Introduction
Breast cancer is the leading cause of cancer-related
death in women worldwide (1).
Although chemotherapy has greatly improved the survival of patients
with breast cancer, drug resistance often occurs (2). Therefore, it is important to identify
new targets for preventing breast cancer metastasis and drug
resistance.
HOXB4, a member of HOX family, is preferentially
expressed in most hematopoietic cell types and elicits the
selective expansion of more primitive populations (3). Recently, HOXB4 was shown to control stem
cell amplification via its unique proline-rich region (4). However, the roles of HOXB4 in cancers
are unclear.
StAR-related lipid transfer domain protein 13
(STARD13) acts as a tumor-suppressor in various types of cancers
(5,6),
its expression level is downregulated in metastatic breast cancer,
potentially mediating a competitive endogenous RNA (ceRNA) network
to inhibit breast cancer metastasis (7). Additionally, STARD13 can act as a ceRNA
for Fas to promote apoptosis of hepatocellular carcinoma cells
(8). Although STARD13 has been
identified as a potential target for miR-125b in breast (6) and gastric cancer (9), the mechanisms regulating the
transcription of STARD13 in breast cancer are unclear.
Here, we found that HOXB4 expression was positively
correlated with STARD13 expression in breast cancer tissues and
cells with different metastatic potentials. Additionally, we showed
that HOXB4 could bind directly to the STARD13 promoter, thus
inducing STARD13 expression in breast cancer. HOXB4 could also
inhibit breast cancer cell migration and the EMT through the
STARD13-RhoA-ROCK signaling pathway. Finally, we showed that forced
expression of HOXB4 enhanced the sensitivity of breast cancer cells
to doxorubicin and reversed resistance in doxorubicin-resistant
cells. Therefore, STARD13 could be a downstream effector of HOBX4
in breast cancer, and HOXB4 could be targeted as a potential
inhibitor of breast cancer metastasis.
Materials and methods
The cancer genome atlas (TCGA) data
and patient samples
Twenty primary breast tumors with lymph node
metastasis, twenty seven metastasis-free primary breast tumors, and
adjacent normal tissues were obtained from the Affiliated Hospital
of Jining Medical University from May 2015 to June 2016. Approval
from the Institute Research Ethics Committee was obtained for the
use of these clinical materials for research purposes. The R2:
Genomics Analysis and Visualization Platform (http://hgserver1.amc.nl/cgi-bin/r2/main.cgi) was used
to download and analyze the HOXB4 and STARD13 mRNA expression
profiling data.
Cell culture
293T, MCF-10A (normal breast epithelial cell lines),
MCF-7 (relative low metastatic breast cancer cell lines),
MDA-MB-435 (relative median metastatic breast cancer cell lines)
and MDA-MB-231 (relative high metastatic breast cancer cell lines)
cells were purchased from the cell bank of the Chinese Academy of
Sciences (Shanghai, China). Doxorubicin resistant MCF-7 cells
(MCF-7-ADR) were purchased from KeyGen Biotech (Nanjing, China).
MCF-10A, MCF-7 and MCF-7-ADR cells were cultured in Dulbecco's
Modified Eagle Medium (Gibco, Grand Island, NY, USA), MDA-MB-435
cells were cultured in 1640 medium (Gibco,), MDA-MB-231 cells were
cultured in L-15 medium (Gibco) with 10% fetal bovine serum, 80
U/ml penicillin and 0.08 mg/ml streptomycin at 37°C under
humidified air with 5% CO2. Y-27632 (B1293), a ROCK
inhibitor, was purchased from ApexBio (Hsinchu City, Taiwan).
Construction of stable cell lines
Lentivirus short hairpin(sh)RNA against human HOXB4
and a scramble non-targeting shRNA (sc-38692) were purchased from
Santa Cruz Biotechnology (Santa Cruz, CA, USA), and inserted into
pLKO.1. HOXB4 coding sequences were amplified by PCR and cloned
into the SpeI and Xbal sites of pLVX-IRES-ZsGreen1, referred to as
Lenti-HOXB4-CDS. The primers were described in Table I. As described previously (8). Quantitative real-time PCR (qRT-PCR) and
western blot analyses were used for verification. Cells infected
with Lenti-HOXB4-CDS were selected by fluorescent cell sorting.
 | Table I.qRT-PCR Primer sequences and the
sequence(s) for PCR. |
Table I.
qRT-PCR Primer sequences and the
sequence(s) for PCR.
| Gene | Sequences (5′ to
3′) |
|---|
| HOXB4-CDS forward
(Spel) |
ACTAGTATGGCTATGAGTTCTTTTTTGATCA |
| HOXB4-CDS reverse
(Xbal) |
TCTAGACTAGAGCGCGCGGGGGCCTCCATTG |
| GAPDH forward |
CGGAGTCAACGGATTTGGTCGTAT |
| GAPDH reverse |
AGCCTTCTCCATGGTGGTGAAGAC |
| HOXB4-qRT-PCR
forward |
ACACACCCAAACAAGGACACAGCA |
| HOXB4-qRT-PCR
reverse |
ACACACACGGAGAGAGGGAGAAAG |
| STARD13-qRT-PCR
forward |
ACAGGAGGGATTCTGGTGTAGGGG |
| STARD13-qRT-PCR
reverse |
AGGGAAGTTTTCATTCATTTGGCG |
| STARD13promoter (for
ChIP) forward |
GAGGAAAAGCAATACACGCACAAA |
| STARD13promoter (for
ChIP) forward |
TCAGGACAGGACCAAGAACAAGGT |
| Vimentin-qRT-PCR
forward |
AGGAACCAATGAGTCCCTGGAACG |
| Vimentin-qRT-PCR
reverse |
CTGCAGAAAGGCACTTGAAAGCTG |
| E-cadherin-qRT-PCR
forward |
CTCACATTTCCCAACTCCTCTCCT |
| E-cadherin-qRT-PCR
reverse |
ACCTTCAGCCATCCTGTTTCTCTT |
Adenovirus vectors construction
The adenovirus vectors containing the STARD13 coding
area (Ad-STARD13-CDS) or STARD13 shRNA (Ad-STARD13-shRNA) were
constructed by Hanbio (Shanghai, China). The constructs were
verified by DNA sequencing.
Promoter activity assay
The STARD13 promoter sequences were introduced into
the pGL3 vector (Promega, Madison, WI, USA) (pGL3-STARD13) for the
STARD13 promoter transcriptional activity assays. The β-gal vector
was used as an internal control. 293T cells were co-transfected
with pGL3-STARD13 (0.2 µg) and β-gal (0.2 µg) for 24 h using
Lipofectamine 2000 (Invitrogen, Carlsbad, CA) in 24 wells plate,
then further infected with Lenti-HOXB4-CDS.
