Introduction
Cervical cancer is the second highest cancer
associated-morbidity and mortality in females worldwide, with
>500,000 novel cases and ~260,000 mortalities caused by cervical
cancer annually (1,2). Cervical cancer is more prevalent in
developing countries, primarily due to the lack of screening
programs, diagnostic procedures and effective therapies (3,4). Patients
with cervical cancer at an early stage are treated with surgery,
followed by radiotherapy and/or chemotherapy (5,6). For
patients with metastatic diseases, or those at a more advanced
stage who are at marked risk of recurrence, concurrent
chemoradiotherapy was recommended as a standard therapy (7,8). However,
the overall survival rate for patients with locally advanced and
metastatic cervical cancer is between 30 and 50%, and between 5 and
15%, respectively (9). Furthermore,
~30% of patients presented with cancer recurrence, lymph node
recurrence and distant metastasis, and obtained an unfavorable
prognosis (10). Although the
mechanism of cervical carcinogenesis and progression has been
hypothesized, the underlying molecular mechanism of cervical
carcinogenesis and progression remains unknown (11). Thus, understanding the molecular
mechanism underlying the initiation and progression of cervical
cancer, and identifying effective therapeutic treatments for
patients with cervical cancer is required.
MicroRNAs (miRNAs) are a group of endogenous
non-protein-coding single-stranded short RNA molecules of between
19 and 25 nucleotides in length (12). miRNAs negatively regulate protein
expression predominantly through binding to the 3′ untranslated
regions (3′UTRs) of target mRNAs in a base-pairing manner,
resulting in the degradation or translation inhibition of mRNA
(13–15). Previous studies have indicated that
miRNAs regulate the activity of >30% of human genes and are
implicated in a variety of physiological and pathological
processes, including cell viability, development, apoptosis,
survival and metastasis (16–18). In addition, an association between
abnormal expression of miRNAs and the initiation and development of
cancers has been identified (19,20).
Furthermore, miRNAs may function as oncogenes or as tumor
suppressors, depending on the nature of their target mRNAs
(21,22). Therefore, targeting miRNAs may be used
to identify a novel therapeutic treatment for patients with
cervical cancer.
The aim of the present study was to investigate the
expression, functions and underlying molecular mechanism of miR-320
in cervical cancer. miR-320 was identified to be markedly
downregulated in cervical cancer tissues and cell lines. In
vitro functional studies revealed that overexpression of
miR-320 inhibited the viability, migration and invasion of cervical
cancer, whereas the inhibition of miR-320 enhanced these processes.
Additionally, forkhead box M1 (FOXM1) was identified as a direct
functional target gene of miR-320 in cervical cancer. The results
of the present study revealed a novel underlying molecular
mechanism involved in the regulation of FOXM1 and cervical
carcinogenesis and development. Thus, miR-320/FOXM1-based targeted
therapy may be a therapeutic strategy for patients with cervical
cancer.
Materials and methods
Tissue samples
The present study was approved by the Medical Ethics
Committee of Beijing Chao-Yang Hospital (Beijing, China), in
accordance with The Declaration of Helsinki. In addition, written
informed consent was obtained from all patients in the present
study, in order for patient information to be stored in the
hospital database and used for the present study. A total of 36
human cervical cancer tissue samples and corresponding healthy
cervical epithelial tissues were obtained from patients with
cervical cancer who underwent surgery at Beijing Chao-Yang
Hospital. None of the patients received chemotherapy and
radiotherapy, or other therapeutic treatments prior to surgery. All
tissue specimens were immediately frozen in liquid nitrogen
following surgery and stored at −80°C.
Cell lines and culture conditions
An immortalized human papillomavirus (HPV)-negative
skin keratinocyte line (HaCaT), four human cervical cancer cell
lines (HeLa, CaSki, C33A and SiHa) and the HEK293T cell line, used
for the luciferase reporter assay, were obtained from the Shanghai
Institute of Biochemistry and Cell Biology (Shanghai, China). All
cells were cultured in Dulbecco's modified Eagle's medium (DMEM) or
RPMI-1640 medium and supplemented with 10% fetal bovine serum (FBS)
and 1% penicillin-streptomycin (all from Gibco; Thermo Fisher
Scientific, Inc., Waltham, MA, USA), at 37°C in a humidified
atmosphere containing 5% CO2.
miRNA/short interfering RNA (siRNA)
transfection
Human miR-320 mimic, a corresponding negative
control (NC), miR-320 inhibitor and NC inhibitor were obtained from
GenePharma Co., Ltd. (Shanghai, China). FOXM1 siRNA and the
scramble control siRNA were purchased from Guangzhou RiboBio Co.,
Ltd. (Guangzhou, China), and were used to knock down FOXM1 in
cervical cancer cells. The miR-320 mimic sequence was
5′-AAAAGCUGGGUUGAGAGGGCGA-3′; the NC, 5′-UUCUCCGAACGUGUCACGUTT-3′;
miR-320 inhibitor, 5′-CCUCUCAACCCAGCUUUU-3′; NC inhibitor,
5′-CAGUACUUUUGUGUAGUACAA-3′; FOXM1 siRNA,
5′-GGACCACUUUUCCCUACUUUTT-3′; scramble control siRNA,
5′-UUCUUCCGAACGUGUCACGUTT-3′. Cells were transfected with 50
pmol/ml miR-320 mimic, NC, miR-320 inhibitor, NC inhibitor, FOXM1
siRNA or scramble control siRNA using Lipofectamine 2000 reagent
(Invitrogen; Thermo Fisher Scientific, Inc.) according to the
manufacturer's protocol. The medium was replaced with culture
medium at 6 h after transfection.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA extraction from tissues or cells was
carried out using TRIzol reagent (Invitrogen; Thermo Fisher
Scientific, Inc.). To determine mature miR-320 and U6 expression,
reverse transcription was conducted using a TaqMan MicroRNA Reverse
Transcription kit, followed by RT-qPCR using a TaqMan miRNA assay
(all from Applied Biosystems; Thermo Fisher Scientific, Inc.). The
thermocycling conditions were as follows: 40 cycles of denaturation
at 95°C for 15 sec and annealing/extension at 60°C for 60 sec. U6
was used as an internal control for miR-320 expression. The primers
were designed as follows: miR-320,
5′-ACACTCCAGCTGGGAAAAGCTGGGTTGAGA-3′ (forward) and
5′-TGGTGTCGTGGAGTCG-3′ (reverse); U6, 5′-CTCGCTTCGGCAGCACATATACT-3′
(forward) and 5′-ACGCTTCACGAATTTGCGTGTC-3′ (reverse). All samples
were performed in triplicate. Relative expression level was
calculated using the 2−ΔΔCq method (23).
