Introduction
The estrogen receptor (ER) and its ligand, estrogen,
serve a critical role in the development and progression of breast
cancer (1). The human ER exists as
two subtypes, ER-α and ER-β, which regulate the transcription of
various target genes upon binding to estrogen response elements
present within the regulatory region of the target genes (2). In the majority of ER-α-positive cases of
breast cancer, the expression level of ER-α is considerably higher
compared with that in normal breast epithelium (3). Accordingly, endocrine therapies, which
target ER activity, are standard treatments for patients with
ER-positive breast cancer in the early and advanced/metastatic
stages. However, despite the substantial benefit from endocrine
treatment, resistance is still common, and it significantly
influences the overall morbidity and mortality of breast cancer
(3).
The fruits and seeds of the Ginkgo biloba
tree have traditionally been used in Chinese medicine with
indications for the treatment of asthma, coughs and enuresis
(4). Ginkgetin, which is a
biflavonoids isolated from G. biloba extract, is known to
have anti-inflammatory and anti-viral activities in vitro
and in vivo (5,6). Of note, ginkgetin was reported to
exhibit cytotoxic effects in ovarian adenocarcinoma and prostate
cancer cells (7–9). However, the anticancer activities and
the underlying mechanism of ginkgetin in breast cancer cells have
not yet been investigated.
The present study examined the cytotoxicity of
ginkgetin against numerous breast cancer cell lines, including
ER-positive and negative cells. It was demonstrated that the
anticancer activity of ginkgetin in breast cancer cells was
associated with the downregulation of the ER and, subsequently, the
blockade of the signaling pathway activated by estrogen. The
results of the present study suggested that ginkgetin may merit
further investigation as a chemotherapeutic agent against breast
cancer.
Materials and methods
Cell culture and reagents
MCF-7, T-47D, and MDA-MB-231 breast cancer cells
were purchased from the American Type Culture Collection (Manassas,
VA, USA). The MCF-7 cells were grown in Dulbecco's modified Eagle's
medium (DMEM) (Invitrogen; Thermo Fisher Scientific, Inc., Waltham,
MA, USA), the T-47D cells were grown in RPMI-1640 growth medium
(Invitrogen; Thermo Fisher Scientific, Inc.), and the MDA-MB-231
cells were grown in Leibovitz's L-15 medium (Invitrogen; Thermo
Fisher Scientific, Inc.) with 10% fetal bovine serum (FBS; Corning
Life Sciences, Tewksbury, MA, USA) at 37°C in 5% CO2
incubator. For treatment with 17β-estradiol (E2), the cells were
grown in media containing no phenol red and 10%
charcoal:dextran-stripped FBS (SciPak Lifesciences, Sacramento, CA,
USA). Antibodies against ER-α (cat. no. sc-8005) and β-actin (cat.
no. sc-130300) were purchased from Santa Cruz Biotechnology, Inc.
(Dallas, TX, USA). Antibodies against cleaved poly (ADP-ribose)
polymerase (PARP; cat. no. 5625), caspase 7 (cat. no. 12827),
cyclin D1 (cat. no. 2978) and survivin (cat. no. 2808) were
purchased from Cell Signaling Technology, Inc. (Danvers, MA, USA).
The antibody against
6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3; cat.
no. 13763-1-AP) was purchased from Proteintech (Chicago, IL, USA).
Short interfering (si)RNAs targeting ER-α and negative control
(scrambled) siRNAs were purchased from Santa Cruz Biotechnology,
Inc. An inhibitor of PFKFB3, 3PO, was purchased from Merck KGaA
(Darmstadt, Germany). Ginkgetin and isoginkgetin were isolated from
dried G. biloba leaves (9).
Transfections and treatments
Cells (2×105) in 1 ml of serum-free
medium were transfected with control siRNA or ER-α siRNA (100 nM)
for 6 h at 37°C in a CO2 incubator using Lipofectamine
(Invitrogen; Thermo Fisher Scientific, Inc.), according to the
manufacturer's protocol. Upon replacing the culture medium with
fresh DMEM with 10% FBS, the cells were treated with 5 µМ ginkgetin
for 24 h at 37°C to proceed with subsequent experiments.
Reverse transcription-quantitative
polymerase chain reaction (RT-qPCR)
Total RNA, from T-47D cells, was isolated using the
TRIzol® reagent (Thermo Fisher Scientific, Inc.),
according to the manufacturer's protocol, and was quantified using
a spectrophotometer. An aliquot of total RNA (2 µg) was transcribed
into complementary (c)DNA primed with oligo dT using an RT2 First
Strand kit (Qiagen GmbH, Hilden, Germany). For RT-qPCR, the cDNA
was amplified using a KAPA SYBR FASR qPCR kit (Kapa Biosystems,
Inc., Wilmington, MA, USA) using specific primer pairs (Origene
Technologies, Inc., Rockville, MD, USA). PFKFB3, cyclin D1, and
β-actin mRNA expression levels were analyzed using a
LightCycler® (Roche Diagnostics, Basel, Switzerland),
and thermocycling was performed according to the manufacturer's
protocols. The primer sequences were as follows: PFKFB3,
5′-CTGCAGAGGAGATGCCCTAC-3′ (forward) and 5′-AGGTCCCTTCTTTGCATCCT-3′
(reverse); cyclin D1, 5′-CCGTCCATGCGGAAGATC-3′ (forward) and
5′-CCTCCTCCTCGCACTTCTGT-3′ (reverse); β-actin,
5′-GGATTCCTATGTGGGCGACGA-3′ (forward) and
5′-CGCTCGGTGAGGATCTTCATG-3′ (reverse). Relative quantification of
PFKFB3 and cyclin D1 expression levels was determined by the
2−ΔΔCq method (10).