Chromatin immunoprecipitation (ChIP)
assay
The ChIP assay was performed to assess in
vivo DNA-protein interactions at the STRAD13 promoter using the
EZ-CHIP™ Kit (EMDMillipore, Darmstadt, Germany). The recovered DNA
was used as the template to amplify the STARD13 promoter. The
primers for detecting the STARD13 promoter containing the putative
HOXB4 binding sites were shown in Table
I.
Cell migration assays
The detailed procedure was referred to the previous
study (6).
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT)
assay
Cells (3×103/well) were plated in 96-well
plates and treated with the IC50 of doxorubicin after adhesion.
Cell viability was assessed after 24, 48, and 72 h of culturing.
MTT (KeyGen Biotech) was added into the medium at 0.25 mg/ml and
the absorbance was measured at 570 nm using a microplate
reader.
Cell adhesion assays
Cell adhesion was assessed as described previously
(10).
Apoptosis assay
Apoptosis was evaluated using flow cytometry with
AnnexinV-FITC and propidium iodide (PI) staining (Vazyme Biotech,
Nanjing, China). Cells with and without HOXB4 overexpressionwere
harvested and washed with ice-cold PBS. The cells were then stained
with Annexin V-FITC and PI (BD Biosciences, Franklin Lakes, NY,
USA) following the manufacturer's protocol. Flow cytometry utilized
an instrument from BD Biosciences.
Quantitative real-time PCR
(qRT-PCR)
Total RNA was prepared from cells using TransZol Up
(Transgen Biotech, Beijing, China) according to the manufacturer's
protocols. Total RNA was reverse transcribed into cDNA using
EasyScript Reverse Transcriptase (M-MLV, RNaseH-) (Transgen
Biotech) following the standard protocols. mRNA expression levels
were determined according to the TransScript Probe qPCR SuperMix
protocols (Transgen Biotech) and performed on an ABI Prism 7500
Detection System (Applied Biosystems, Foster City, CA, USA). The
primers for qRT-PCR were listed in Table
I. The expression of each transcript was calculated using the
2−ΔΔct method.
Western blotting
The detailed western blotting procedure was
described previously (11).
Antibodies against Bcl-2 (sc-509), Bax (sc-4239) and STARD13
(sc-67843) were purchased from Santa Cruz Biotechnology. Antibodies
against HOXB4 (ab76093), E-cadherin (ab40772), vimentin (ab8978)
and β-actin (ab8227) were purchased from Abcam (Cambridge, UK),
Blots were washed and incubated with a peroxidase-conjugated
secondary antibody. Chemiluminescence was detected using Super
Signal West Pico (Thermo Fisher Scientific, Waltham, MA, USA)
followed by exposure with Tanon 5200.
Immunohistochemistry
Immunohistochemistry procedures were described
previously (7).
Statistical analysis
All data are presented as means ± SD from three
independent experiments. Differences between the groups were
analyzed with One-way ANOVA and then Tukey's test, and *P<0.05
or less was considered significant.
Results
HOXB4 expression was positively
correlated with STARD13 expression in patients with breast
cancer
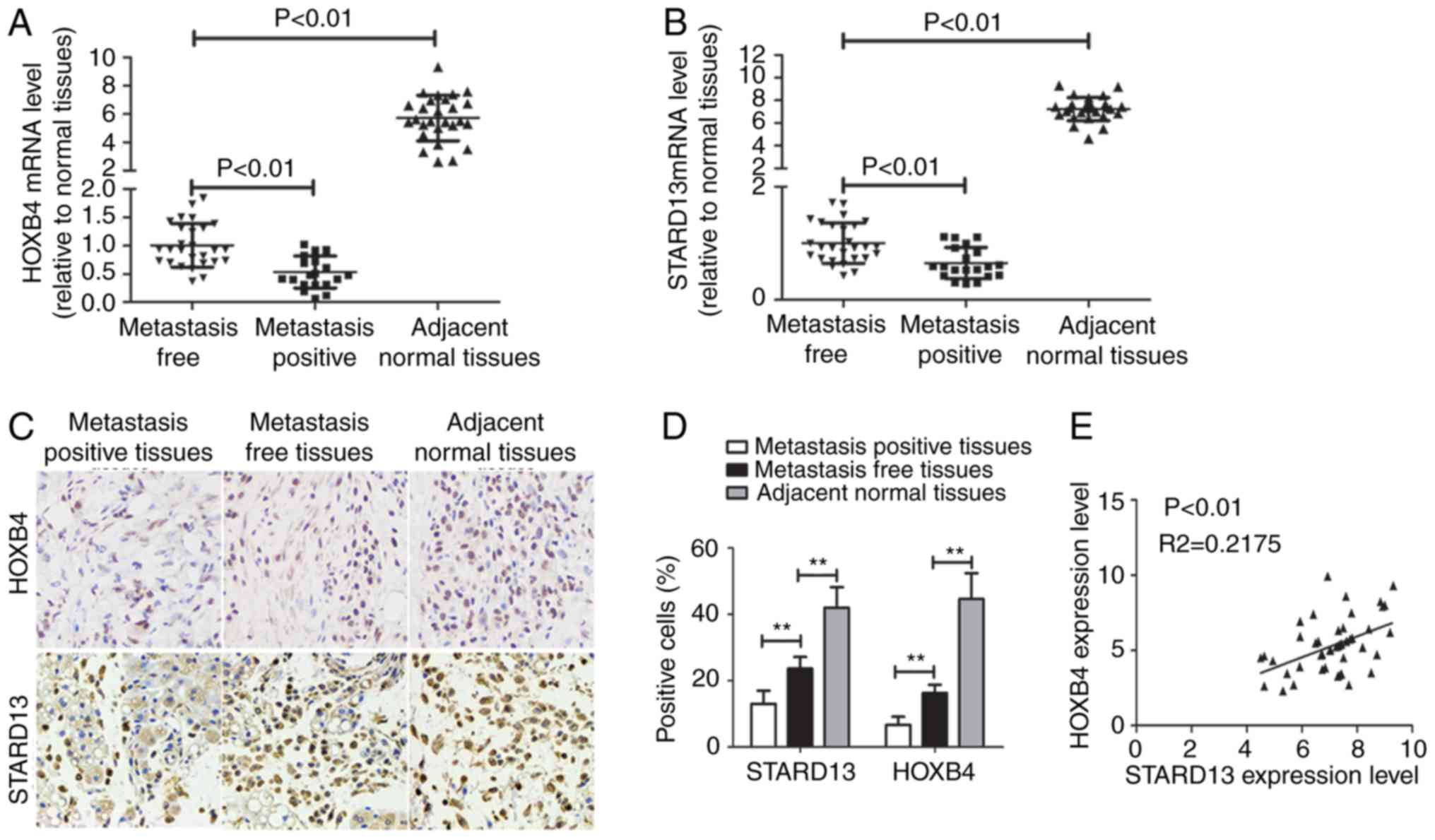
Immunohistochemistry and qRT-PCR assays were used to
examine the expression levels of HOXB4 and STARD13 in breast cancer
and adjacent normal tissues. HOXB4 and STARD13 expression were both
decreased (Fig. 1A-D) and positively
correlated in breast cancer tissues (Fig.