MTT assay
Cell viability was determined using an MTT assay (5
mg/ml; Sigma-Aldrich; Merck KGaA, Darmstadt, Germany). At 24 h
after transfection, cells were collected and seeded into 96-well
plates at a density of 4,000 cells/well and cultured for 24, 48, 72
and 96 h. A total of 20 µl MTT solution was added to each well
prior to incubation for an additional 4 h. Subsequently, 200 µl
dimethyl sulfoxide (Sigma-Aldrich; Merck KGaA) was used to dissolve
the formazan precipitates. The absorbance at 490 nm was determined
using an automatic multi-well spectrophotometer (Bio-Rad
Laboratories, Inc., Hercules, CA, USA). All experiments were
carried out in triplicate.
Transwell cell migration and invasion
assay
Transfected cells (1×105) in 200 µl
serum-free DMEM were placed into the upper Transwell chamber (8 µm;
Millipore, Billerica, MA, USA), which were pre-coated with or
without Matrigel (BD Biosciences, San Jose, CA, USA). The lower
compartment was filled with 500 µl DMEM medium containing 20% FBS
to act as a chemoattractant. Following incubation at 37°C for 24 h,
cells that had not migrated or invaded to the lower surface of the
filters were removed using cotton swabs. Subsequently, the cells
that had migrated or invaded to the bottom surface of the Transwell
chambers were fixed with 100% methanol for 10 min, stained with
0.5% crystal violet solution for 10 min, and washed with PBS, at
room temperature. The capacity for cell migration and invasion was
determined by counting 5 fields per membrane with an optical
microscope (magnification, ×200; Olympus IX53; Olympus Corporation,
Tokyo, Japan). Each experiment was performed in triplicate.
miR-320 target prediction
The potential targets of miR-320 were predicted
using the PicTar (pictar.mdc-berlin.de), TargetScan (www.targetscan.org) and miRanda (www.microrna.org/microrna) databases.
Dual-luciferase reporter assay
pGL3-FOXM1-3′UTR wild-type (Wt) or pGL3-FOXM1-3′UTR
mutant (Mut) were purchased from GenePharma Co., Ltd. HEK293T cells
were seeded into 24-well plates. Following incubation at 37°C
overnight, cells were transfected with miR-320 mimic or NC, and
co-transfected with pGL3-FOXM1-3′UTR Wt or PGL3-FOXM1-3′UTR Mut
using Lipofectamine 2000 reagent. Luciferase activities were
determined consecutively 48 h post-transfection using
Dual-Luciferase Reporter assays (Promega Corporation, Madison, WI,
USA), according to the manufacturer's protocol. The Renilla
luciferase activities were normalized to the firefly luciferase
activities. All experiments were carried out in triplicate.
Western blot analysis
Western blot analysis was used to evaluate the
expression level of FOXM1. Cells were collected and lysed in
ice-cold radioimmunoprecipitation assay buffer (Beyotime Institute
of Biotechnology, Haimen, China) 72 h post-transfection. The
concentration of total protein was measured using a Bicinchoninic
Acid Protein assay kit (Thermo Fisher Scientific, Inc.). Equal
amounts of protein (30 µg) were separated using SDS-PAGE (10% gel)
and subsequently transferred onto polyvinylidene fluoride membranes
(Millipore). Following incubation with Tris-buffered
saline-Tween-20 (TBST) containing 5% non-fat dry milk at room
temperature for 1 h, the membranes were probed with the following
primary antibodies: Mouse anti-human monoclonal FOXM1 (1:1,000
dilution; cat. no. sc-166709; Santa Cruz Biotechnology, Inc.,
Dallas, TX, USA) and β-actin (1:1,000 dilution; cat. no. sc-130301;
Santa Cruz Biotechnology, Inc.). Following incubation at 4°C
overnight, the membranes were washed with TBST three times,
followed by incubation with corresponding horseradish
peroxidase-conjugated secondary antibody (1:1,000 dilution; cat.
no. A0216; Beyotime Institute of Biotechnology) for 1 h at room
temperature. The protein bands were determined using the an
enhanced chemiluminescence kit (Pierce; Thermo Fisher Scientific,
Inc.).
Statistical analysis
Data are presented as the mean ± standard deviation
and compared with Student's t-test, or one-way analysis of variance
followed by the SNK multiple comparison test, using SPSS software
(version 13.0; SPSS, Inc., Chicago, IL, USA). P<0.05 was
considered to indicate a statistically significant difference.
Results
Expression of miR-320 is downregulated
in cervical cancer tissue samples and cell lines
To explore whether miR-320 was involved in the
carcinogenesis and progression of cervical cancer, the expression
levels of miR-320 in human cervical cancer tissues and
corresponding healthy cervical epithelial tissues were determined.