Measurement of cell viability
Cell viability was determined by evaluating the
mitochondrial conversion of MTT to a colored product. The cells
were treated with drugs as indicated, and the medium was exchanged
with serum-free DMEM or RPMI-1640 containing 1 mM MTT. Following 1
h of incubation at 37°C, the cells were solubilized in dimethyl
sulfoxide. The amount of formazan, which is the converted form of
MTT, was determined by evaluating the absorbance at 590 nm.
Western blotting
The cells were harvested and lysed for 20 min at 4°C
in radioimmunoprecipitation assay buffer (50 mM Tris-HCl pH 7.5,
150 mM NaCl, 1% Nonidet P-40, 0.5% sodium deoxycholate and 0.1%
SDS) supplemented with a protease inhibitor cocktail (GenDEPOT,
Barker, TX, USA). Determination of total protein was performed by
Bradford method (11). Equal amounts
of protein (20–50 µg) were separated by 10% SDS-PAGE and
transferred to a nitrocellulose membrane. The membranes were
blocked by incubation with 2.5% skimmed milk for 30 min at RT,
followed by incubation overnight at 4°C with the appropriate
primary antibodies (diluted 1:1,000; anti-cleaved PARP,
anti-caspase 7, anti-ER-α, anti-cyclin D1, anti-survivin,
anti-PFKFB3, anti-β-actin) and incubation for 1.5 h at 4°C with the
secondary antibody (Santa Cruz Biotechnology, Inc.; diluted
1:10,000; mouse anti-rabbit IgG-horseradish peroxidase; cat. no.
sc-2357). Immunoreactive proteins were visualized using enhanced
chemiluminescence reagents (GE Healthcare, Chicago, IL, USA).
Evaluation of apoptosis
Apoptosis was determined by fluorescence-activated
cell sorting analysis using an Annexin V-FITC Apoptosis Detection
kit (BioVision, Inc., Milpitas, CA, USA), according to the
manufacturer's protocol. Briefly, the MCF-7 and T-47D cells were
incubated for 24 h at 37°C with ginkgetin and then treated with
trypsin (Invitrogen; Thermo Fisher Scientific, Inc.) for 5 min at
37°C. The cells were resuspended with binding buffer (10 mM
4-(2-hydroxyethyl)-1-piperazineethanesulphonic acid/NaOH, pH 7.4,
140 mM NaCl, 2.5 mM CaCl2) including Annexin
V-fluorescein isothiocyanate and propidium iodide (PI). Cell
fluorescence was then analyzed by flow cytometry using a
FACScaliber and Cell Quest software (version 3.3; BD Biosciences,
San Jose, CA, USA).
Statistical analysis
All data presented are representative of at least
three separate experiments. Results are expressed as the mean ±
standard deviation. Statistical differences among groups were
determined using the Student's t-test (for two groups) or one-way
analysis of variance, followed by the post-hoc Tukey's test, for
>2 groups using Prism 7 software (GraphPad Software, La Jolla,
CA, USA). P<0.05 was considered to indicate a statistically
significant difference.
