Introduction
As a developing research area, the microbiome has
been the focus of multiple studies in previous years. The
non-spore-forming, anaerobic gram-negative bacterium
Fusobacterium nucleatum is part of the normal flora of the
human oral cavity and gut mucosa, but is an established
opportunistic pathogen in periodontal diseases (1–4) and
several inflammatory diseases, including inflammatory bowel disease
(5–8),
liver abscesses (9,10) and chorioamnionitis (11). Two previous studies have reported an
overabundance of F. nucleatum in colorectal cancer tissues
compared with adjacent normal tissues (12,13).
Following this, a previous study demonstrated that F.
nucleatum activates the E cadherin/β-catenin signaling pathway
via FadA adhesion, promoting colorectal cancer growth (14). Fusobacterium subspecies (spp.),
including F. nucleatum, are also present at increased levels
in human colorectal, pancreatic and other types of cancer (12,13,15–20).
To the best of our knowledge, there are only five previous studies
reporting the presence of Fusobacterium spp. in colorectal
and pancreatic cancer tissues and there are no published studies
that associate Fusobacterium spp. with esophageal, gastric,
hepatocellular and other gastroenterological cancer (Table I) (15,16,19,20,21).
 | Table I.Detection rates of
Fusobacterium spp. in gastroenterological cancer tissues
from previous studies. |
Table I.
Detection rates of
Fusobacterium spp. in gastroenterological cancer tissues
from previous studies.
|
|
|
|
|
|
Fusobacterium detection rate,
% |
|
|---|
|
|
|
|
|
|
|
|
|---|
| Authors | Type of cancer | No. of cases | Tissue
fixation | Bacterial
strain | Tumor tissue | Normal tissue | (Refs.) |
|---|
| Tahara et
al, 2014 | Colorectal
cancer | 149 | Frozen tissue | F.
nucleatum | 52.3 (78/149) | 30.3 (27/89) | (15) |
| Mima et al,
2015 | Colorectal
cancer | 598 | FFPE | F.
nucleatum | 13 (76/598) | 3.4 (19/558) | (16) |
| Ito et al,
2015 | Colorectal
cancer | 511 | FFPE | F.
nucleatum | 56
(286/511) | − | (19) |
| Mitsuhashi et
al, 2015 | Pancreatic
cancer | 283 | FFPE | Fusobacterium
species | 8.8 (25/283) | 28 (7/25) | (20) |
| Viljoen et
al, 2015 | Colorectal
cancer | 71 | FFPE | F. nucleatum
spp. polymorphum | 82 (58/71) | 81 (48/59) | (21) |
Elevated levels of F. nucleatum DNA in
colorectal cancer tissue are associated with certain molecules and
cell functions, including microsatellite instability, the CpG
island methylator phenotype and hMLH1 (15), and are also associated with a lower
density of T cells (16). A number of
previous studies have associated high levels of F. nucleatum
DNA content with poor patient prognosis (17,18),
however, other previous studies have reported that there is no
association between the quantity of F. nucleatum DNA and
patient survival rate (12,19). In one previous study, the DNA status
of Fusobacterium spp. in pancreatic cancer tissue was
independently associated with the poor prognosis of patients
(20).
However, whether F. nucleatum is present in
other types of gastroenterological cancer, including esophageal,
gastric or liver cancer, has yet to be investigated.
In the present study, quantitative polymerase chain
reaction (qPCR) method was evaluated to determine if it was able to
detect the quantity of F. nucleatum DNA from an oral cavity.
Subsequently, a qPCR assay was also used to analyze whether it
similarly detects the existence of Fusobacterium in
formalin-fixed paraffin-embedded (FFPE) tissues and frozen tissues.
Finally, the quantity of F. nucleatum DNA in 20
paraffin-embedded digestive cancer specimens and 20 matched normal
specimens was evaluated.
Materials and methods
Tissue samples
The test specimens were 20 FFPE tissue samples of
esophageal (squamous cell carcinoma), gastric, colorectal, liver
and pancreatic cancer, and 20 normal matched paraffin embedded
specimens. All specimens were obtained by surgical resection at
Kumamoto University Hospital (Kumamoto, Japan). The sampled
patients were not administered preoperative treatment. A single
pathologist, who was blind to the clinical and molecular data of
the patients, evaluated hematoxylin-eosin-stained tissue sections
of each cancer case and recorded the pathological features. Tumor
staging was conducted as described in the Cancer Staging Manual
(7th edition) published by the American Joint Committee on Cancer
(22). Written informed consent was
obtained from each patient and the present study was approved by
the Institutional Review Board of Kumamoto University Hospital
(Approval no. 1272).