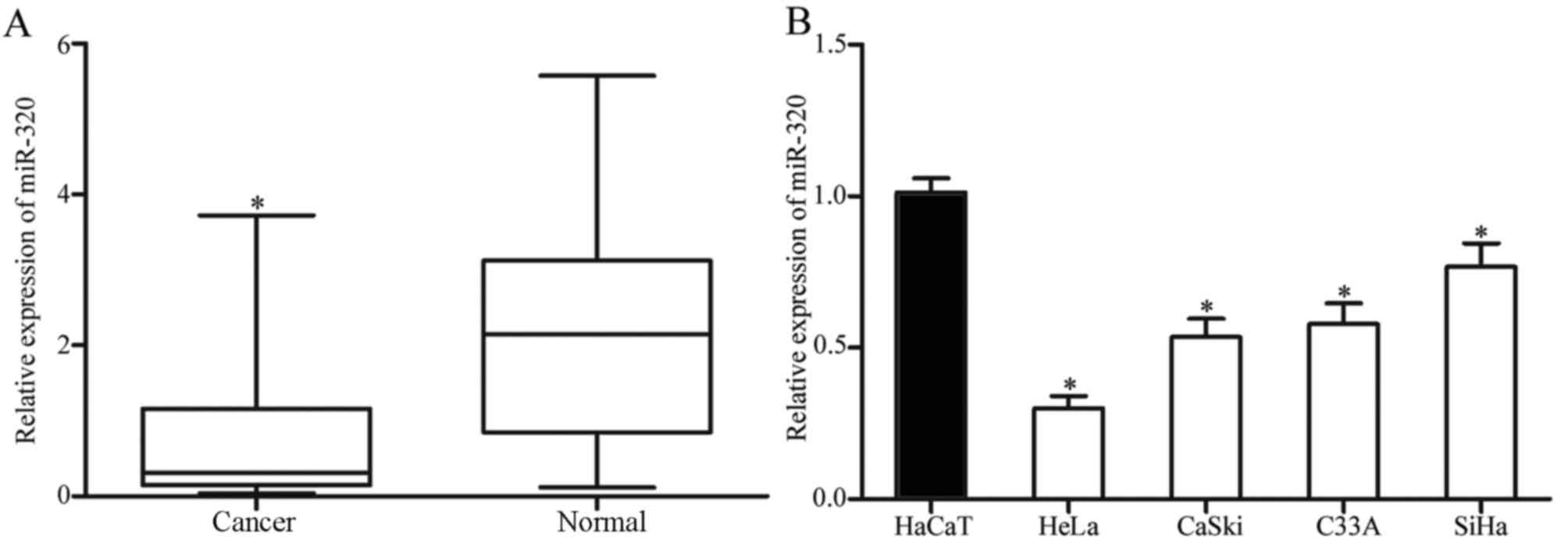
As presented in Fig. 1A, miR-320 was
markedly downregulated in cervical cancer tissue samples, compared
with that in corresponding healthy cervical epithelial tissues.
Consistent with the results identified in cervical cancer tissues,
the expression level of miR-320 was decreased in HeLa, CaSki, C33A
and SiHa cell lines, compared with that in an immortalized
HPV-negative skin keratinocyte line (HaCaT; Fig. 1B). Thus, miR-320 was identified to be
downregulated in cervical cancer.
Overexpression of miR-320 suppresses
viability, migration and invasion of cervical cancer cells
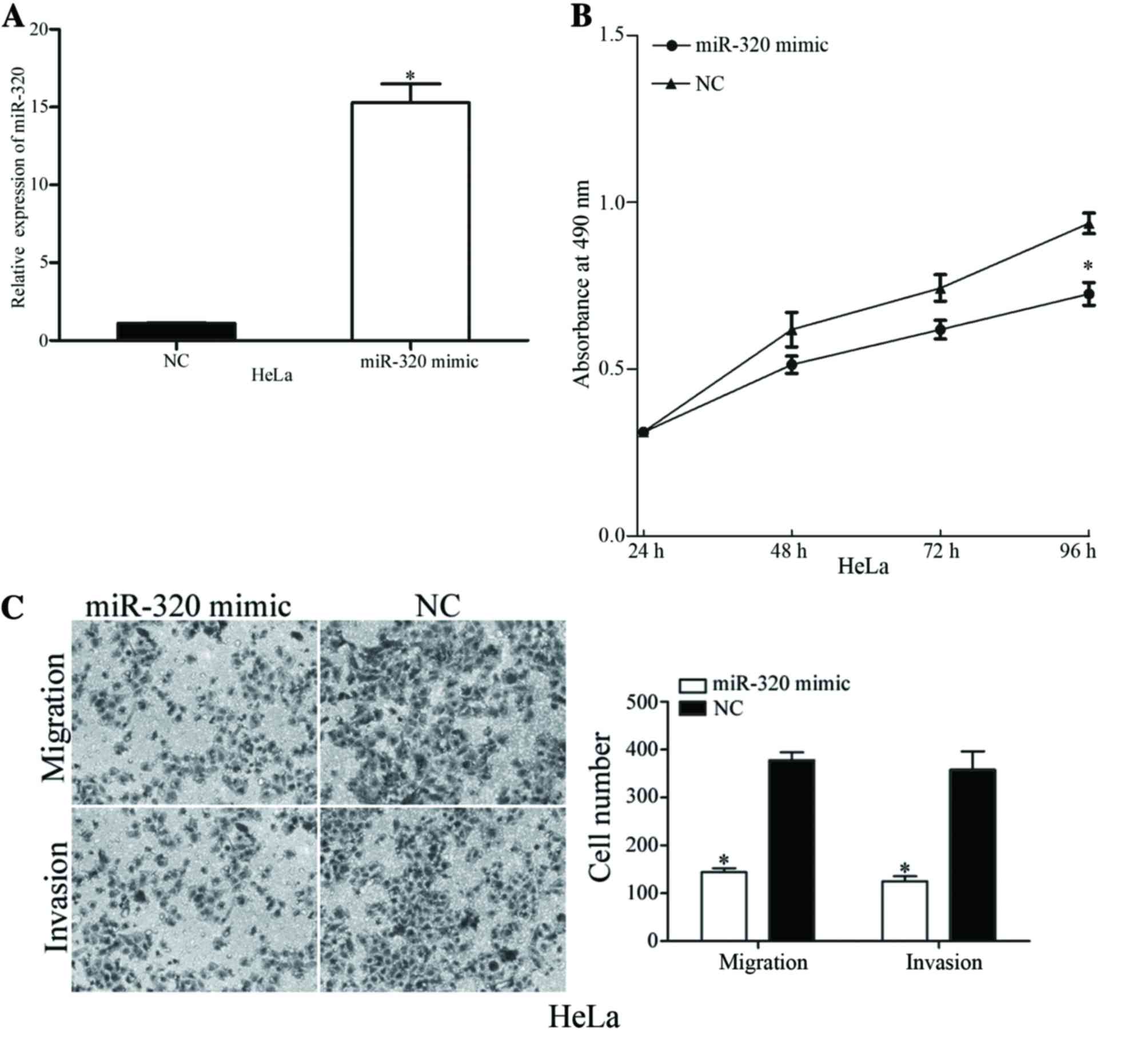
To identify the functions of miR-320 in cervical
cancer, miR-320 mimic or NC was transfected into HeLa cells, which
exhibited decreased expression levels of miR-320. Following
transfection, miR-320 expression was markedly overexpressed in HeLa
cells (Fig. 2A). The viability,
migration and invasion of miR-320-overexpressing cells was
evaluated using MTT, migration and invasion assays, respectively.
