corporation, Tokyo, Japan) was used during the whole
process. A high frequency electrosurgical generator model VIO300 D
(Erbe Elektromedizin GmbH, Tubingen, Germany) was used, which was
set at a cutting current (Endocut mode, effect 2, 40 W) for mucosal
incision and at a coagulating current for submucosal dissection
(Swift Coagulation mode, effect 4, 40 W). Firstly, the margin of
the duodenal tumor was marked according to the chromoendoscopy
using indigo carmine dye spraying. Following submucosal injection
of sodium hyaluronate (Bausch and Lomb Freda Pharmaceutical Co.,
Ltd., Shangdong, China), containing 0.005 and 0.0025% indigo
carmine, and epinephrine, respectively, a mucosal incision was made
outside the tumor margin using a Dual knife (KD-650Q; Olympus
Corporation) (Fig. 1C). A direct
dissection of the submucosal layer was performed to obtain the
complete specimen and subsequently, a complete en bloc resection
was accomplished (Fig. 1C and D). The
lesion was removed and visible vessels located in the bottom of the
ulcer were clamped using hemostatic forceps (FD-410LR; Olympus
Corporation). The surface of the wound was closed using three
titanium clips (Micro-Tech Co., Ltd., Nanjing, China). The
pathological results validated the HGD with negative horizontal and
vertical margins using hematoxylin and eosin staining (Fig. 1E). The patient was administered a
proton pump inhibitor (PPI) for 1 week, and was observed in
hospital in case of delayed complications, including bleeding and
perforation. The patient was discharged and transited to
follow-up.
Discussion
The small intestine accounts for 75% of the entire
digestive tract and tumors which arise from it constitutes ~5% of
all gastrointestinal (GI) tract tumors. However, the duodenum,
which only accounts for 4% of the small intestine, has a relatively
higher proportion of associated tumors compared with tumors of the
jejunum and ileum. Furthermore, although accounting for only 0.3%
of malignant digestive tract tumors, duodenal carcinoma constitutes
50% of all malignant small intestinal tumors (12). Owing to the rarity, non-specific signs
and symptoms, and the fact that the duodenum is typically
overlooked during upper gastrointestinal endoscopy, duodenal tumors
pose diagnostic difficulties. Almost all non-ampullary neoplasms
are incidentally discovered during routine endoscopy, as was the
case in the present report.
The exact etiology of duodenal tumors remains
unknown. Previous studies have demonstrated that patients with FAP,
Crohn's disease, celiac disease, Lynch syndrome or Peutz-Jeghers
syndrome exhibit an increased risk of developing duodenal carcinoma
(13,14). In the present case report, no
associations with the aforementioned diseases were identified and
therefore this lesion appeared a sporadic duodenal tumor. It was
identified that patients with a duodenal carcinoma exhibited a
5-year survival rate of <30% and the prognosis of patients with
an advanced stage was even poorer (5,15).
However, if such lesions are identified at an early stage and
subsequently treated through endoscopic curative resection or
operation, the outcome for patients is often markedly improved
(16). It is important to identify
duodenal tumors at an early stage; however, due to the lack of
specific clinical manifestations, and the fact that the duodenum is
typically overlooked during endoscopy exam, this rare type of
lesion is difficult to identify at an early stage. Furthermore, a
definition for early NADC has not been established according to the
depth of tumor invasion and the risk of lymph node metastasis
(17). Previous studies have used the
regulations for early colorectal or gastric cancer (18,19) and
for tumor invasion into the lamina propria, muscularis mucosa
(T1a), or submucosa (T1b), neglecting lymph node metastasis
(20). It is easier to determine the
margins of early duodenal flat lesion using endoscopy examination
than to distinguish the depth of tumor infiltration, for example
T1a from T1b duodenal cancer. However, developments in endoscopic
technology (including high-resolution endoscopy and image-enhanced
endoscopy) and the establishment of a series of criteria, enabling
the identification of GI tumors (the Vienna classification), have
allowed for the identification of early superficial duodenal tumor
lesions (9,11). In addition, these developments enable
duodenal tumor lesions to be resected without operation (9,11).