DNA extraction and qPCR for F.
nucleatum DNA content
Genomic DNA in the oral cavity was obtained using a
cotton swab. The patients were not allowed to eat or drink 30 min
prior to sample collection and the cotton swap was scraped against
the inside of each cheek 5–6 times. The collected swab was
air-dried for >2 h. The genomic DNA from the oral cavity was
extracted using QIAamp DNA Mini kit (Qiagen GmbH, Hilden, Germany).
Genomic DNA from the FFPE tissues and from the frozen
gastroenterological cancer tissues was extracted using the QIAamp
DNA FFPE Tissue kit (Qiagen GmbH) and the QIAamp DNA Mini kit
(Qiagen GmbH), respectively. The nusG gene of F.
nucleatum and the reference human gene solute carrier organic
anion transporter family member 2A1 (SLCO2A1) were amplified using
custom-made TaqMan primer/probe sets (Applied Biosystems; Thermo
Fisher Scientific, Inc., Waltham, MA, USA) as previously described
(18). The primer and probe sequences
used for the custom TaqMan Gene Expression assay were as follows:
F. nucleatum forward primer,
5′-TGGTGTCATTCTTCCAAAAATATCA-3′; F. nucleatum reverse
primer, 5′-AGATCAAGAAGGACAAGTTGCTGAA-3′; F. nucleatum FAM
probe, 5′-ACTTTAACTCTACCATGTTCA-3′; SLCO2A1 forward primer,
5′-ATCCCCAAAGCACCTGGTTT-3′; SLCO2A1 reverse primer,
5′-AGAGGCCAAGATAGTCCTGGTAA-3′; SLCO2A1 VIC probe,
5′-CCATCCATGTCCTCATCTC-3′. The PCR mix consisted of 1X TaqMan
Environmental Master Mix 2.0 (Applied Biosystems; Thermo Fisher
Scientific, Inc.), 0.5 pmol forward and reverse primer, 0.1 pmol
probe, nuclease-free water (Invitrogen; Thermo Fisher Scientific,
Inc.) and 12.5 ng genomic DNA in a total volume of 10 µl. Assays
were performed in a 384-well optical PCR plate. The DNA was
amplified and detected with the LightCycler 480 Instrument II
(Roche Diagnostics, Basel, Switzerland) under the following
reaction conditions: Initial denaturation at 95°C for 10 min, 15
sec at 95°C and 60 sec at 60°C. The quantity of F. nucleatum
DNA in each tissue was normalized relative to SLCO2A1 using the
2−ΔΔCq method (where ΔCq is the mean Cq of F.
nucleatum minus the mean Cq of SLCO2A1) (16,23). All
RT-qPCR reactions were performed in triplicate.
Statistical analysis
All statistical analyses were performed by the JMP
program, version 10 (SAS Institute, Inc., Cary, NC, USA). All
P-values were 2-sided. The mean quantity of F. nucleatum DNA
was compared with paired Student's t-tests for variables
with two categories. P<0.05 was considered to indicate a
statistically significant difference.
Results
Literature review
An online search of MEDLINE (PubMed) was performed
for all articles published in the English language. The following
Medical Subject Headings terms were used in combination:
‘Fusobacterium esophageal cancer’, ‘Fusobacterium gastric cancer’,
‘Fusobacterium colorectal cancer’, ‘Fusobacterium hepatocellular
carcinoma’, and ‘Fusobacterium pancreatic cancer’. The latest
search was performed on December 2015. Among them, five studies
which had detection rates of Fusobacterium spp. in cancer tissues
were identified. In total, four previous studies have reported
detectable levels of F. nucleatum in colorectal cancer
tissues (15,16,19,21). The
F. nucleatum detection rate was 13–82% in colorectal tumor
tissue and 3.4–81% in adjacent normal tissue (Table I). A single previous study detected
F. nucleatum in pancreatic cancer (the detection rate was
8.8% in tumor tissue and 28% in adjacent normal tissue) (20). However, the expression status of
Fusobacterium DNA in esophageal, gastric and liver cancer
remains to be elucidated.