1E). Importantly, HOXB4 and STARD13 expression levels were
downregulated in breast cancer tissues with lymph node metastasis
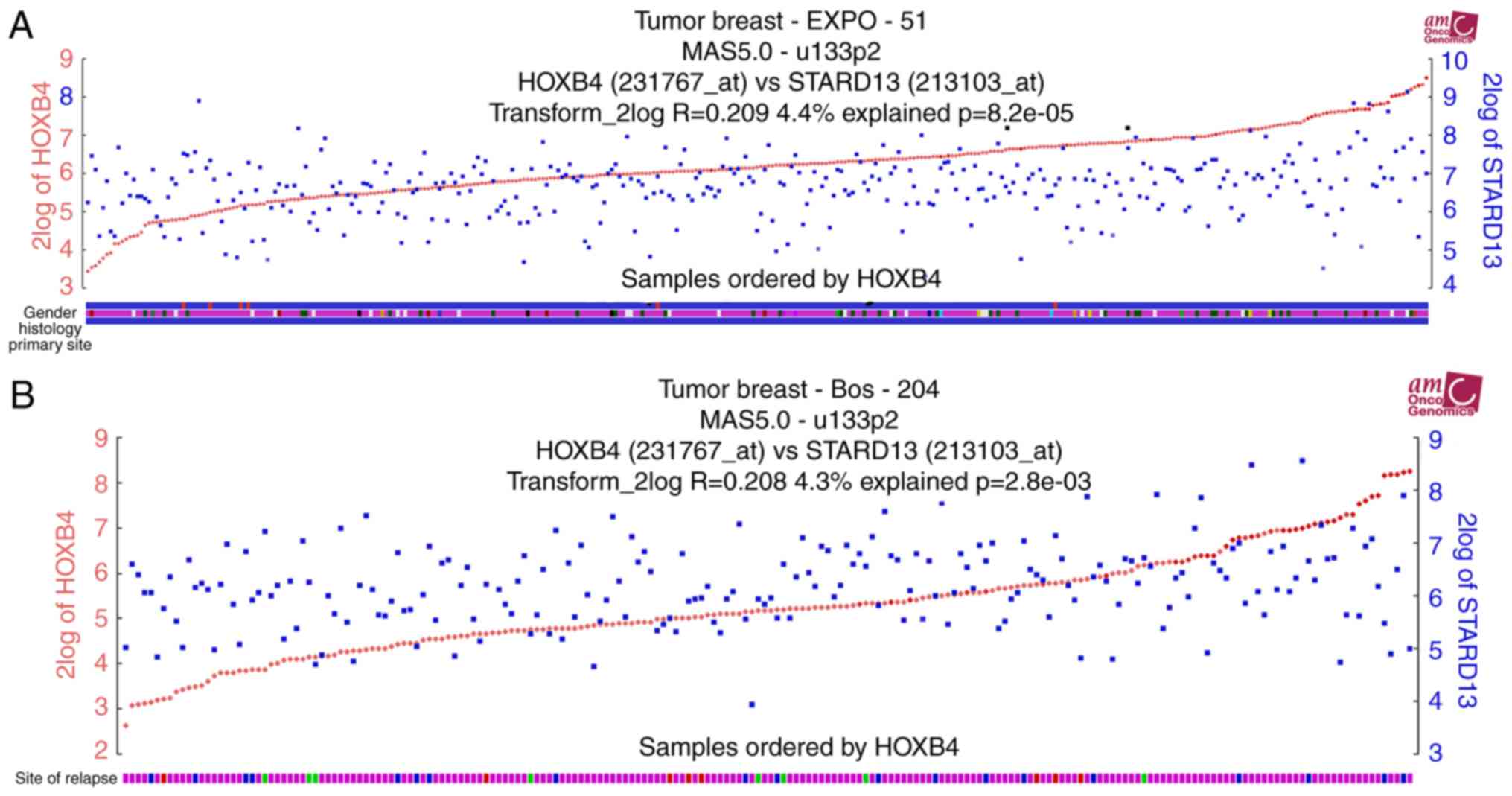
compared with primary tissues. TCGA data analysis was further
performed to determine expression profiles in patients with breast
cancer. Notably, HOXB4 and STARD13 expression levels were
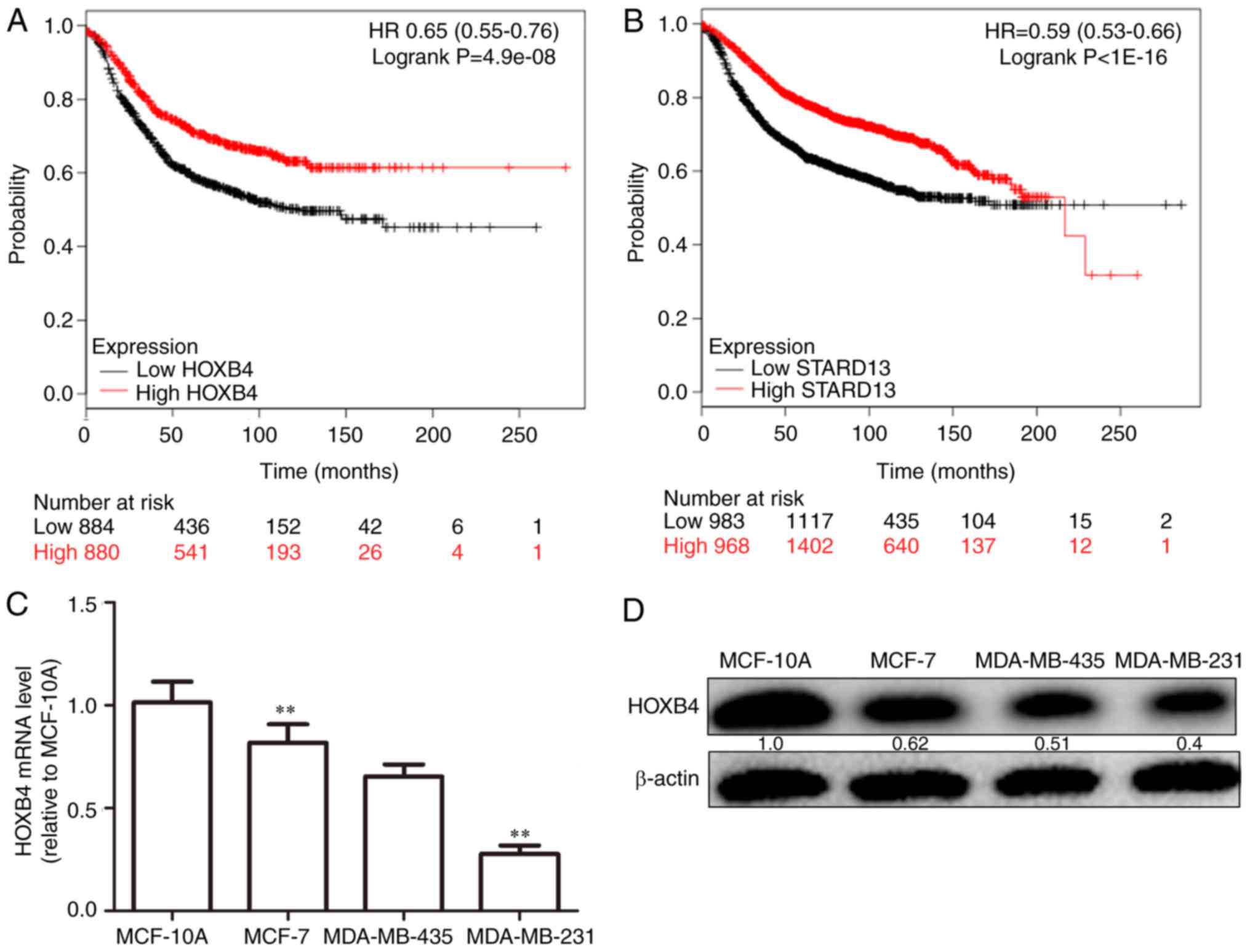
positively correlated in two different clinical samples (Fig. 2A and B). Additionally, higher HOXB4
and STARD13 expression was correlated with longer survival in
patients with breast cancer (Fig. 3A and
B). Next, as shown in Fig. 3C and
D, HOXB4 mRNA and protein levels were higher in normal MCF-10A
breast epithelial cells than in breast cancer cells. In addition,
HOXB4 expression was negatively correlated with cell metastatic
ability (MCF-7 cells, showing low metastatic ability, vs.,
MDA-MB-435 cells, showing moderate metastatic ability, and
MDA-MB-231 cells, showing high metastatic ability), consistent with
STARD13 expression patterns reported in a previous study (7), and this result was similar with the