To overcome the limitations of traditional
white-light endoscopy with chomoendoscopy, ME-NBI may be used to
identify the potential malignant lesion. NBI refers to an imaging
technique for endoscopic diagnostic medical tests, where light of
specific blue and green wavelengths is used to enhance the details
of certain aspects of the mucosa surface (21). ME-NBI has been revealed to enhance
visualization of the micromucosal and microcirculatory structure
for a detailed assessment of the early lesions, and may be used to
differentiate digestive tract lesions more accurately compared with
conventional endoscopy (22,23). However, there are a limited number of
studies where ME-NBI has been used in association with SNADT. In
2006, Uchiyama et al (24)
reported that ampullary polyps may be classified, using NBI, as:
Type I, oval-shaped villi; type II, pinecone/leaf-shaped villi; or
type III, irregular/non-structured. A previous study categorized
the surface pattern of SNADT as preserved, micrified or absent and
subsequently divided these into two types, mono and mixed, using a
novel diagnostic algorithm for ME-NBI (10). In addition, vessels were defined to
possess one of the following patterns: Absent, network,
intrastructural vascular (ISV) with dilated, tortuous in the
mucosal structure or unclassified (10). All mixed-type lesions identified were
classified as category 4 (mucosal high-grade neoplasia) or category
5 (submucosal invasion by carcinoma) tumors, on the basis of the
revised Vienna classification (10).
Additionally, ~50% (10/23) of the monotype lesions were identified
to be category 3 (mucosal low-grade neoplasia) tumors (10). However, among the monotype lesions,
the proportion of category 4/5 tumors was 100% in lesions with an
unclassified vascular pattern, 64.3% in lesions with an ISV
pattern, 33.3% in lesions with an absent pattern, and 25.5% (1/4)
in lesions with a network pattern (10). Thus, the results of this study are
useful for the identification of benign and malignant lesions in
SNADTs (10). Furthermore, a
multicenter study revealed that a significantly higher number of
high-grade dysplasia or superficial adenocarcinomas compared with
low-grade dysplasia were identified in tumors with a diameter >5
mm with solely or predominantly red coloration (9). According to the aforementioned
categorical descriptions, the lesion in the present study was
identified to possess a mixed type surface with an unclassified
pattern vessel and may be a category 4/5 tumor.
Comprehensive observation of the surface and
vascular patterns of SNADT, using enhanced endoscopy, and ME-NBI
classification maybe used to perform histological diagnosis of a
lesion. However, owing to the limited number of SNADT cases, the
additional advantages of magnifying endoscopy in early duodenal
cancer remain unknown and additional studies are required.
Furthermore, due to the thin wall of the bowel, unintended fibrosis
induced by common biopsy may affect subsequent ER, and the
diagnostic accuracy of enhanced endoscopy and biopsy has been
identified to be similar (78 and 75%, respectively) (9,25).
Therefore, in the present case report, the ESD procedure was
performed to remove the lesion for pathological analysis instead of
forcep biopsy.
The conventional treatment methods for duodenal
tumor are local surgical excision or radical surgery, which are
both characterized by significant proportions of recurrence and
relatively higher morbidity and mortality compared with endoscopy
therapy (26). Surgical resection is
invasive, it is difficult to determine the site and extent of the
lesion from outside the intestine, and resect it locally.
Endoscopic resection for the treatment of SNADT is less invasive,
and a number of endoscopic resection techniques, including snare
polypectomy, EMR, ESD and APC ablative methods for treatment of
SNADT are available. However, the endoscopic therapeutic methods
for SNADT are not standardized, and clinicians are required to
determine whether EMR or ESD may be used on the basis of the size
and location of the lesion, and the operability of patients. Small
duodenal adenomas maybe removed en bloc by conventional EMR;
however, ESD is a more reliable option, as the removal of this type
of malignant tumor is difficult using conventional EMR. ESD is
recognized as a promising minimally invasive approach, which is
curative and safe, even for large lesions in the stomach,
esophagus, and colon (27–29). In contrast, performing ESD in the
duodenum is technically challenging, due to the distinct anatomical
characteristics of the duodenum, and has not been accepted as a
radical method of local resection until the present moment
(30). There have been a limited
number of studies on the use of ESD to treat of SNADTs; however, a
complete resection rate between 80 and 100% with no recurrence was
revealed (17,31). In the present case report, although
the patient was discharged with no complications, there has been a
relatively high proportion of bleeding and perforation identified
in duodenal ESD, attributed to the extensive second-order arterial
blood supply, and thin wall of the duodenum (17,31–33).