Validation of qPCR for F.
nucleatum
A cheek swab from a healthy researcher (Dr Kensuke
Yamamura, Department of Gastroenterology, Kumamoto University,
Kumamoto, Japan) was submitted for genomic DNA determination of the
oral cavity. F. nucleatum and SLCO2A1 in the oral cavity
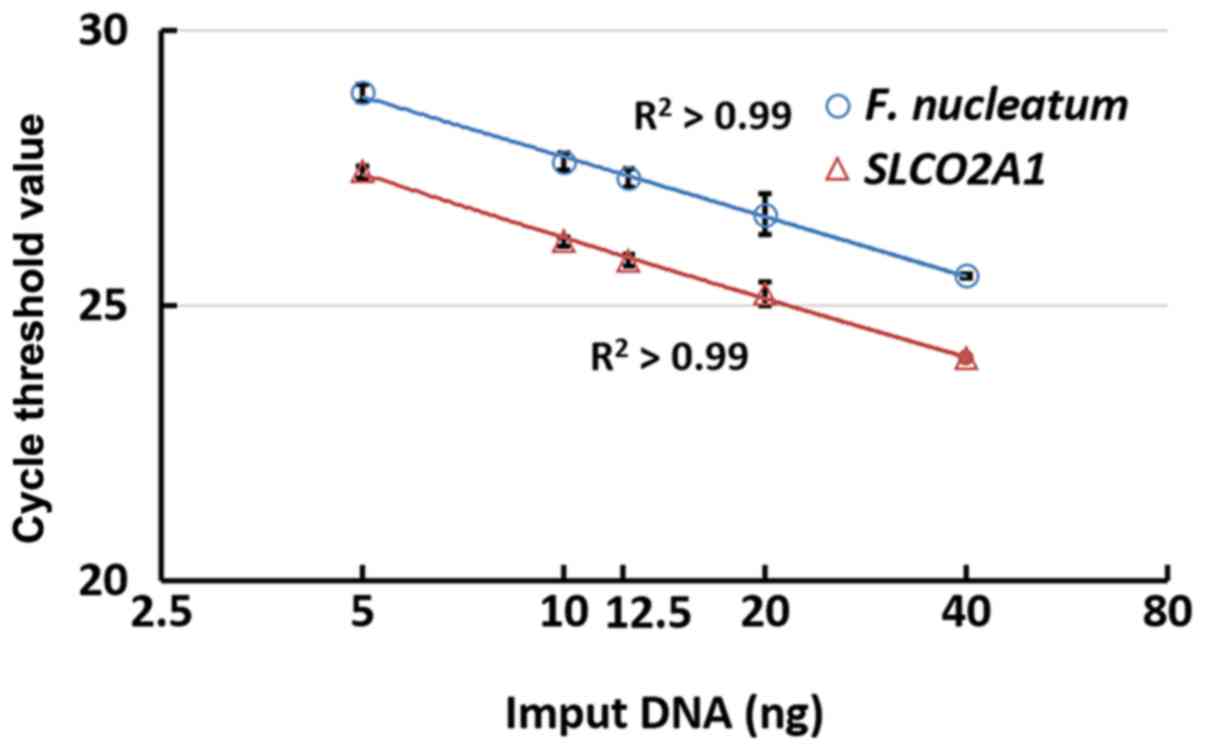
were evaluated using qPCR in a 2-fold dilution series (5, 10, 12.5,
20 and 40 ng). The assays were quantified using the coefficient of
determination (r2) between 5 and 40 ng. In the
qPCR assays of oral F. nucleatum and SLCO2A1, the cycle
threshold (Cq) values linearly decreased with the quantity of input
DNA (on a linear-log scale, r2>0.99; Fig. 1). These results demonstrated that qPCR
has the ability to quantify F. nucleatum DNA in the oral
cavity.
qPCR of F. nucleatum in frozen tissue
and FFPE
F. nucleatum DNA in FFPE and frozen tissues
of 10 esophageal squamous cell carcinoma (ESCC) cases were
investigated. In the 5 tissues that were positive for F.
nucleatum, the organism was also detected in the matched FFPE
tissues. Similarly, in the 5 Fusobacterium-negative ESCC
cases, F. nucleatum was not detected in the matched FFPE
tissue (Table II). Therefore, the
qPCR results were consistent between the frozen tissues and FFPE
tissues. These results suggested that F. nucleatum may be
accurately assayed in FFPE tissues.
 | Table II.Consistency of quantitative
polymerase chain reaction detection of Fusobacterium
nucleatum in tumor FFPEs and frozen tissues of esophageal
cancer. |
Table II.