results obtained from clinical samples.
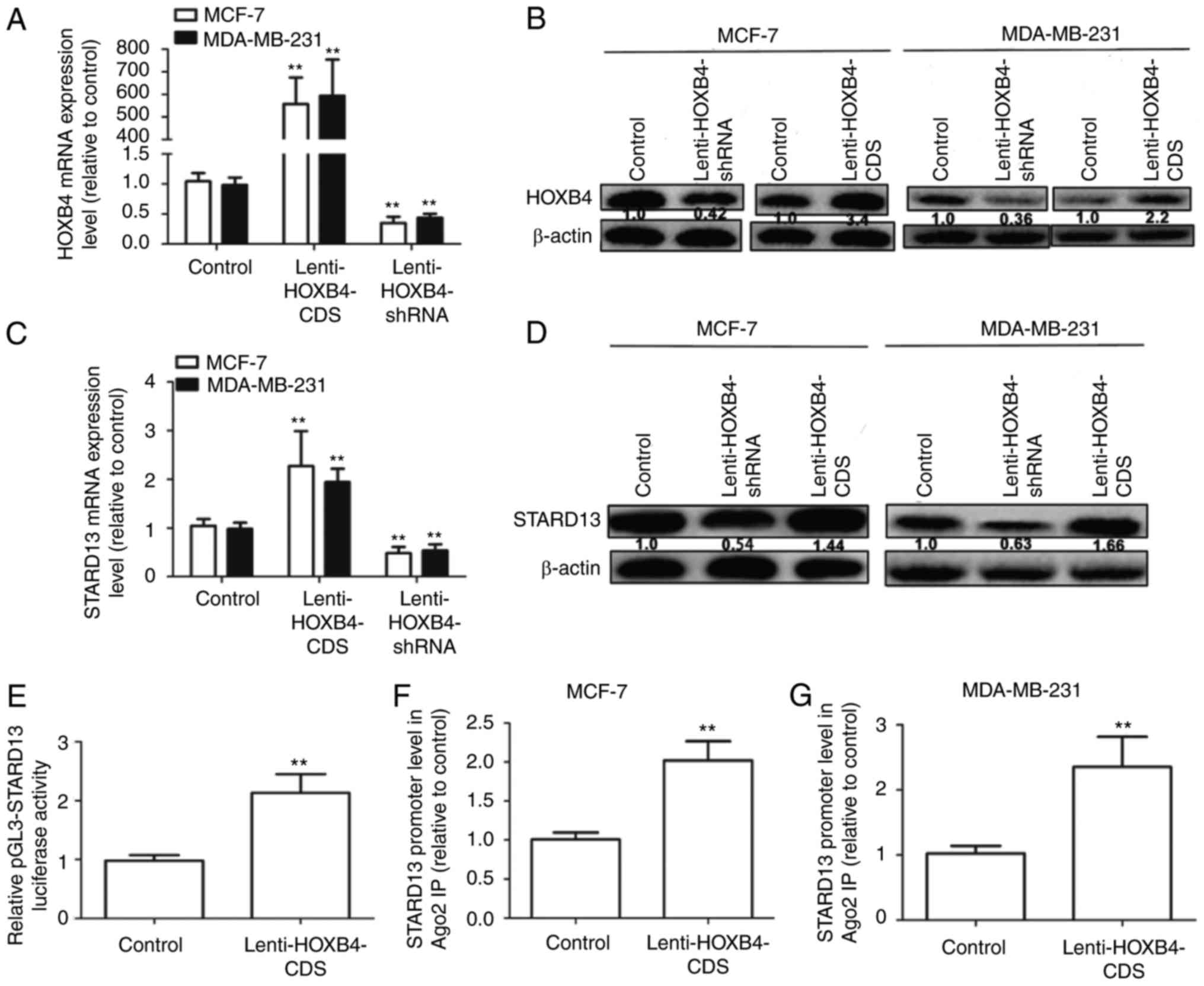
HOXB4 promoted STARD13 expression by
directly binding to the STARD13 promoter
MCF-7 cells (showing low metastatic ability) and
MDA-MB-231 cells (showing high metastatic ability) were used for
further studies. We constructed MCF-7 and MDA-MB-231 cells with
stable expression of HOXB4 or HOXB4 shRNA. As shown in Fig. 4A and B, infection with Lenti-HOXB4-CDS
or Lenti-HOXB4-shRNA markedly upregulated or downregulated HOXB4
expression respectively, in MCF-7 and MDA-MB-231 cells. STARD13
expression levels were significantly upregulated in cells
overexpressing HOXB4 (Fig. 4C and D),
but were downregulated in cells with HOXB4 knockdown. The Genomatix
Software Suite (https://www.genomatix.de/cgi-bin) was used to predict
which transcription factors could bind to the STARD13 promoter. As
expected, HOXB4 had five potential binding sites in the STARD13
promoter. We then assessed whether HOXB4 could enhance STARD13
promoter activity. Infection with Lenti-HOXB4-CDS could promote
pGL3-STARD13 activity in 293T cells (Fig.