The MTT assay results revealed that overexpression of miR-320
suppressed the viability of HeLa cells, compared with the NC
(Fig. 2B). Furthermore, the migration
and invasion assay demonstrated that upregulation of miR-320
decreased the migratory and invasive potential of HeLa cells
(Fig. 2C). The results of the present
study indicated that miR-320 exhibited a suppressive role in the
viability and metastasis of cervical cancer cells.
Underexpression of miR-320 enhances
viability, migration and invasion of cervical cancer cells
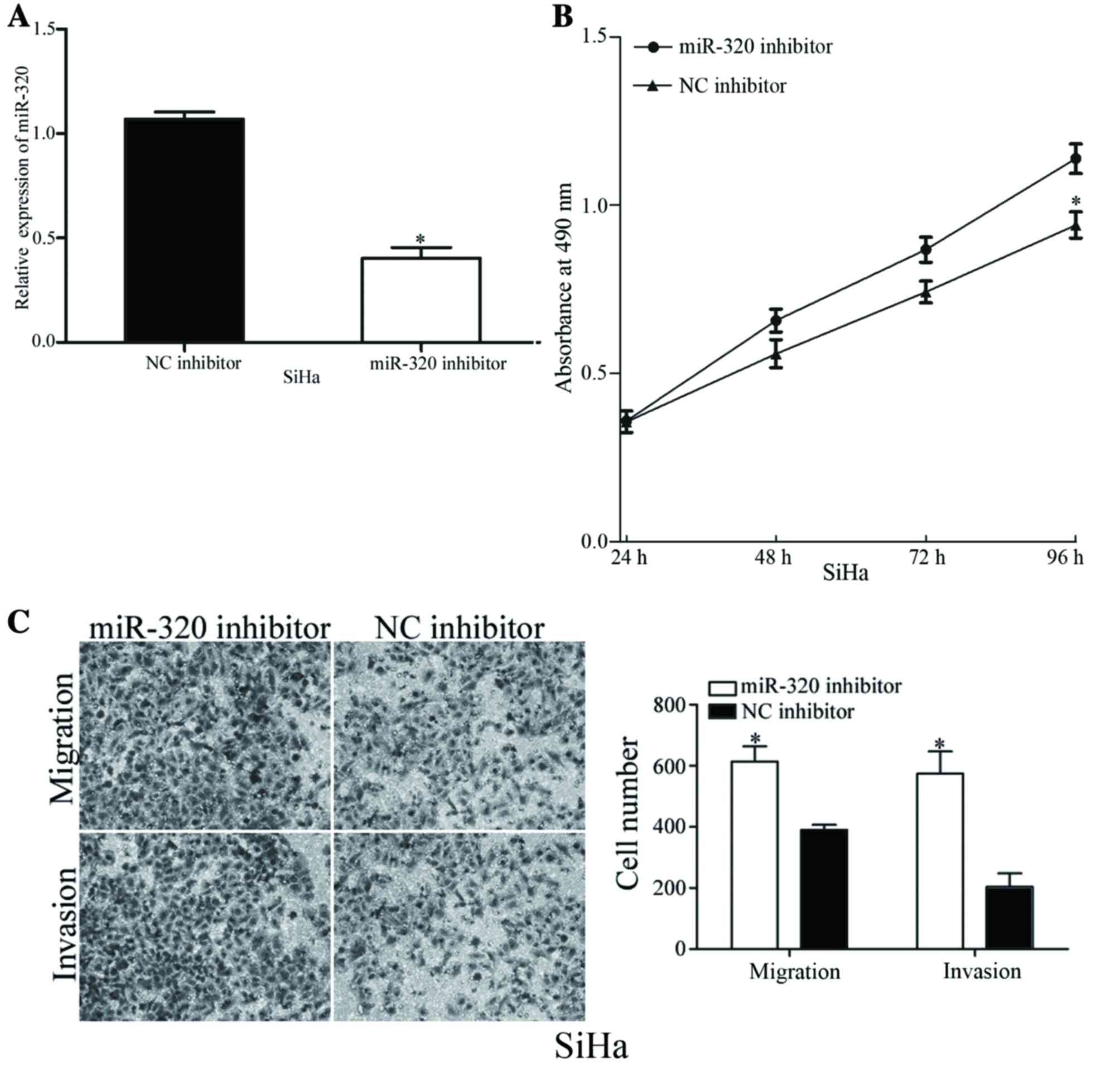
To validate the functions of miR-320 in the
viability and metastasis of cervical cancer cells, SiHa cells,
which exhibited increased expression levels of miR-320, were
transfected with an miR-320 inhibitor or NC inhibitor. As presented
in Fig. 3A, the expression level of
miR-320 was markedly decreased in miR-320 inhibitor-transfected
SiHa cells. The viability, migration and invasion of
miR-320-underexpressing cells was analyzed using MTT, migration and
invasion assays, respectively. Decreasing the expression levels of
miR-320 resulted in an increased viability rate and improved cell
migratory and invasive abilities of SiHa cells (Fig. 3B and C). The results of the present
study demonstrated the inhibitory functions of miR-320 in the
viability, migration and invasion of cervical cancer cells.
miR-320 regulates FOXM1 expression by
directly targeting 3′UTR of FOXM1
To identify the molecular mechanism underlying the
suppressive functions of miR-320 in cell viability, migration and
invasion, PicTar, TargetScan and miRanda databases were used to
predict the target genes of miR-320. Bioinformatics analysis
predicated that FOXM1 was a direct target gene of miR-320 (Fig. 4A). Western blot analysis revealed that
overexpression of miR-320 decreased the expression level of FOXM1
protein in HeLa cells, whereas underexpression of miR-320 increased
the expression level of FOXM1 in SiHa cells (Fig. 4B).