Consistency of quantitative
polymerase chain reaction detection of Fusobacterium
nucleatum in tumor FFPEs and frozen tissues of esophageal
cancer.
| Variable | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 | Case 8 | Case 9 | Case 10 |
|---|
| FFPE | − | − | − | − | − | + | + | + | + | + |
| Frozen tissue | − | − | − | − | − | + | + | + | + | + |
| Concordance | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes | Yes |
F. nucleatum in gastroenterological
cancer tissue
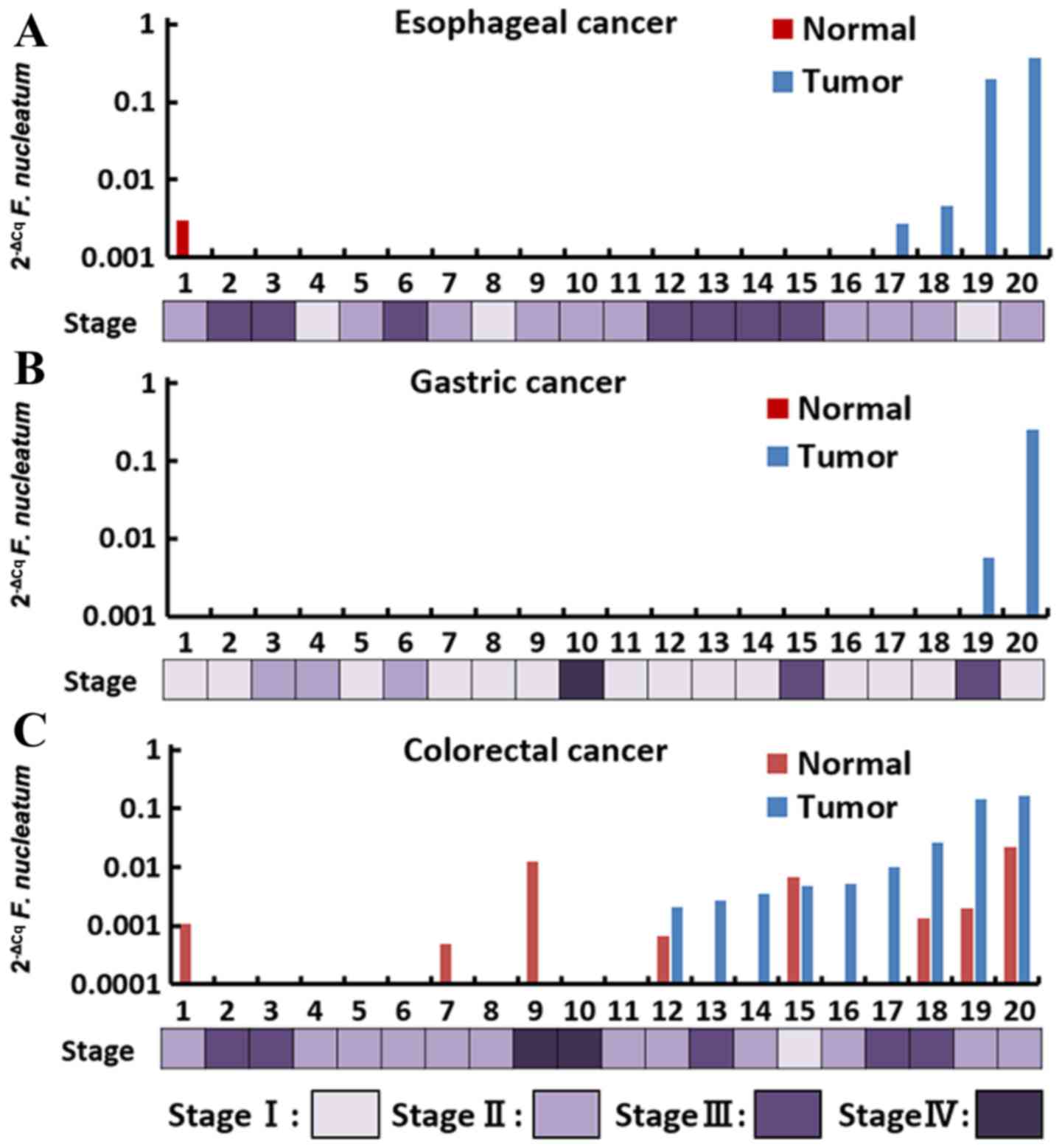
20 FFPE tumors and their adjacent non-tumorous
tissues in each cancer were analyzed using qPCR assays. F.