4E). ChIP analysis was performed to confirm whether HOXB4 could
bind to the STARD13 promoter directly. The HOXB4 binding complex
was pulled down with HOXB4 antibodies in MCF-7 and MDA-MB-231
cells, regardless of HOXB4 overexpression. We then examined the
bound STARD13 promoter sequence containing HOXB4 binding sites by
qRT-PCR. The STARD13 promoter sequence containing the HOXB4 binding
sites was increased in cells with HOXB4 overexpression (Fig. 4F and G). These results suggest that
HOXB4 could directly bind to the STARD13 promoter in breast cancer
cells.
HOXB4 inhibited breast cancer cell
migration, adhesion ability and the EMT in a STARD13-dependent
manner
Because STARD13 is involved in breast cancer
metastasis and the EMT (6,7), we speculated that HOXB4 could hold
similar roles in a STARD13-dependent manner. We induced HOXB4
overexpression in MDA-MB-231 cells and HOXB4 knockdown in MCF-7
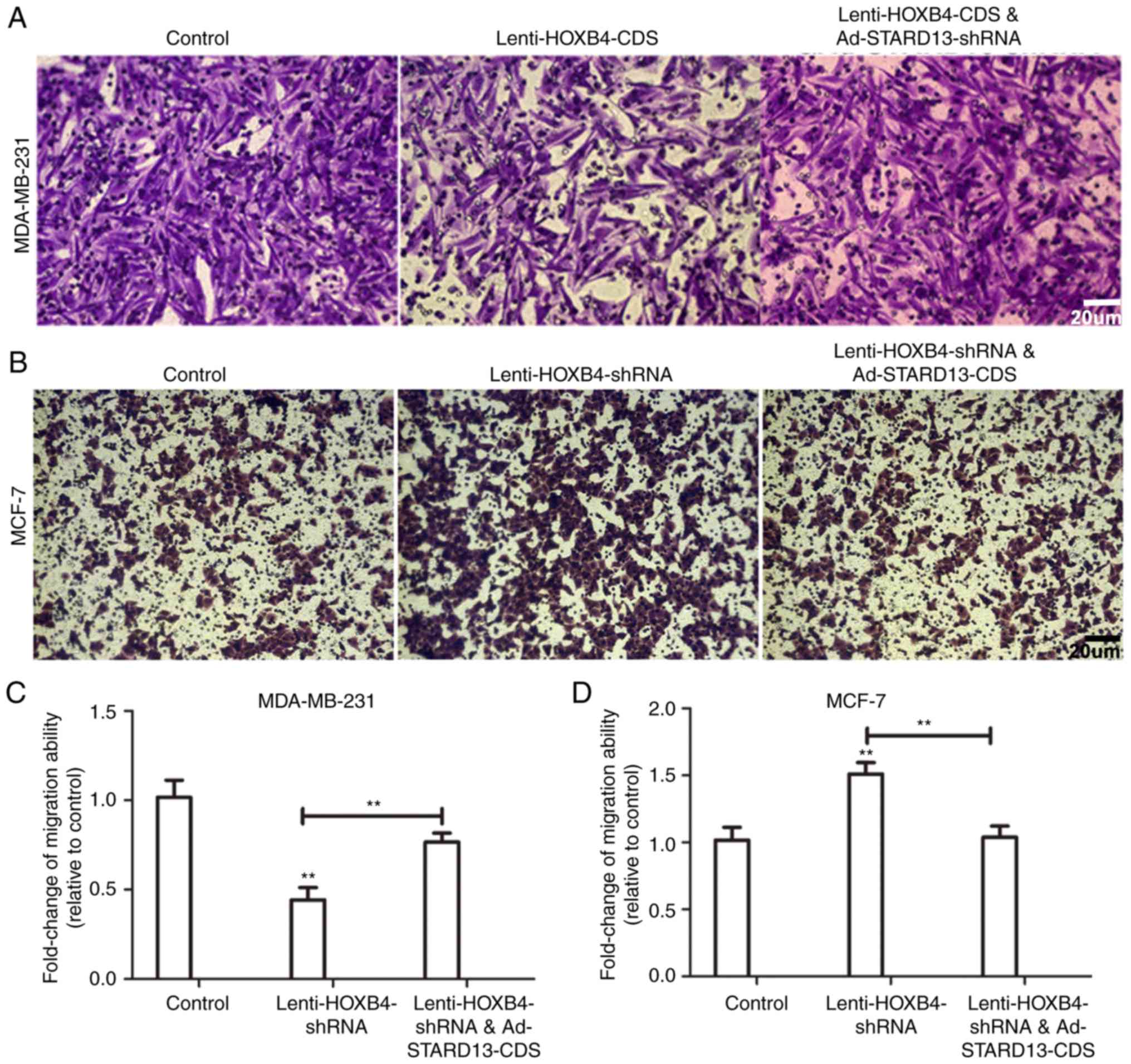
cells (Fig. 5). Decreased migration
ability was observed in Leni-HOXB4-CDS-infected MDA-MB-231 cells,
and this ability was attenuated when cells were co-infected with
Ad-STARD13-shRNA (Fig. 5A and C).