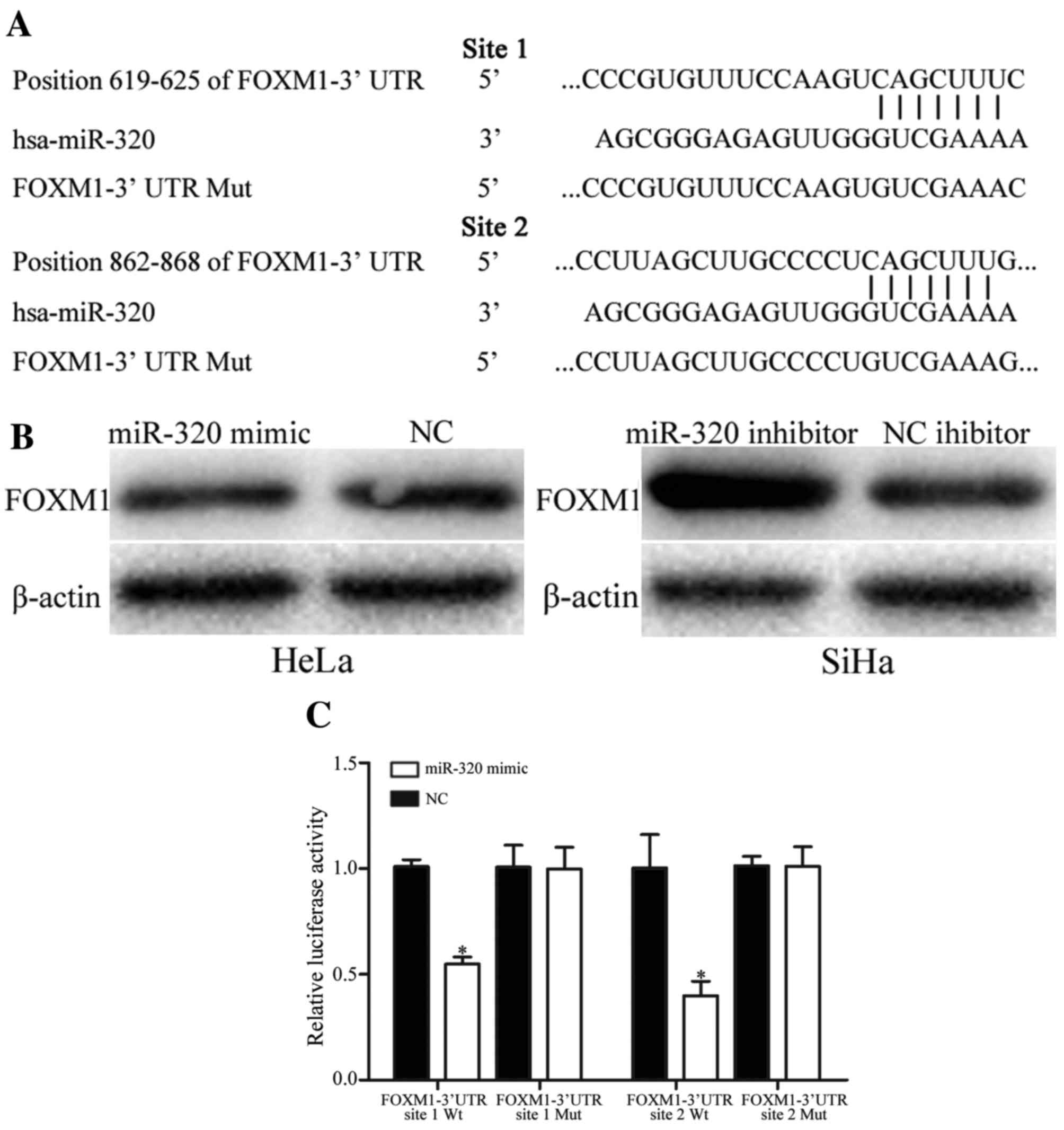 | Figure 4.miR-320 regulates FOXM1 expression
levels by directly targeting the 3′UTR of FOXM1 in cervical cancer.
(A) miR-320-binding site in the 3′UTR of FOXM1 identified using
PicTar, TargetScan and miRanda databases, and the FOXM1 3′UTR
mutant sequence. (B) Western blot analysis of FOXM1 protein
expression in HeLa cells following transfection with the miR-320
mimic or NC, and SiHa cells transfected with miR-320 inhibitor or
NC inhibitor. β-actin was used as a loading control. (C) miR-320
decreased the FOXM1-3′UTR site 1 Wt and FOXM1-3′UTR site 2 Wt
luciferase activity, but not the FOXM1-3′UTR site 1 Mut and
FOXM1-3′UTR site 2 Mut luciferase activity in HEK293T cells.
*P<0.05 vs. respective controls. miR, microRNA; FOXM1, forkhead
box M1; UTR, untranslated region; NC, negative control; Wt,
wild-type; Mut, mutant; hsa, human. |
Furthermore, the binding of miR-320 on the 3′UTR of
FOXM1 was evaluated using a dual-luciferase reporter assay in
HEK293T cells. The results demonstrated that HEK293T cells
co-transfected with miR-320 mimic and FOXM1-3′UTR Wt markedly
decreased the luciferase activities, whereas no effect was observed
when the 3′UTR of FOXM1 was mutated (Fig.