Results
Ginkgetin inhibits cell growth and
induces apoptosis in human breast cancer cell lines
In previous studies, ginkgetin was cytotoxic against
tumor cells with a half-maximal inhibitory concentration
(IC50) of ~10 µM (7–9). Thus, the
present study exposed MCF-7 and T-47D human breast cancer cells to
5 or 10 µM of ginkgetin to evaluate its cytotoxicity against breast
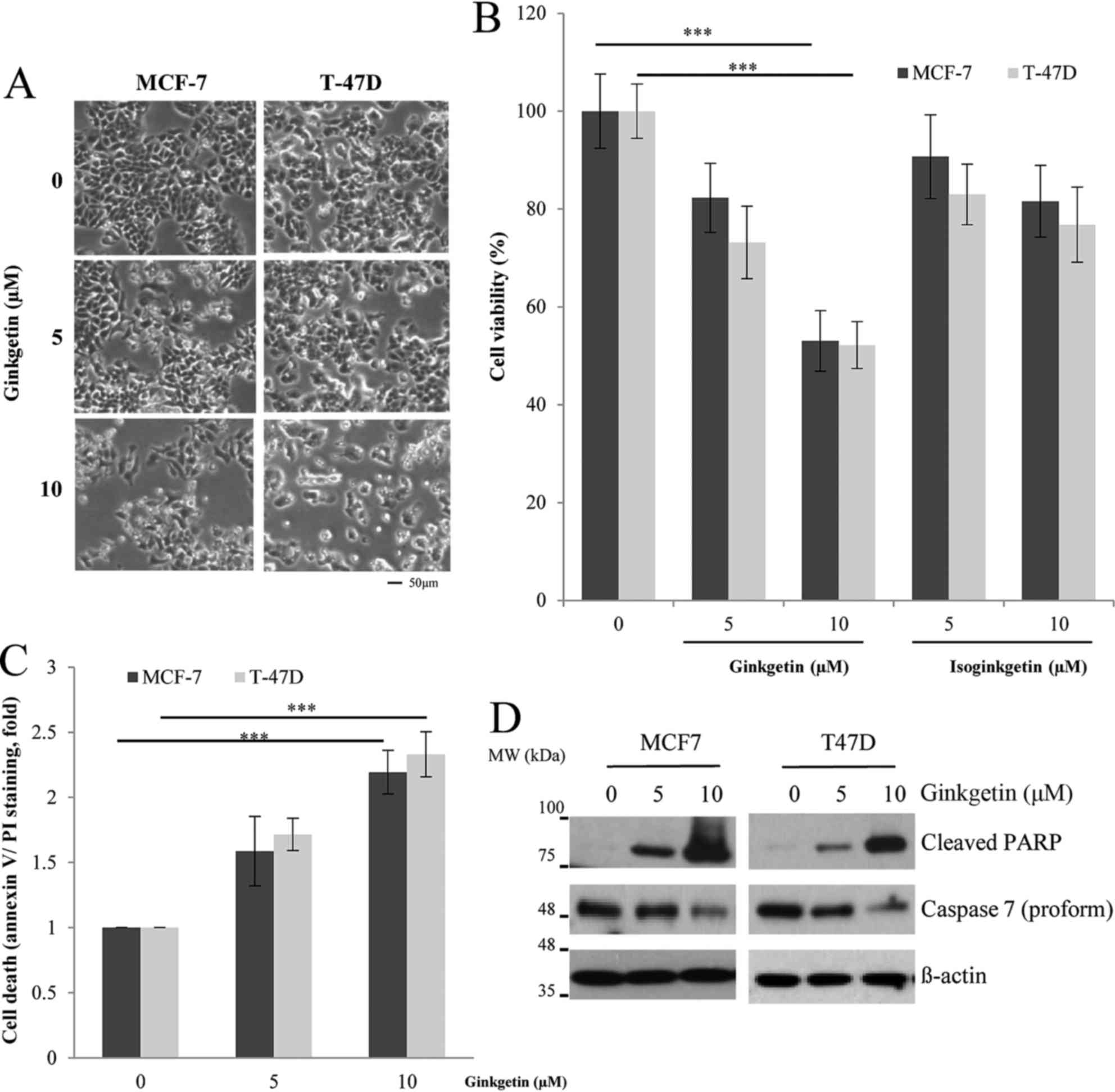
cancer cells. As presented in Fig.
1A, the cells treated with ginkgetin demonstrated a decrease in
cell number and an increased indication of apoptosis, including
apoptotic bodies and cell shrinkage, as observed under an inverted
microscope. The results of the MTT assay revealed that ginkgetin
reduced cell viability by ~50% in both cell lines at a
concentration of 10 µM (Fig. 1B;
P<0.001). Subsequently, the present study investigated whether
isoginkgetin, a derivative of ginkgetin isolated from G.
biloba extract, has similar cytotoxic effects on breast cancer
cells. However, 10 µM isoginkgetin decreased the viability of the
MCF-7 and T-47D cells by 17 and 25%, respectively. Thus, the
results of the present study are consistent with a previous study
demonstrating that ginkgetin was more effective in exerting
antitumor activity compared with that of its derivatives (9). To confirm whether ginkgetin induces
apoptosis in breast cancer cells, the apoptotic cells were
evaluated by flow cytometry. The populations of annexin V and
PI-positive cells increased with ginkgetin treatment in both cell
types in a dose-dependent manner (Fig.
1C; P<0.001). In addition, the reduced procaspase 7 and
cleaved PARP were detected by western blotting (Fig. 1D), indicating that ginkgetin induced
apoptotic cell death in breast cancer cells.
Ginkgetin impairs the ER signaling
pathway via downregulation of ER-α expression
Certain flavonoids are known to affect the ER
signaling pathways in ER-positive breast cancer cells (12,13). To
investigate the possibility that the cytotoxicity of ginkgetin in
breast cancer cells acts via ER regulation, the present study
analyzed the expression level of ER-α in ginkgetin-treated cells.