Duodenal ESD may be performed with caution in selected patients to
avoid serious complications and follow-up is required.
Previous studies have demonstrated that if the
perforation is in the course of the surgery, the hole maybe closed
using an endoscopic titanium clip or sutured using anylon snare,
followed by the administration of antibiotics and fasting for
several days (8,34–37).
Abdominal computed tomography may be performed in all patients with
perforation to identify whether the retroperitoneal perforation has
occurred. If the perforation cannot be closed and the ESD procedure
cannot be performed, the patient may undergo surgery. In addition,
hematemesis or melena, caused by delayed post-surgical bleeding,
requires additional endoscopy and a hemostatic procedure, using
hemostatic forceps, or clips, similar to performing a resection.
All patients are recommended to be administered a PPI for ≥2 months
following ESD, and undergo follow-up endoscopic examinations 2 and
6 months following ESD, and subsequently every 12 months. In
addition, abdominal computed tomography or ultrasonography may be
performed annually to identify lymph node and distant metastases,
if the final pathological diagnosis of the specimen revealed
characteristics of malignancy (8,34–37).
The present case report identified and diagnosed an
early SNADT using ME-NBI endoscopy, which was completely removed.
Additional studies with a larger number of cases are required to
acquire information on the diagnostic and treatment of SNADT, using
endoscopy.
Acknowledgements
The present case report was supported by the Project
of Health Department of Jiangsu Province (China) (grant no.
H201363), the Social Development Project of Taizhou City (Jiangsu,
China) (grant no. TS025) and the Project of Jiangsu University
(2014).
References
|
1
|
Alwmark A, Andersson A and Lasson A:
Primary carcinoma of the duodenum. Ann Surg. 191:13–18. 1980.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Yokoyama T, Saito D, Kondo H, Kido M,
Hosokawa K, Shirao K, Yokota T, Yamaguchi H, Oguro Y, Ishikawa T,
et al: Endoscopic diagnosis of malignant lesions of the duodenum.
Stomach Int. 28:641–649. 1993.
|
|
3
|
Vasen HF, Möslein G, Alonso A, Aretz S,
Bernstein I, Bertario L, Blanco I, Bülow S, Burn J, Capella G, et
al: Guidelines for the clinical management of familial adenomatous
polyposis (FAP). Gut. 57:704–713. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Perzin KH and Bridge MF: Adenomas of the
small intestine: A clinicopathologic review of 51 cases and a study
of their relationship to carcinoma. Cancer. 48:799–819. 1981.
View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Bakaeen FG, Murr MM, Sarr MG, Thompson GB,
Farnell MB, Nagorney DM, Farley DR, van Heerden JA, Wiersema LM,
Schleck CD and Donohue JH: What prognostic factors are important in
duodenal adenocarcinoma? Arch Surg. 135:635–641. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim CH and Cho YS: Nonampullary duodenal
adenoma: Current understanding of its diagnosis, pathogenesis and
clinical management. World J Gastroenterol. 22:853–861. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Lépilliez V, Chemaly M, Ponchon T,
Napoleon B and Saurin JC: Endoscopic resection of sporadic duodenal
adenomas: An efficient technique with a substantial risk of delayed
bleeding. Endoscopy. 40:806–810. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Ono H, Nonaka S, Uedo N, Kaise M, Oyama T,
Doyama H, Kokawa A, Kaneko K, Kodashima S, Tanabe S, et al:
Clinical issues of duodenal EMR/ESD. Stomach Int. 46:1669–1677.
2011.
|
|
9
|
Goda K, Kikuchi D, Yamamoto Y, Takimoto K,
Kakushima N, Morita Y, Doyama H, Gotoda T, Maehata Y and Abe N:
Endoscopic diagnosis of superficial non-ampullary duodenal
epithelial tumors in Japan: Multicenter case series. Dig Endosc.