4C). The results of the present study suggested that miR-320
negatively regulated FOXM1 expression by directly targeting the
3′UTR of FOXM1.
miR-320 suppresses the viability,
migration and invasion of cervical cancer cells via the regulation
of FOXM1
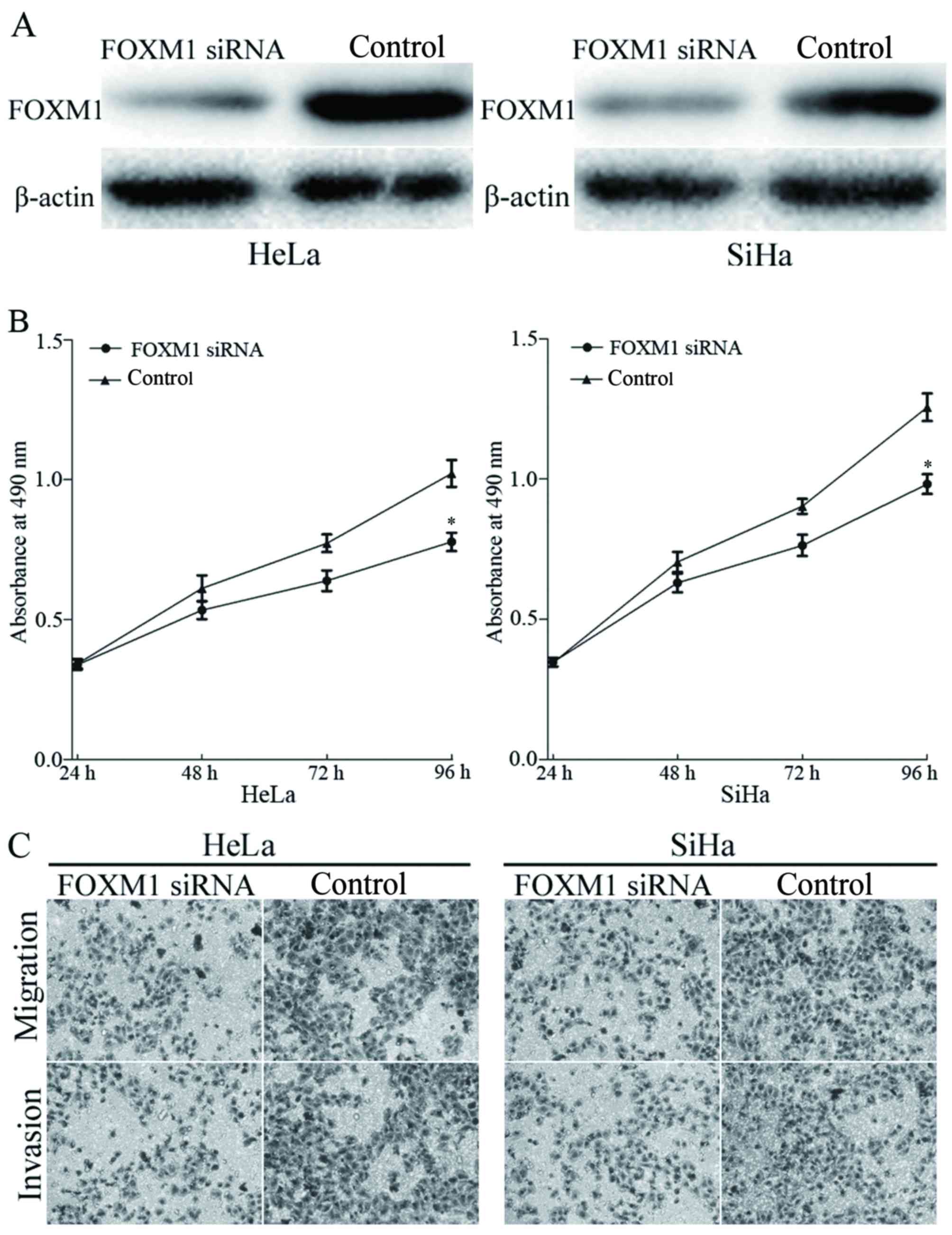
FOXM1 was identified as a direct target of miR-320
in cervical cancer. To elucidate whether miR-320 functions through
FOXM1, FOXM1 siRNA was transfected into HeLa and SiHa cells.
Transfection with FOXM1 siRNA resulted in marked downregulation of
FOXM1 expression in HeLa and SiHa cells (Fig. 5A). The effects of FOXM1 siRNA on cell
viability, migration and invasion was evaluated using MTT,
migration and invasion assays, respectively. The results identified
that knockdown of FOXM1 inhibited viability (Fig. 5B), migration and invasion (Fig. 5C) of HeLa and SiHa cells, compared
with the control groups. The results indicated that miR-320, by
knocking down FOXM1 expression, inhibited the viability, migration
and invasion of cervical cancer cells.
Discussion
Cervical cancer is one of the most common types of
gynecological cancer worldwide (24).
Currently, the standard therapeutic treatments for patients are
surgical resection, followed by chemotherapy and radiotherapy.
However, the overall survival rate remains low (25). Therefore, the development of novel
therapeutic strategies by targeting the molecules involved in
carcinogenesis and development of cervical cancer are required to
improve the prognosis for patients with cervical cancer. In the
present study, miR-320 was identified to be downregulated in
cervical cancer tissues and cell lines. Functional studies revealed
that the overexpression of miR-320 markedly decreased the
viability, migration and invasion of cervical cancer cells, whereas
the underexpression of miR-320 led to the opposite results. In
addition, FOXM1 was demonstrated to be a functional target of
miR-320 in cervical cancer. The results of the present study
suggested that miR-320 may be a therapeutic target for patients
with cervical cancer.
miR-320 has been reported to be downregulated in a
number of types of human cancer, and serves important functions in
carcinogenesis and cancer progression. Sun et al (26) identified that miR-320 was markedly
downregulated in glioma tissues, compared with that in healthy
tissues, and overexpression of miR-320 inhibited cell viability and
metastasis by the downregulation of E2F transcription factor 1.