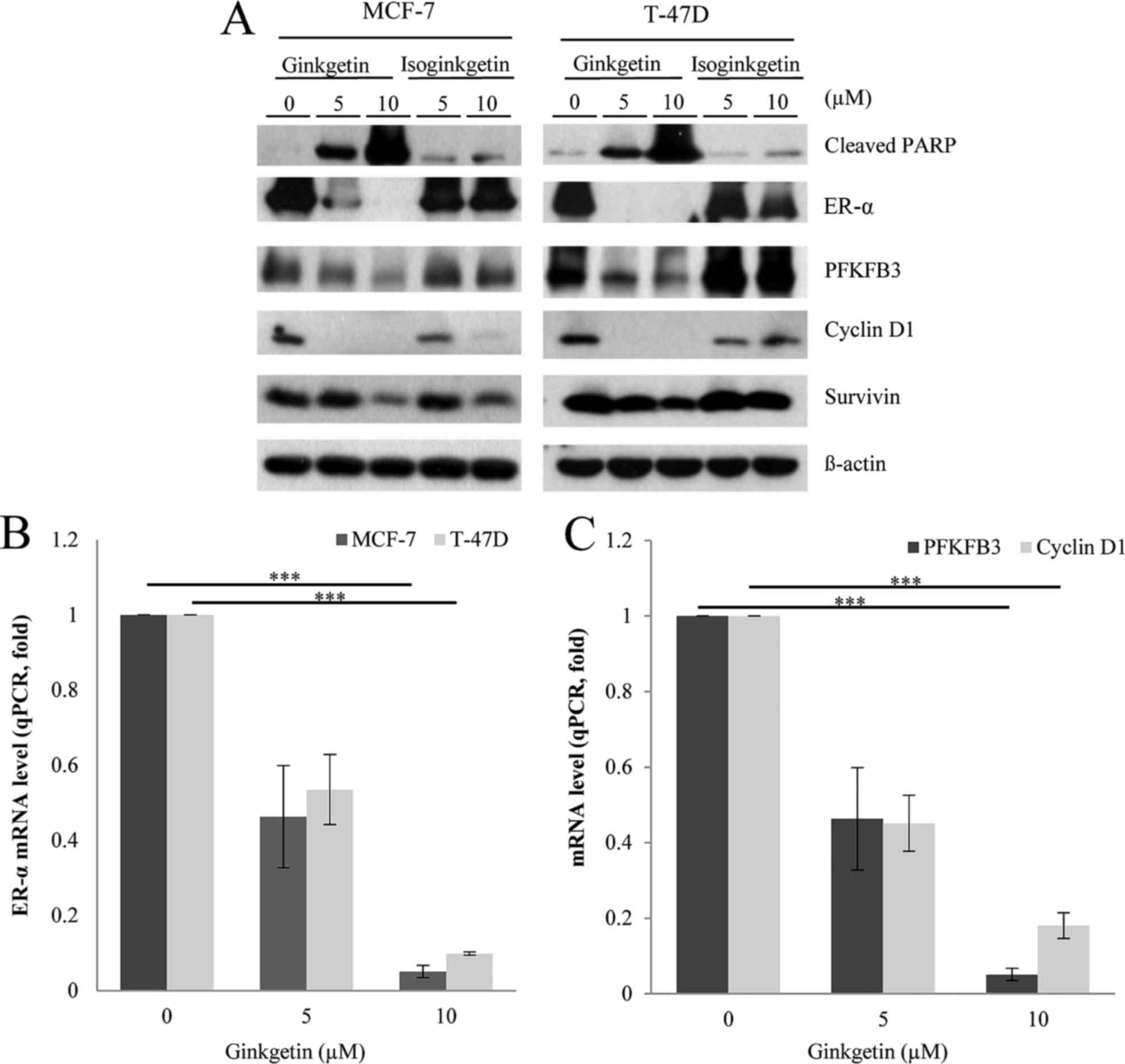
Western blot analysis demonstrated that ginkgetin markedly reduced
the ER-α expression level in MCF-7 and T-47D cells in a
dose-dependent manner (Fig. 2A). To
confirm the inhibition of the ER-α signaling pathways by ginkgetin,
the present study determined the expression levels of downstream
effectors in ginkgetin-treated cells. Previous studies reported
that ER-α directly induced the expression of PFKFB3, cyclin D1 and
survivin following estrogen binding to the receptor of breast
cancer cells for their survival and growth (14–17). As
presented in Fig. 2A, ginkgetin also
reduced the expression level of PFKFB3, cyclin D1 and survivin in
both cell lines. Isoginkgetin had a small effect on the expression
level of ER-α and its effectors, although the expression levels of
cyclin D1 and survivin were suppressed in the isoginkgetin-treated
MCF-7 cells (Fig. 2A). Since
progesterone reprograms ER-α binding events to novel chromatin loci
and transcriptional targets (18),
the discrepancy in the effects of isoginketin may be due to the
differences in the levels of progesterone receptor between these
two cell lines. The other possibility is that isoginkgetin may
downregulate these molecules by other mechanisms that may be
elucidated in the future. To further investigate the decrease in
ER-α expression levels in breast cancer cells treated with
ginkgetin, the present study performed RT-qPCR to analyze the
corresponding mRNA expression levels. Treatment with ginkgetin
decreased ER-α mRNA expression levels dose-dependently, which was
consistent with the observed reduction in ER-α protein expression
levels (Fig. 2B; P<0.001).