nucleatum was detected in 4 (20%) cases of esophageal cancer, 2
(10%) cases of gastric cancer and 9 (45%) cases of colorectal
cancer (Fig. 2; Table III). In esophageal and colorectal
cancer, F. nucleatum was also detected in adjacent non-tumor
tissue, whereas F. nucleatum was not detected in the liver
and pancreatic cancer tissues and their adjacent non-tumor tissues.
Among all cancer cases that were positive for F. nucleatum,
the level of F. nucleatum DNA content in esophageal and
colorectal cancer ranged from 2.68×10−3 to
365.2×10−3 (median, 101.3×10−3) and from
2.10×10−3 to 166.7×10−3 (median,
5.08×10−3), respectively.
 | Table III.Quantitative polymerase chain
reaction results of Fusobacterium nucleatum in
gastroenterological cancer and adjacent normal tissues. |
Table III.
Quantitative polymerase chain
reaction results of Fusobacterium nucleatum in
gastroenterological cancer and adjacent normal tissues.
|
|
Fusobacterium detection rate,
% |
|---|
|
|
|
|---|
| Type of cancer | Tumor tissue | Normal tissue | Tumor and normal
tissues |
|---|
| Esophageal
cancer | 20 (4/20) | 5
(1/20) | 0 |
| Gastric cancer | 10 (2/20) | 0 | 0 |
| Colorectal
cancer | 45 (9/20) | 40 (8/20) | 25 (5/20) |
| Liver cancer | 0 | 0 | 0 |
| Pancreatic
cancer | 0 | 0 | 0 |
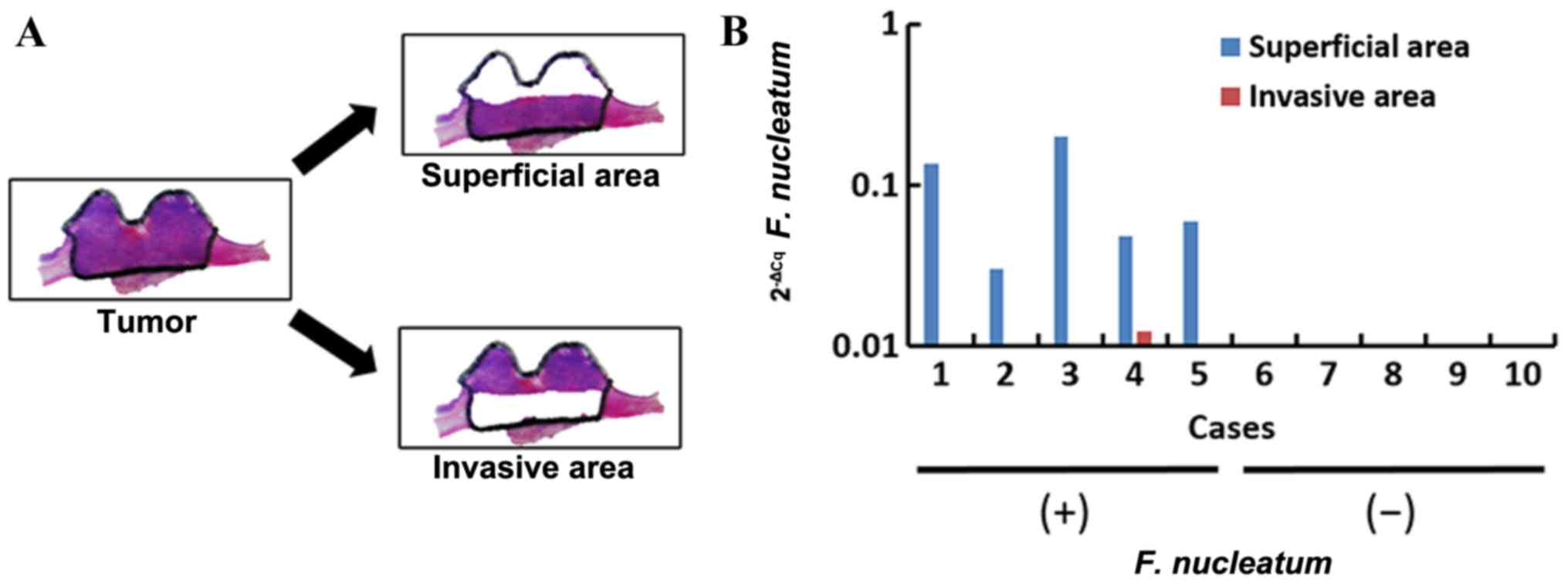
Heterogeneity of F. nucleatum in
esophageal cancer tissue
To evaluate the heterogeneity of the F.