Cheng et al (27) reported
that miR-320 expression levels were decreased in human osteosarcoma
tissues, and increased miR-320 expression in osteosarcoma cells
suppressed viability in vitro and in vivo by directly
targeting fatty acid synthase. Furthermore, Wu et al
(28) demonstrated that miR-320,
which was decreased in oral squamous cell carcinoma tissues and
cell lines, suppressed the tumorigenicity of oral squamous cell
carcinoma cells in vitro and tumor angiogenesis in
vivo, via inhibition of neuropilin 1. In addition, Wu et
al (28) reported that miR-320
was downregulated in prostate cancer, and that the upregulation of
miR-320 inhibited carcinogenesis and progression of prostate cancer
through the downregulation of the Wnt/β-catenin signaling pathway.
The overexpression of miR-320 in prostate stem-like
tumor-initiating cells markedly inhibited the stem cell-like
properties of prostate cancer cells, including tumorsphere
formation, chemoresistance and tumorigenic abilities (29). In the present study, it was revealed
that miR-320 was downregulated in cervical cancer and functioned as
a tumor suppressor in cervical cancer by inhibiting the viability,
migration and invasion, via directly targeting FOXM1.
miRNAs typically exert biological functions through
the negative regulation of target mRNAs, via directly binding to
the 3′UTRs of target mRNAs (30). In
the present study, three bioinformatics algorithms were used to
predict the target genes of miR-320. FOXM1 was identified to
contain two miR-320 seed matches at positions between 619 and 625,
and between 862 and 868 of the 3′UTR of FOXM1. The dual-luciferase
reporter assay validated that miR-320 directly targeted the 3′UTR
of FOXM1. Western blot analysis revealed that the overexpression of
miR-320 decreased FOXM1 expression levels in cervical cancer,
whereas underexpression of miR-320 increased FOXM1 expression
levels. Furthermore, the functions of FOXM1 siRNA were similar to
those induced by miR-320 in cervical cancer, suggesting that FOXM1
was a functional target of miR-320 in cervical cancer. The
identification of target genes of miR-320 is required to elucidate
the functions of miR-320 in the carcinogenesis and progression of
cervical cancer, and may provide novel therapeutic targets for the
treatment of patients with cervical cancer.
FOXM1 serves a function in tumor initiation and
progression (31). FOXM1 has been
reported to be upregulated in a number of types of tumor, including
lung, breast, liver, pancreatic and cervical cancer, and
glioblastoma (32,33). FOXM1 serves a function in the
regulation of a number of biological processes, including cell
viability, cell cycle progression, cell differentiation, DNA damage
repair, tissue homeostasis, angiogenesis and apoptosis (34). In cervical cancer, FOXM1 was markedly
overexpressed in cervical cancer tissues, and increased expression
levels of FOXM1 was associated with the tumor late stage and cell
viability marker Ki67 (35).
In functional studies, enforced FOXM1 expression
increased the migration and invasion of cervical cancer cells,
whereas the knockdown of FOXM1 exhibited the opposite effect in
cervical metastasis (33).
Furthermore, Chen et al (36)
reported that the underexpression of FOXM1 suppressed the
viability, invasion and angiogenesis of cervical cancer cells in
vitro and in vivo (36).
Therefore, the overexpression of FOXM1 protein in cervical cancer
was associated with the carcinogenesis and progression of cervical
cancer, and may be investigated as a novel therapeutic target for
cervical cancer. In the present study, overexpression of miR-320
was identified to target FOXM1, and inhibit viability and
metastasis of cervical cancer, suggesting that miR-320/FOXM1-based
targeted therapy may be a novel therapeutic strategy for the
treatment of patients with cervical cancer.
The results of the present study demonstrated that
miR-320 was downregulated in cervical cancer tissues and cell
lines. Additionally, it was revealed that miR-320 acted as a tumor
suppressor and exhibited a marked suppressive effect on the
viability, migration and invasion of cervical cancer in
vitro. Furthermore, FOXM1 was identified as a direct target
gene of miR-320 in cervical cancer. The overexpression of miR-320
may be a novel therapeutic target for the treatment of cervical
cancer.
References
|
1
|
Arbyn M, Castellsagué X, de Sanjosé S,
Bruni L, Saraiya M, Bray F and Ferlay J: Worldwide burden of
cervical cancer in 2008. Ann Oncol. 22:2675–2686. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Siegel R, Naishadham D and Jemal A: Cancer
statistics, 2012. CA Cancer J Clin. 62:10–29. 2012.PubMed/NCBI
|
|
3
|
Su SY, Huang JY, Ho CC and Liaw YP:
Evidence for cervical cancer mortality with screening program in
Taiwan, 1981–2010: Age-period-cohort model. BMC Public Health.
13:132013. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Chen J, Yao D, Li Y, Chen H, He C, Ding N,
Lu Y, Ou T, Zhao S, Li L and Long F: Serum microRNA expression
levels can predict lymph node metastasis in patients with
early-stage cervical squamous cell carcinoma. Int J Mol Med.
32:557–567. 2013.PubMed/NCBI
|
|
5
|
Yee GP, de Souza P and Khachigian LM:
Current and potential treatments for cervical cancer. Curr Cancer
Drug Targets. 13:205–220. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Wang F, Liu M, Li X and Tang H: MiR-214
reduces cell survival and enhances cisplatin-induced cytotoxicity
via down-regulation of Bcl2l2 in cervical cancer cells. FEBS Lett.
587:488–495. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Rose PG, Bundy BN, Watkins EB, Thigpen JT,
Deppe G, Maiman MA, Clarke-Pearson DL and Insalaco S: Concurrent
cisplatin-based radiotherapy and chemotherapy for locally advanced
cervical cancer. N Engl J Med. 340:1144–1153. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Thomas GM: Improved treatment for cervical
cancer-concurrent chemotherapy and radiotherapy. N Engl J Med.