Conversely, ginkgetin had no effect on ER-β mRNA and protein levels
(data not shown), indicating the specificity of ginkgetin in
regulating ER-α expression in breast cancer cells. The mRNA
expression levels of PFKFB3 and cyclin D1 were also downregulated
by ginkgetin in T-47D cells, which was consistent with a decrease
in the expression level of these molecules at the protein level
(Fig. 2C; P<0.001). Taken
together, the results of the present study suggested that ginkgetin
may inhibit the ER signaling pathways of breast cancer cells by
downregulating ER-α at the transcriptional level.
The cytotoxicity of ginkgetin is
dependent on ER-α expression
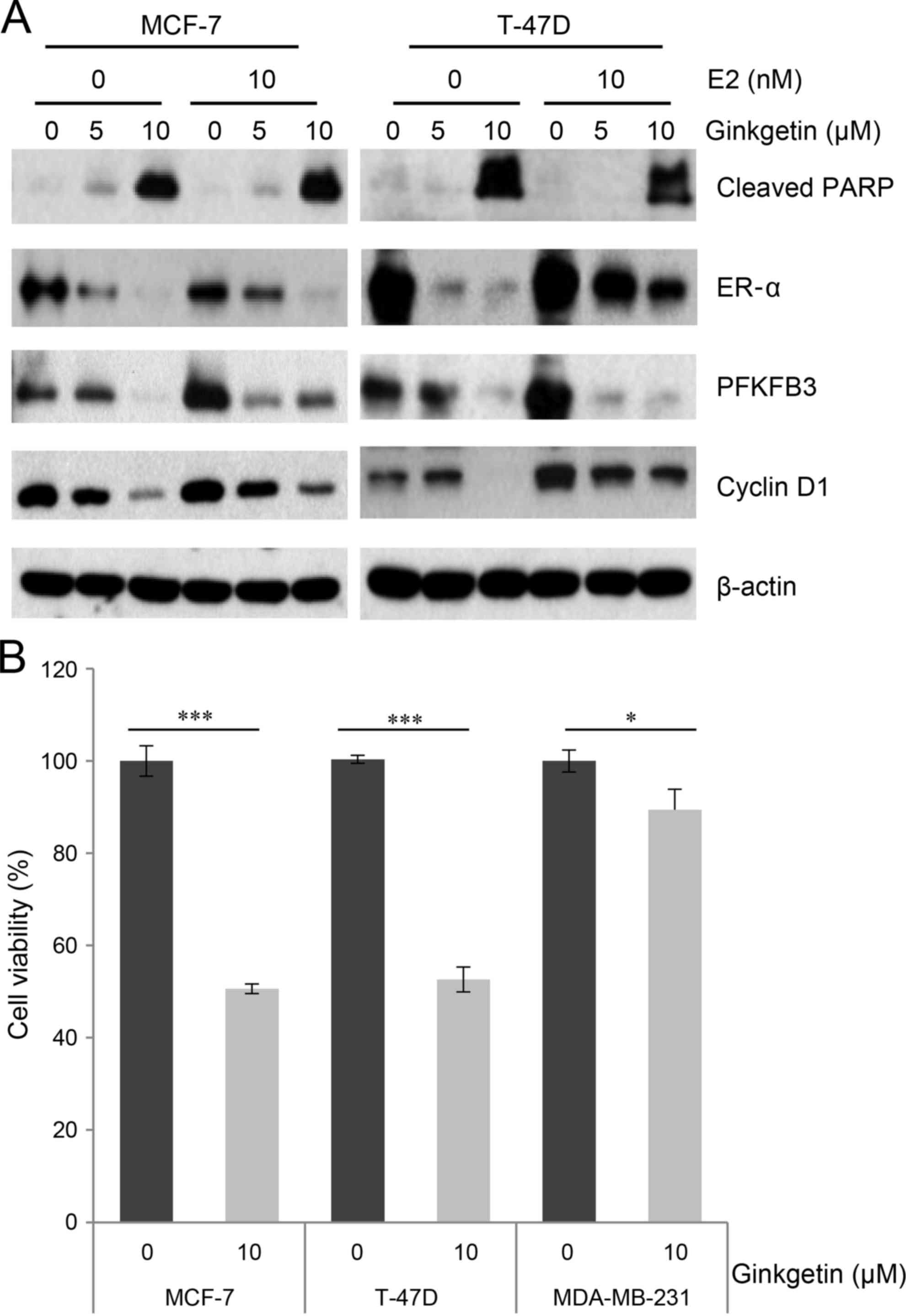
E2, which is the most potent endogenous estrogen,
binds to the ER and subsequently stimulates ER proliferation
pathways in cultured ER-positive breast cancer cells (19). To further investigate the cytotoxicity
of ginkgetin in response to E2 treatment, cells were exposed to
ginkgetin with or without E2 treatment. The expression levels of
PFKFB3, in MCF-7 and T-47D cells, and cyclin D1, in T-47D cells,
were increased by E2 treatment in the absence of ginkgetin,
indicating stimulation of the ER signaling pathways (Fig. 3A). However, E2 treatment combined with
5 µM ginkgetin did not induce the expression of PFKFB3, but
decreased it in the two cell lines. Additional investigations are
required to determine the underlying molecular mechanism. Of note,
the ER signaling pathways activated by E2 were also markedly
attenuated by ginkgetin treatment in MCF-7 and T-47D cells
(Fig. 3A). Ginkgetin effectively
induced PARP cleavage and repressed ER-α expression levels, even in
E2-treated cells. These results indicated that the cytotoxicity of
ginkgetin may be fatal for breast cancer cells and is sufficient to
abrogate growth stimulation by E2. To further elucidate the role of
ER-α, the present study investigated the cytotoxicity of ginkgetin
in MDA-MB-231 cells, which have a relatively low expression of
ER-α; thus, these cells are often utilized as a negative control
for the investigation of ER-α involvement (20). The MTT assay demonstrated that the
growth inhibitory effect of ginkgetin in MDA-MB-231 cells was lower
compared with the corresponding effect in MCF-7 or T-47D cells
(Fig. 3B), supporting the negative
correlation between ER-α expression level and ginkgetin
cytotoxicity. Of note, the relatively minor reduction of cell
growth in MDA-MD-231 cells resulting from ginkgetin treatment
suggests an ER-independent cytotoxic effect of ginkgetin in breast
cancer cells (Fig. 3B).