Similarly, cell migration was increased in
Lenti-HOXB4-shRNA-infected MCF-7 cells, and this effect was
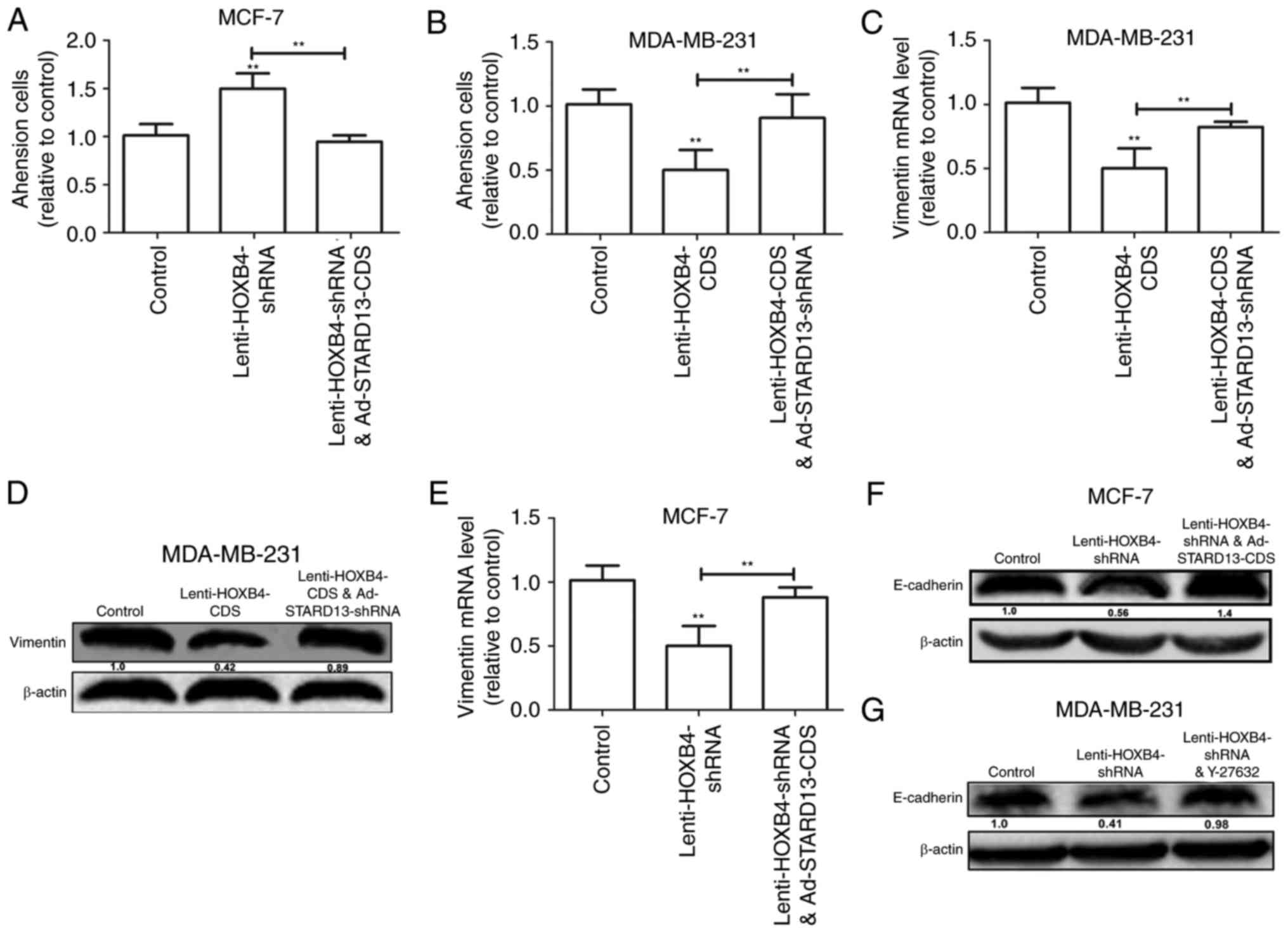
reversed with Ad-STARD13-CDS co-infection (Fig. 5B and D). Adhesion of tumor cells to
the extra-cellular matrix is thought to be the initial step in
tumor invasion (12). HOXB4
knockdown-induced downregulation of cell adhesion was reversed by
overexpression of STARD13 in MCF-7 cells (Fig. 6A). In contrast, Overexpression of
HOXB4 decreased the adhesion ability of MDA-MB-231 cells, which was
attenuated by STARD13 knockdown (Fig.
6B). Moreover, forced expression of HOXB4 inhibited the
expression of vimentin in MDA-MB-231 cells (Fig. 6C and D). This effect was also
attenuated by Ad-STARD13-shRNA infection. In contrast, knockdown of
HOXB4 decreased the expression levels of E-cadherin in MCF-7 cells,
and Ad-STARD13-CDS infection reversed this effect (Fig. 6E and F). Because STARD13 can inhibit
the activity of RhoA (13). Treatment
of HOXB4-knockdown MCF-7 cells with Y-27632 (an inhibitor of ROCK)
completely blocked the HOXB4 knockdown-dependent downregulation of
E-cadherin (Fig. 5G). These results
demonstrate that ectopic expression of HOXB4 could inhibit breast
cancer cell migration and the EMT process in a STARD13-dependent
manner.