26:(Suppl 2). S23–S29. 2014. View Article : Google Scholar
|
|
10
|
Kikuchi D, Hoteya S, Iizuka T, Kimura R
and Kaise M: Diagnostic algorithm of magnifying endoscopy with
narrow band imaging for superficial non-ampullary duodenal
epithelial tumors. Dig Endosc. 26:(Suppl 2). S16–S22. 2014.
View Article : Google Scholar
|
|
11
|
Dixon MF: Gastrointestinal epithelial
neoplasia: Vienna revisited. Gut. 51:130–131. 2002. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Kerremans RP, Lerut J and Penninckx FM:
Primary malignant duodenal tumors. Ann Surg. 190:179–182. 1979.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Heniford BT, Iannitti DA, Evans P, Gagner
M and Henderson JM: Primary nonampullary/periampullary
adenocarcinoma of the duodenum. Am Surg. 64:1165–1169.
1998.PubMed/NCBI
|
|
14
|
Dabaja BS, Suki D, Pro B, Bonnen M and
Ajani J: Adenocarcinoma of the small bowel: Presentation,
prognostic factors, and outcome of 217 patients. Cancer.
101:518–526. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Howe JR, Karnell LH, Menck HR and
Scott-Conner C: The American College of Surgeons Commission on
Cancer and the American Cancer Society. Adenocarcinoma of the small
bowel: Review of the National Cancer Data Base, 1985–1995. Cancer.
86:2693–2706. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Yoshimura N, Goda K, Tajiri H, Ikegami M,
Nakayoshi T and Kaise M: Endoscopic features of nonampullary
duodenal tumors with narrow-band imaging. Hepatogastroenterology.
57:462–467. 2010.PubMed/NCBI
|
|
17
|
Kakushima N, Ono H, Takao T, Kanemoto H
and Sasaki K: Method and timing of resection of superficial
non-ampullary duodenal epithelial tumors. Dig Endosc. 26:35–40.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Japanese Society for Cancer of the Colon
and Rectum: Japanese Classification of Colorectal Carcinoma. 8th.
Kanehara Shuppan, Tokyo: pp. 9–10. 2013
|
|
19
|
Japanese Gastric Cancer Association:
Japanese Classification of Gastric Carcinoma. 14th. Kanehara
Shuppan, Tokyo: pp. 7–8. 2010
|
|
20
|
Takahashi T, Ando T, Kabeshima Y, Kawakubo
H, Shito M, Sugiura H and Omori T: Borderline cases between
benignancy and malignancy of the duodenum diagnosed successfully by
endoscopic submucosal dissection. Scand J Gastroenterol.
44:1377–1383. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Gono K, Obi T, Yamaguchi M, Ohyama N,
Machida H, Sano Y, Yoshida S, Hamamoto Y and Endo T: Appearance of
enhanced tissue features in narrow-band endoscopic imaging. J
Biomed Opt. 9:568–577. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Nakanishi H, Doyama H, Takemura K, Yoshida
N, Tsuji K, Takeda Y, Asahina Y, Kito Y, Ito R, Hayashi T, et al:
Detection of pharyngeal cancer in the overall population undergoing
upper GI endoscopy by using narrow-band imaging: A single-center
experience, 2009–2012. Gastrointest Endosc. 79:558–564. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Miwa K, Doyama H, Ito R, Nakanishi H,
Hirano K, Inagaki S, Tominaga K, Yoshida N, Takemura K, Yamada S,
et al: Erratum to: Can magnifying endoscopy with narrow band
imaging be useful for low grade adenomas in preoperative biopsy
specimens? Gastric Cancer. 18:4462015. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Uchiyama Y, Imazu H, Kakutani H, Hino S,
Sumiyama K, Kuramochi A, Tsukinaga S, Matsunaga K, Nakayoshi T,
Goda K, et al: New approach to diagnosing ampullary tumors by
magnifying endoscopy combined with a narrow-band imaging system. J
Gastroenterol. 41:483–490. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Kakushima N, Kanemoto H, Sasaki K, Kawata
N, Tanaka M, Takizawa K, Imai K, Hotta K, Matsubayashi H and Ono H:
Endoscopic and biopsy diagnoses of superficial, nonampullary,
duodenal adenocarcinomas. World J Gastroentero. 21:5560–5567. 2015.