Inhibition of ER-α and PFKFB3 enhances
the cytotoxicity of ginkgetin in ER-positive breast cancer
cells
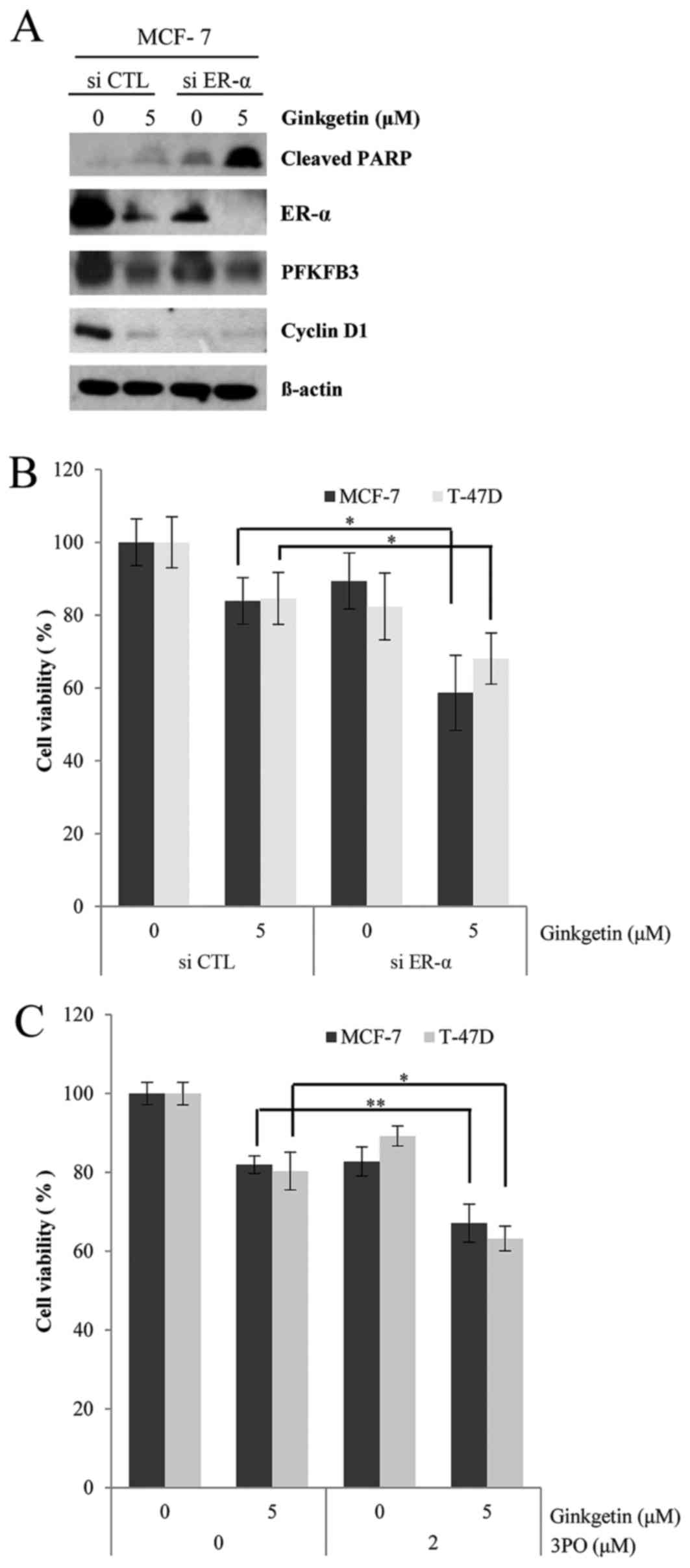
The viability of ER-positive breast cancer cells is
dependent on ER-α expression level (3); therefore, the present study determined
whether ER-α siRNA enhanced the sensitivity of ER-positive breast
cancer cells to ginkgetin. As presented in Fig. 4A, ER-α siRNA markedly enhanced
ginkgetin-induced PARP cleavage in MCF-7 cells, along with the
downregulation of PFKFB3 and cyclin D1. In the MTT assays, ER-α
siRNA had a greater effect on the cytotoxicity of ginkgetin
compared with that of the negative control siRNA (Fig. 4B). Finally, the present study employed
3PO, which is a specific inhibitor of PFKFB3, to determine whether
inhibition of the ER downstream signaling pathways also promoted
the cytotoxicity of ginkgetin. As expected, ginkgetin-induced
inhibition was further augmented by 3PO treatment (Fig. 4C). Therefore, the present study
suggested that the cytotoxic effects of ginkgetin on breast cancer
cells may be mediated by the downregulation of ER-α, which may
suppress the survival of ER-positive breast cancer cells.