Overexpression of HOXB4 enhanced
doxorubicin sensitivity in breast cancer cells
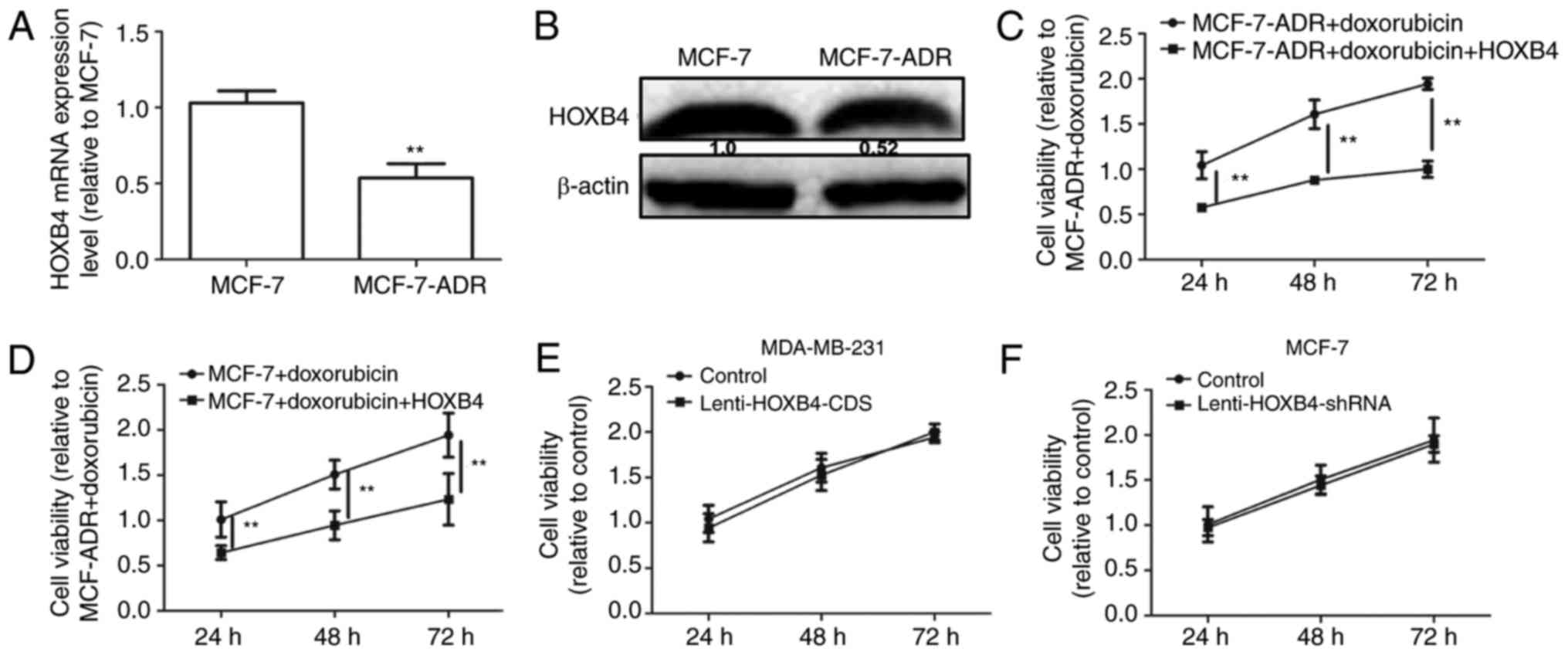
A previous study reported that the EMT could lead to
chemoresistance in cancer cells (14). HOXB4 expression levels were detected
in MCF-7 and MCF-7-ADR cells. As shown in Fig. 7A and B, HOXB4 expression levels were
downregulated in MCF-7-ADR compared to MCF-7 cells. We then
analyzed HOXB4 overexpression in MCF-7-ADR cells with
Lenti-HOXB4-CDS infection. The MTT assay showed that forced
expression of HOXB4 attenuated doxorubicin resistance in MCF-7-ADR
cells (Fig. 7C). Additionally,
overexpression of HOXB4 enhanced doxorubicin sensitivity in MCF-7
cells (Fig. 7D). However, the
proliferation ability was not altered in MDA-MB-231 cells with or
without HOXB4 overexpression and in MCF-7 cells with or without
HOXB4 knockdown (Fig. 7E and F).
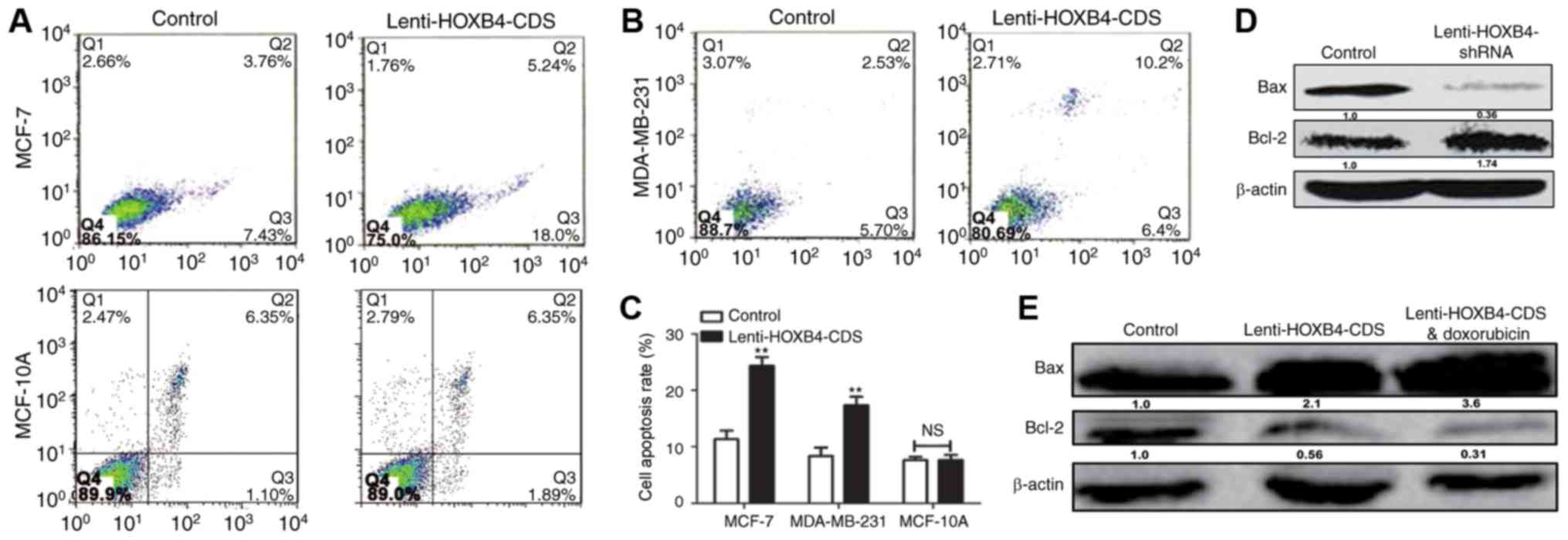
Furthermore, increased apoptosis was observed in HOXB4
overexpressing cells (Fig. 8A-C),
while ectopic expression of HOXB4 had no effect on MCF-10A cell
apoptosis. MDA-MB-231 cells with HOXB4 overexpression exhibited
increased expression of Bax and decreased expression of Bcl-2,
which was enhanced by additive doxorubicin treatment (Fig. 8D). Conversely, HOXB4 knockdown
inhibited Bax expression but increased Bcl-2 expression in MCF-7
cells (Fig. 8E). Hence, our results
indicate that forced expression of HOXB4 could increase doxorubicin
sensitivity and attenuate doxorubicin resistance in MCF-7
cells.
Discussion
Upregulation of HOXB4 promotes is strongly
associated with the overall survival of patients with acute myeloid
leukemia (15). However, the roles of
HOXB4 in somatic tumors are unclear.