View Article : Google Scholar
|
|
26
|
Farnell MB, Sakorafas GH, Sarr MG, Rowland
CM, Tsiotos GG, Farley DR and Nagorney DM: Villous tumors of the
duodenum: Reappraisal of local vs. extended resection. J
Gastrointest Surg. 4:13–21. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
27
|
Yahagi N, Fujishiro M, Kakushima N,
Kobayashi K, Hashimoto T, Oka M, Iguchi M, Enomoto S, Ichinose M,
Niwa H and Omata M: Endoscopic submucosal dissection for early
gastric cancer using the tip of an electrosurgical snare (thin
type). Dig Endosc. 16:34–38. 2004. View Article : Google Scholar
|
|
28
|
Oyama T, Tomori A, Hotta K, Morita S,
Kominato K, Tanaka M and Miyata Y: Endoscopic submucosal dissection
of early esophageal cancer. Clin Gastroenterol Hepatol. 3:(7 Suppl
1). S67–S70. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Yamamoto H, Yahagi N and Oyama T:
Mucosectomy in the colon with endoscopic submucosal dissection.
Endoscopy. 37:764–768. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Hoteya S, Yahagi N, Iizuka T, Kikuchi D,
Mitani T, Matsui A, Ogawa O, Yamashita S, Furuhata T, Yamada A, et
al: Endoscopic submucosal dissection for nonampullary large
superficial adenocarcinoma/adenoma of the duodenum: Feasibility and
long-term outcomes. Endosc Int Open. 1:2–7. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Matsumoto S, Miyatani H and Yoshida Y:
Endoscopic submucosal dissection for duodenal tumors: A
single-center experience. Endoscopy. 45:136–137. 2013.PubMed/NCBI
|
|
32
|
Takeuchi M, Kobayashi M, Shioji K, Togashi
T, Hashimoto S, Sato Y, Narisawa R and Aoyagi Y: Prevention and
management of complications in endoscopic resection for
non-ampullary duodenal neoplasms (in Japanese with an English
abstract). Endosc Digest. 22:1561–1158. 2010.
|
|
33
|
Honda T, Yamamoto H, Osawa H, Yoshizawa M,
Nakano H, Sunada K, Hanatsuka K and Sugano K: Endoscopic submucosal
dissection for superficial duodenal neoplasms. Dig Endos.
21:270–274. 2009. View Article : Google Scholar
|
|
34
|
Doyama H, Tominaga K, Yoshida N, Takemura
K and Yamada S: Endoscopic tissue shielding with polyglycolic acid
sheets, fibrin glue and clips to prevent delayed perforation after
duodenal endoscopic resection. Dig Endos. 26:(Suppl 2). 41–45.
2014. View Article : Google Scholar
|
|
35
|
Takimoto K, Imai Y and Matsuyama K:
Endoscopic tissue shielding method with polyglycolic acid sheets
and fibrin glue to prevent delayed perforation after duodenal
endoscopic submucosal dissection. Dig Endosc. 26:(Suppl 2). 46–49.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Inoue T, Uedo N, Yamashina T, Yamamoto S,
Hanaoka N, Takeuchi Y, Higashino K, Ishihara R, Iishi H, Tatsuta M,
et al: Delayed perforation: A hazardous complication of endoscopic
resection for non-ampullary duodenal neoplasm. Dig Endos.
26:220–227. 2014. View Article : Google Scholar
|
|
37
|
Mori H, Shintaro F, Kobara H, Nishiyama N,
Rafiq K, Kobayashi M, Nakatsu T, Miichi N, Suzuki Y and Masaki T:
Successful closing of duodenal ulcer after endoscopic submucosal
dissection with over-the-scope clip to prevent delayed perforation.
Dig Endosc. 25:459–461. 2013. View Article : Google Scholar : PubMed/NCBI
|