nucleatum DNA within tumor tissues, the F. nucleatum DNA
in the superficial and invasive areas of the 5 esophageal cancer
tissues that were positive for F. nucleatum were evaluated
(Fig. 3A). High levels of
Fusobacterium nucleatum DNA was observed in superficial
areas, but low levels were observed in invasive areas. In the
superficial areas, the quantity of F. nucleatum DNA ranged
from 30.1×10−3 to 200.3×10−3, whereas in
invasive areas it was 12.4×10−3 at its highest (P=0.02;
Fig. 3B). Therefore, the F.
nucleatum DNA may distribute heterogeneously within a single
tumor.
Discussion
F. nucleatum has received increased
recognition as an opportunistic pathogen in periodontal diseases,
but also in human cancer. As the microbiome has a number of
important effects on the functions of the human body, the clinical
significance of the discovery of F. nucleatum cannot be
overemphasized. To the best of our knowledge, the present study has
reported the first detection of F. nucleatum DNA in
esophageal, gastric and liver cancer tissues. The present study has
demonstrated that the qPCR assay may reliably detect F.
nucleatum DNA from oral swabs, as F. nucleatum is among
the most prevalent species in the oral cavity (1,2,24). The association between cycle threshold
and input DNA in the qPCR assay of F. nucleatum was linear
(r2>0.99). Furthermore, the FFPE and frozen
tissues prepared from the same esophageal tumor yielded a similar
level of detection accuracy. Typically, the fixation process
chemically alters the nucleic acids in a sample by inducing
covalent DNA cross-linking and fragmentation. These alterations may
reduce the efficacy of analysis using PCR and DNA sequencing
methods (25,26). In the present study, the results of
the FFPE and frozen tissues were concordant, which suggested that
in the two types of tissue preparations, qPCR accurately detected
F. nucleatum DNA in gastroenterological cancer tissues.
F. nucleatum DNA was successfully detected in
gastrointestinal tract cancer tissues (esophageal, gastric and
colorectal cancer), but F. nucleatum was not detected in
pancreatic and liver cancer tissues. In previous studies, the
detection rates of F. nucleatum were 13–82% in colorectal
cancer (15,16,19,21) and
8.8% in pancreatic cancer (20).
These previous studies are concordant with the data from the
present study that uses colorectal cancer tissues, but these
results contradict the pancreatic cancer results in the current
study. Although F. nucleatum is part of the normal flora of
the oropharyngeal and gastrointestinal tracts, F. nucleatum
also expresses FadA, a bacterial cell surface adhesion protein that
activates the WNT signaling pathway in colorectal cancer cells, and
consequently promotes tumor growth (14). Therefore, it may reasonably be
expected that the detection rate of F. nucleatum is higher
in gastroenterological cancer compared with liver and pancreatic
cancer. However, the presence of F. nucleatum in esophageal
and gastric cancer tissues remains to be investigated.
In addition, the F. nucleatum expression
levels in superficial and invasive areas of esophageal cancer
tissues were compared, and an increased level was observed in
superficial areas. This result suggested that F. nucleatum
may not be able to infiltrate into the invasive area and may only
contribute to the tumor growth through the side of the
gastrointestinal tract. As the differential distribution of F.
nucleatum has not been previously reported, the low level of
F. nucleatum in invasive areas remains to be fully
elucidated. The involvement of F. nucleatum in tumor growth
requires further investigation.
In conclusion, F. nucleatum DNA was detected
in esophageal, gastric and colorectal cancer, but not in pancreatic
and liver cancer. This suggested that F. nucleatum may be
associated with the progression of gastroenterological tract
cancer, but not the progression of pancreatic and liver cancer.