In the present study, we investigated the effects of
HOXB4 on breast cancer metastasis, the EMT process and doxorubicin
sensitivity. Bioinformatics analysis was used to predict the
potential targets of HOXB4. STARD13 attracted our interest because
it has suppressive roles in breast cancer. Further analysis of
clinical samples and TCGA data, as well as ChIP assays, confirmed
our prediction that HOXB4 could bind to the STARD13 promoter
directly. Our results obtained from gain and loss of function
approaches indicated that HOXB4 was negatively correlated with the
migratory abilities of breast cancer cells in vitro. For
MCF-7 cells with low metastatic ability and high expression of
HOXB4, knockdown of HOXB4 permitted these cells to gain higher
metastatic potential. In MDA-MB-231 cells with low expression of
HOXB4, cell migration ability was significantly inhibited with
HOXB4 overexpression. Importantly, the inhibitory effects of HOXB4
on cell migration was dependent on STARD13 expression.
The EMT occurs aberrantly during tumor progression
and initiates the metastasis cascade and drug resistance (7,16). Here,
we found that enforced expression of HOXB4 strongly suppressed the
EMT process in breast cancer cells. To demonstrate the mechanisms
through which HOXB4 regulates the EMT, we used an inhibitor of
ROCK. We showed that HOXB4 regulated E-cadherin expression through
the STARD13-RhoA-ROCK signaling pathway. Interestingly, the
proliferation ability of cells was unchanged as HOXB4 expression
was altered, indicating that HOXB4 may affect the EMT process in
breast cancer and thereby modulate cell migration ability. Finally,
our results showed that overexpression of HOXB4 could promote
apoptosis, enhance doxorubicin sensitivity and attenuate
doxorubicin resistance in MCF-7 cells. These results suggested that
HOXB4 modulated doxorubicin sensitivity, possibly through
regulating the EMT process or cell apoptosis, but not through
modulating cell proliferation. In contrast to our results, a
previous study reported that HOXB4 knockdown reversed multidrug
resistance of human myelogenous leukemia K562/ADM cells (17). This difference may be due to the
different types of tumors, suggesting that HOXB4 may have various
roles depending on the tumors. Further studies are needed to
elucidate the roles of HOXB4 in other tumors.
To the best of our knowledge, this is the first
study to demonstrate the potential role of HOXB4 in breast cancer.
These results provided strong evidence in support of the inhibitory
activities of HOXB4 in the EMT, migration process and doxorubicin
resistance in breast cancer r. It is possible that these
characteristics may be exploited for application in gene therapy of
cancer.
References
|
1
|
Giordano SB and Gradishar W: Breast
cancer: Updates and advances in 2016. Curr Opin Obstet Gynecol.
29:12–17. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Lou PJ, Lai PS, Shieh MJ, Macrobert AJ,
Berg K and Bown SG: Reversal of doxorubicin resistance in breast
cancer cells by photochemical internalization. Int J Cancer.
119:2692–2698. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Helgason CD, Sauvageau G, Lawrence HJ,
Largman C and Humphries RK: Overexpression of HOXB4 enhances the
hematopoietic potential of embryonic stem cells differentiated in
vitro. Blood. 87:2740–2749. 1996.PubMed/NCBI
|
|
4
|
Cusan M, Vegi NM, Mulaw MA, Bamezai S,
Kaiser LM, Deshpande AJ, Greif PA, Quintanilla-Fend L, Göllner S,
Müller-Tidow C, et al: Controlled stem cell amplification by HOXB4
depends on its unique proline-rich region near the N-terminus.
Blood. 129:319–323. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Petzold KM, Naumann H and Spagnoli FM: Rho
signalling restriction by the RhoGAP Stard13 integrates growth and
morphogenesis in the pancreas. Development. 140:126–135. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Tang F, Zhang R, He Y, Zou M, Guo L and Xi
T: MicroRNA-125b induces metastasis by targeting STARD13 in MCF-7
and MDA-MB-231 breast cancer cells. PLoS One. 7:e354352012.
View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Li X, Zheng L, Zhang F, Hu J, Chou J, Liu
Y, Xing Y and Xi T: STARD13-correlated ceRNA network inhibits EMT
and metastasis of breast cancer. Oncotarget. 7:23197–23211. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Zhang H, Wang F and Hu Y: STARD13 promotes
hepatocellular carcinoma apoptosis by acting as a ceRNA for Fas.
Biotechnol Lett. 39:207–217. 2017. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Chang S, He S, Qiu G, Lu J, Wang J, Liu J,
Fan L, Zhao W and Che X: MicroRNA-125b promotes invasion and
metastasis of gastric cancer by targeting STARD13 and NEU1. Tumour
Biol. 37:12141–12151. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yang J, Li T, Gao C, Lv X, Liu K, Song H,
Xing Y and Xi T: FOXO1 3′UTR functions as a ceRNA in repressing the
metastases of breast cancer cells via regulating miRNA activity.
FEBS Lett. 588:3218–3224. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Zheng L, Li X, Meng X, Chou J, Hu J, Zhang
F, Zhang Z, Xing Y, Liu Y and Xi T: Competing endogenous RNA
networks of CYP4Z1 and pseudogene CYP4Z2P confer tamoxifen
resistance in breast cancer. Mol Cell Endocrinol. 427:133–142.
2016. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Wang B, Zheng L, Chou J, Li C, Zhang Y,
Meng X and Xi T: CYP4Z1 3′UTR represses migration of human breast
cancer cells. Biochem Biophys Res Commun. 478:900–907. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Nagaraja GM and Kandpal RP: Chromosome
13q12 encoded Rho GTPase activating protein suppresses growth of
breast carcinoma cells, and yeast two-hybrid screen shows its
interaction with several proteins. Biochem Biophys Res Commun.
313:654–665. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Xu T, Zhang J, Chen W, Pan S, Zhi X, Wen
L, Zhou Y, Chen BW, Qiu J, Zhang Y, et al: ARK5 promotes
doxorubicin resistance in hepatocellular carcinoma via
epithelial-mesenchymal transition. Cancer Lett. 377:140–148. 2016.
View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Li L, Zhao CT, Cui BL, Wu SL, Liu XD, Su
Z, Yang J, Wang W, Cui ZG and Zhao HG: Expression of HOXB4, PRDM16
and HOXA9 in patients with acute myeloid leukemia and its clinical
significance. Zhongguo Shi Yan Xue Ye Xue Za Zhi. 24:326–331.
2016.(In Chinese). PubMed/NCBI
|
|
16
|
Sun W and Tang L: MDM2 increases drug
resistance in cancer cells by inducing EMT independent of p53. Curr
Med Chem. 23:4529–4539. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Wang H, Jia XH, Chen JR, Yi YJ, Wang JY,
Li YJ and Xie SY: HOXB4 knockdown reverses multidrug resistance of
human myelogenous leukemia K562/ADM cells by downregulating P-gp,
MRP1 and BCRP expression via PI3K/Akt signaling pathway. Int J
Oncol. 49:2529–2537. 2016. View Article : Google Scholar : PubMed/NCBI
|