Discussion
Breast cancer is one of the most common types of
cancer and is the leading cause of cancer-associated mortalities
among females worldwide (19). For
developing breast cancer therapies, ER-α has been utilized as a
target molecule due to its high expression in ~70% of all breast
tumors (20). The activation of the
ER by estrogens serves a critical role in cancer initiation and
progression, and ER antagonists have demonstrated efficacy in the
treatment of breast cancer. Endocrine therapy modalities are based
on three main strategies: i) Depriving the tumor of its ligand by
systemically depleting estrogen production using aromatase
inhibitors or ovarian suppression; ii) inhibiting estrogen binding
to the ER by using selective ER modulators, including tamoxifen; or
iii) degrading the ER using selective ER down-regulators (SERD),
including fulvestrant, which results in a more complete inhibition
of the ER signaling pathway (21).
Fulvestrant, the only SERD approved by the USA Food and Drug
Administration to treat patients with breast cancer, has the
100-fold affinity of tamoxifen for ER with no adverse effect on
endometrial ERs (21). Recently, an
orally bioavailable SERD, TAS-108, has been through phase II
clinical studies, and phase III studies are currently being planned
(22).
Ginkgetin has a wide spectrum of biological
functions, including anti-inflammatory, antifungal, anti-influenza,
neuroprotective and antitumor activities (4,6); however,
these antitumor activities have been reported in limited cancer
types (7–9), and the investigation of the toxicity of
ginkgetin in other types of cancer is required to fully understand
the underlying mechanism of its effects. The present study reported
evidence of a mechanism of ginkgetin-induced cell death in
ER-positive breast cancer cells via the downregulation of ER-α
expression level. Ginkgetin selectively inhibited the growth of
ER-positive breast cancer cells and did not affect ER-negative
(MDA-MB-231) or normal cells (MCF-10A; data not shown). Therefore,
the present study hypothesized that ginkgetin, at an effective dose
against tumor cells, may not have severe toxicity toward breast
tissues with low ER expression level. Of note, human epidermal
growth factor receptor 2 (HER2) -positive (BT-474) breast cancer
cells were less sensitive to ginkgetin compared with HER2-negative
(MCF-7 and T-47D) cells, suggesting that HER2 expression level may
be associated with the antitumor activity of ginkgetin (data not
shown). Further studies are required to determine the mechanism
underlying the resistance to ginkgetin in HER2-postive breast
cancer cells.
The present study indicated that ginkgetin
suppressed ER-α expression at the mRNA and protein levels. Evidence
from previous studies indicated that the therapeutic effect of
ginkgetin may involve the modification of gene expression,
including genes implicated in antioxidant and stress responses
(4). In addition, the apoptosis of
human ovarian adenocarcinoma cells induced by ginkgetin was
mediated mainly by hydrogen peroxide generated most likely via the
autooxidation of ginkgetin (7). Thus,
the present study speculated that oxidative stress may regulate the
activity of the regulator(s) responsible for the gene expression of
ER-α. Further experiments are required to determine whether an
antioxidant affects the cytotoxicity of ginkgetin in breast cancer
cells.
In conclusion, the present study demonstrated that
the downregulation of ER-α expression in breast cancer cells by
treatment with ginkgetin serves a key role in inducing apoptotic
cell death. The results provided novel insight into the action of
ginkgetin, which may inhibit the ER signaling pathway in breast
cancer. Further studies will provide further evidence for ginkgetin
as a promising candidate with minimal adverse effects for drug
development against breast cancer.
Acknowledgements
The present study was supported by the National
R&D Program through the Korea Institute of Radiological and
Medical Sciences funded by the Ministry of Science, ICT &
Future Planning in the Republic of Korea (grant nos. 1711021781 and
1711021931).
References
|
1
|
Weatherman RV, Fletterick RJ and Scanlan
TS: Nuclear-receptor ligands and ligand-binding domains. Annu Rev
Biochem. 68:559–581. 1999. View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Acconcia F, Totta P, Ogawa S, Cardillo I,
Inoue S, Leone S, Trentalance A, Muramatsu M and Marino M: Survival
versus apoptotic 17beta-estradiol effect: Role of ER alpha and ER
beta activated non-genomic signaling. J Cell Physiol. 203:193–201.