Acknowledgements
The present study was supported in part by 27th SGH
Foundation.
References
|
1
|
Griffen AL, Beall CJ, Campbell JH,
Firestone ND, Kumar PS, Yang ZK, Podar M and Leys EJ: Distinct and
complex bacterial profiles in human periodontitis and health
revealed by 16S pyrosequencing. ISME J. 6:1176–1185. 2012.
View Article : Google Scholar : PubMed/NCBI
|
|
2
|
Loozen G, Ozcelik O, Boon N, De Mol A,
Schoen C, Quirynen M and Teughels W: Inter-bacterial correlations
in subgingival biofilms: A large-scale survey. J Clin Periodontol.
41:1–10. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
3
|
Feng X, Zhang L, Xu L, Meng H, Lu R, Chen
Z, Shi D and Wang X: Detection of eight periodontal microorganisms
and distribution of Porphyromonas gingivalis fimA genotypes in
Chinese patients with aggressive periodontitis. J Periodontol.
85:150–159. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
4
|
Liu P, Liu Y, Wang J, Guo Y, Zhang Y and
Xiao S: Detection of fusobacterium nucleatum and fadA adhesin gene
in patients with orthodontic gingivitis and non-orthodontic
periodontal inflammation. PLoS One. 9:e852802014. View Article : Google Scholar : PubMed/NCBI
|
|
5
|
Ohkusa T, Okayasu I, Ogihara T, Morita K,
Ogawa M and Sato N: Induction of experimental ulcerative colitis by
Fusobacterium varium isolated from colonic mucosa of patients with
ulcerative colitis. Gut. 52:79–83. 2003. View Article : Google Scholar : PubMed/NCBI
|
|
6
|
Minami M, Ando T, Okamoto A, Sasaki N,
Ohkura T, Torii K, Hasegawa T, Ohta M and Goto H: Seroprevalence of
Fusobacterium varium in ulcerative colitis patients in Japan. FEMS
Immunol Med Microbiol. 56:67–72. 2009. View Article : Google Scholar : PubMed/NCBI
|
|
7
|
Tahara T, Shibata T, Kawamura T, Okubo M,
Ichikawa Y, Sumi K, Miyata M, Ishizuka T, Nakamura M, Nagasaka M,
et al: Fusobacterium detected in colonic biopsy and
clinicopathological features of ulcerative colitis in Japan. Dig
Dis Sci. 60:205–210. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
8
|
Strauss J, Kaplan GG, Beck PL, Rioux K,
Panaccione R, Devinney R, Lynch T and Allen-Vercoe E: Invasive
potential of gut mucosa-derived Fusobacterium nucleatum positively
correlates with IBD status of the host. Inflamm Bowel Dis.
17:1971–1978. 2011. View Article : Google Scholar : PubMed/NCBI
|
|
9
|
Song YG, Shim SG, Kim KM, Lee DH, Kim DS,
Choi SH, Song JY, Kang HL, Baik SC, Lee WK, et al: Profiling of the
bacteria responsible for pyogenic liver abscess by 16S rRNA gene
pyrosequencing. J Microbiol. 52:504–509. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
10
|
Yoneda M, Kato S, Mawatari H, Kirikoshi H,
Imajo K, Fujita K, Endo H, Takahashi H, Inamori M, Kobayashi N, et
al: Liver abscess caused by periodontal bacterial infection with
Fusobacterium necrophorum. Hepatol Res. 41:194–196. 2011.
View Article : Google Scholar : PubMed/NCBI
|
|
11
|
Bohrer JC, Kamemoto LE, Almeida PG and
Ogasawara KK: Acute chorioamnionitis at term caused by the oral
pathogen Fusobacterium nucleatum. Hawaii J Med Public Health.
71:280–281. 2012.PubMed/NCBI
|
|
12
|
Castellarin M, Warren RL, Freeman JD,
Dreolini L, Krzywinski M, Strauss J, Barnes R, Watson P,
Allen-Vercoe E, Moore RA, et al: Fusobacterium nucleatum infection
is prevalent in human colorectal carcinoma. Genome Res. 22:299–306.