340:1198–1200. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Mayr NA, Huang Z, Wang JZ, Lo SS, Fan JM,
Grecula JC, Sammet S, Sammet CL, Jia G, Zhang J, et al:
Characterizing tumor heterogeneity with functional imaging and
quantifying high-risk tumor volume for early prediction of
treatment outcome: Cervical cancer as a model. Int J Radiat Oncol
Biol Phys. 83:972–979. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Kogo R, How C, Chaudary N, Bruce J, Shi W,
Hill RP, Zahedi P, Yip KW and Liu FF: The microRNA-218~Survivin
axis regulates migration, invasion and lymph node metastasis in
cervical cancer. Oncotarget. 6:1090–1100. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Castellsaguè X, Díaz M, de Sanjosè S,
Muñoz N, Herrero R, Franceschi S, Peeling RW, Ashley R, Smith JS,
Snijders PJ, et al: Worldwide human papillomavirus etiology of
cervical adenocarcinoma and its cofactors: Implications for
screening and prevention. J Natl Cancer Inst. 98:303–315. 2006.
View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Ambros V: The functions of animal
microRNAs. Nature. 431:350–355. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
He L and Hannon GJ: MicroRNAs: Small RNAs
with a big role in gene regulation. Nat Rev Genet. 5:522–531. 2004.
View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Valencia-Sanchez MA, Liu J, Hannon GJ and
Parker R: Control of translation and mRNA degradation by miRNAs and
siRNAs. Genes Dev. 20:515–524. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Winter J, Jung S, Keller S, Gregory RI and
Diederichs S: Many roads to maturity: Microrna biogenesis pathways
and their regulation. Nat Cell Biol. 11:228–234. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Aigner A: MicroRNAs (miRNAs) in cancer
invasion and metastasis: Therapeutic approaches based on
metastasis-related miRNAs. J Mol Med (Berl). 89:445–457. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Rottiers V and Näär AM: MicroRNAs in
metabolism and metabolic disorders. Nat Rev Mol Cell Biol.
13:239–250. 2012. View
Article : Google Scholar : PubMed/NCBI
|
|
18
|
Cho WC: MicroRNAs: Potential biomarkers
for cancer diagnosis, prognosis and targets for therapy. Int J
Biochem Cell Biol. 42:1273–1281. 2010. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Wang L, Chang L, Li Z, Gao Q, Cai D, Tian
Y, Zeng L and Li M: miR-99a and −99b inhibit cervical cancer cell
proliferation and invasion by targeting mTOR signaling pathway. Med
Oncol. 31:9342014. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Croce CM and Calin GA: miRNAs, cancer, and
stem cell division. Cell. 122:6–7. 2005. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Babashah S and Soleimani M: The oncogenic
and tumour suppressive roles of microRNAs in cancer and apoptosis.
Eur J Cancer. 47:1127–1137. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Zhang B, Pan X, Cobb GP and Anderson TA:
microRNAs as oncogenes and tumor suppressors. Dev Biol. 302:1–12.
2007. View Article : Google Scholar : PubMed/NCBI
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Jemal A, Bray F, Center MM, Ferlay J, Ward
E and Forman D: Global cancer statistics. CA Cancer J Clin.
61:69–90. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
de Freitas AC, Gomes Leitão Mda C and
Coimbra EC: Prospects of molecularly-targeted therapies for
cervical cancer treatment. Curr Drug Targets. 16:77–91. 2015.
View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sun JY, Xiao WZ, Wang F, Wang YQ, Zhu YH,
Wu YF, Miao ZL and Lin YC: MicroRNA-320 inhibits cell proliferation
in glioma by targeting E2F1. Mol Med Rep. 12:2355–2359.
2015.PubMed/NCBI
|
|
27
|
Cheng C, Chen ZQ and Shi XT: MicroRNA-320
inhibits osteosarcoma cells proliferation by directly targeting
fatty acid synthase. Tumour Biol. 35:4177–4183. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
28
|
Wu YY, Chen YL, Jao YC, Hsieh IS, Chang KC
and Hong TM: miR-320 regulates tumor angiogenesis driven by
vascular endothelial cells in oral cancer by silencing neuropilin
1. Angiogenesis. 17:247–260. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
29
|
Hsieh IS, Chang KC, Tsai YT, Ke JY, Lu PJ,
Lee KH, Yeh SD, Hong TM and Chen YL: MicroRNA-320 suppresses the
stem cell-like characteristics of prostate cancer cells by
downregulating the Wnt/beta-catenin signaling pathway.
Carcinogenesis. 34:530–538. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
30
|
Siciliano V, Garzilli I, Fracassi C,
Criscuolo S, Ventre S and di Bernardo D: MiRNAs confer phenotypic
robustness to gene networks by suppressing biological noise. Nat
Commun. 4:23642013. View Article : Google Scholar : PubMed/NCBI
|
|
31
|
Kaestner KH, Knochel W and Martinez DE:
Unified nomenclature for the winged helix/forkhead transcription
factors. Genes Dev. 14:142–146. 2000.PubMed/NCBI
|
|
32
|
Koo CY, Muir KW and Lam EW: FOXM1: From
cancer initiation to progression and treatment. Biochim Biophys
Acta. 1819:28–37. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
33
|
He SY, Shen HW, Xu L, Zhao XH, Yuan L, Niu
G, You ZS and Yao SZ: FOXM1 promotes tumor cell invasion and
correlates with poor prognosis in early-stage cervical cancer.
Gynecol Oncol. 127:601–610. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
34
|
Hou Y, Li W, Sheng Y, Li L, Huang Y, Zhang
Z, Zhu T, Peace D, Quigley JG, Wu W, et al: The transcription
factor Foxm1 is essential for the quiescence and maintenance of
hematopoietic stem cells. Nat Immunol. 16:810–818. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
35
|
Chan DW, Yu SY, Chiu PM, Yao KM, Liu VW,
Cheung AN and Ngan HY: Over-expression of FOXM1 transcription
factor is associated with cervical cancer progression and
pathogenesis. J Pathol. 215:245–252. 2008. View Article : Google Scholar : PubMed/NCBI
|
|
36
|
Chen H, Zou Y, Yang H, Wang J and Pan H:
Downregulation of FoxM1 inhibits proliferation, invasion and
angiogenesis of HeLa cells in vitro and in vivo. Int
J Oncol. 45:2355–2364. 2014.PubMed/NCBI
|