2005. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Saji S, Okumura N, Eguchi H, Nakashima S,
Suzuki A, Toi M, Nozawa Y, Saji S and Hayashi S: MDM2 enhances the
function of estrogen receptor alpha in human breast cancer cells.
Biochem Biophys Res Commun. 281:259–265. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Smith JV and Luo Y: Studies on molecular
mechanisms of Ginkgo biloba extract. Appl Microbiol Biotechnol.
64:465–472. 2004. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Hayashi K, Hayashi T and Morita N:
Mechanism of action of the antiherpesvirus biflavone ginkgetin.
Antimicrob Agents Chemother. 36:1890–1893. 1992. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Lim H, Son KH, Chang HW, Kang SS and Kim
HP: Effects of anti-inflammatory biflavonoid, ginkgetin, on chronic
skin inflammation. Biol Pharm Bull. 29:1046–1049. 2006. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Su Y, Sun CM, Chuang HH and Chang PT:
Studies on the cytotoxic mechanisms of ginkgetin in a human ovarian
adenocarcinoma cell line. Naunyn Schmiedebergs Arch Pharmacol.
362:82–90. 2000. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
You OH and Kim SH, Kim B, Sohn EJ, Lee HJ,
Shim BS, Yun M, Kwon BM and Kim SH: Ginkgetin induces apoptosis via
activation of caspase and inhibition of survival genes in PC-3
prostate cancer cells. Bioorg Med Chem Lett. 23:2692–2695. 2013.
View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Jeon YJ, Jung SN, Yun J, Lee CW, Choi J,
Lee YJ, Han DC and Kwon BM: Ginkgetin inhibits the growth of DU-145
prostate cancer cells through inhibition of signal transducer and
activator of transcription 3 activity. Cancer Sci. 106:413–240.
2015. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2(−Delta Delta C(T)) Method. Methods. 25:402–408. 2001.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bradford MM: A rapid and sensitive method
for the quantitation of microgram quantities of protein utilizing
the principle of protein-dye binding. Anal Biochem. 72:248–254.
1976. View Article : Google Scholar : PubMed/NCBI
|
|
12
|
Sak K: Cytotoxicity of dietary flavonoids
on different human cancer types. Pharmacogn Rev. 8:122–146. 2014.
View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Zheng N, Zhang P, Huang H, Liu W, Hayashi
T, Zang L, Zhang Y, Liu L, Xia M, Tashiro S, et al: ERα
down-regulation plays a key role in silibinin-induced autophagy and
apoptosis in human breast cancer MCF-7 cells. J Pharmacol Sci.
128:97–107. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Altucci L, Addeo R, Cicatiello L, Dauvois
S, Parker MG, Truss M, Beato M, Sica V, Bresciani F and Weisz A:
17beta-Estradiol induces cyclin D1 gene transcription,
p36D1-p34cdk4 complex activation and p105Rb phosphorylation during
mitogenic stimulation of G(1)-arrested human breast cancer cells.
Oncogene. 12:2315–2324. 1996.PubMed/NCBI
|
|
15
|
Frasor J, Danes JM, Komm B, Chang KC,
Lyttle CR and Katzenellenbogen BS: Profiling of estrogen up- and
down-regulated gene expression in human breast cancer cells:
Insights into gene networks and pathways underlying estrogenic
control of proliferation and cell phenotype. Endocrinology.
144:4562–4574. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Zhu J, Lu X, Hua KQ, Sun H, Yu YH and Feng
YJ: Oestrogen receptor α mediates 17β-estradiol enhancement of
ovarian cancer cell motility through up-regulation of survivin
expression. Arch Gynecol Obstet. 286:729–737. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Imbert-Fernandez Y, Clem BF, O'Neal J,
Kerr DA, Spaulding R, Lanceta L, Clem AL, Telang S and Chesney J:
Estradiol stimulates glucose metabolism via
6-phosphofructo-2-kinase (PFKFB3). J Biol Chem. 289:9440–9448.
2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mohammed H, Russell IA, Stark R, Rueda QM,
Hickey TE, Tarulli GA, Serandour AA, Birrell SN, Bruna A, Saadi A,
et al: Progesterone receptor modulates ERα action in breast cancer.
Nature. 523:313–317. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Foster JS, Henley DC, Ahamed S and
Wimalasena J: Estrogens and cell-cycle regulation in breast cancer.
Trends Endocrinol Metab. 12:320–327. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Clark GM, Osborne CK and McGuire WL:
Correlations between estrogen receptor, progesterone receptor, and
patient characteristics in human breast cancer. J Clin Oncol.
2:1102–1109. 1984. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Ingle JN: Pharmacogenomics of endocrine
therapy in breast cancer. J Hum Genet. 58:306–312. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Iwata H, Nakayama T, Yamamoto N, Sato Y,
Tokuda Y, Aogi K, Saji S, Watanabe K, Saito T, Yoshida M, et al:
Randomized phase II study of three doses of oral TAS-108 in
postmenopausal patients with metastatic breast cancer. Cancer Sci.
103:1708–1713. 2012. View Article : Google Scholar : PubMed/NCBI
|