2012. View Article : Google Scholar : PubMed/NCBI
|
|
13
|
Kostic AD, Gevers D, Pedamallu CS, Michaud
M, Duke F, Earl AM, Ojesina AI, Jung J, Bass AJ, Tabernero J, et
al: Genomic analysis identifies association of Fusobacterium with
colorectal carcinoma. Genome Res. 22:292–298. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
14
|
Rubinstein MR, Wang X, Liu W, Hao Y, Cai G
and Han YW: Fusobacterium nucleatum promotes colorectal
carcinogenesis by modulating E-cadherin/beta-catenin signaling via
its FadA adhesin. Cell Host Microbe. 14:195–206. 2013. View Article : Google Scholar : PubMed/NCBI
|
|
15
|
Tahara T, Yamamoto E, Suzuki H, Maruyama
R, Chung W, Garriga J, Jelinek J, Yamano HO, Sugai T, An B, et al:
Fusobacterium in colonic flora and molecular features of colorectal
carcinoma. Cancer Res. 74:1311–1318. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
16
|
Mima K, Sukawa Y, Nishihara R, Qian ZR,
Yamauchi M, Inamura K, Kim SA, Masuda A, Nowak JA, Nosho K, et al:
Fusobacterium nucleatum and T cells in colorectal carcinoma. JAMA
Oncol. 1:653–661. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
17
|
Flanagan L, Schmid J, Ebert M, Soucek P,
Kunicka T, Liska V, Bruha J, Neary P, Dezeeuw N, Tommasino M, et
al: Fusobacterium nucleatum associates with stages of colorectal
neoplasia development, colorectal cancer and disease outcome. Eur J
Clin Microbiol Infect Dis. 33:1381–1390. 2014. View Article : Google Scholar : PubMed/NCBI
|
|
18
|
Mima K, Nishihara R, Qian ZR, Cao Y,
Sukawa Y, Nowak JA, Yang J, Dou R, Masugi Y, Song M, et al:
Fusobacterium nucleatum in colorectal carcinoma tissue and patient
prognosis. Gut. 65:1973–1980. 2016. View Article : Google Scholar : PubMed/NCBI
|
|
19
|
Ito M, Kanno S, Nosho K, Sukawa Y,
Mitsuhashi K, Kurihara H, Igarashi H, Takahashi T, Tachibana M,
Takahashi H, et al: Association of Fusobacterium nucleatum with
clinical and molecular features in colorectal serrated pathway. Int
J Cancer. 137:1258–1268. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
20
|
Mitsuhashi K, Nosho K, Sukawa Y, Matsunaga
Y, Ito M, Kurihara H, Kanno S, Igarashi H, Naito T, Adachi Y, et
al: Association of Fusobacterium species in pancreatic cancer
tissues with molecular features and prognosis. Oncotarget.
6:7209–7220. 2015. View Article : Google Scholar : PubMed/NCBI
|
|
21
|
Viljoen KS, Dakshinamurthy A, Goldberg P
and Blackburn JM: Quantitative profiling of colorectal
cancer-associated bacteria reveals associations between
fusobacterium spp., enterotoxigenic Bacteroides fragilis (ETBF) and
clinicopathological features of colorectal cancer. PLoS One.
10:e01194622015. View Article : Google Scholar : PubMed/NCBI
|
|
22
|
Compton CC, Byrd DR, Garcia-Aguilar J,
Kurtzman SH, Olawaiye A and Washington MK: The AJCC cancer staging
atlas. 2nd edition. Springer; New York, NY: 2012, View Article : Google Scholar
|
|
23
|
Livak KJ and Schmittgen TD: Analysis of
relative gene expression data using real-time quantitative PCR and
the 2-ΔΔCT method. Methods. 25:402–408. 2001. View Article : Google Scholar : PubMed/NCBI
|
|
24
|
Field CA, Gidley MD, Preshaw PM and
Jakubovics N: Investigation and quantification of key periodontal
pathogens in patients with type 2 diabetes. J Periodontal Res.
47:470–478. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
25
|
Do H and Dobrovic A: Dramatic reduction of
sequence artefacts from DNA isolated from formalin-fixed cancer
biopsies by treatment with uracil-DNA glycosylase. Oncotarget.
3:546–558. 2012. View Article : Google Scholar : PubMed/NCBI
|
|
26
|
Sah S, Chen L, Houghton J, Kemppainen J,
Marko AC, Zeigler R and Latham GJ: Functional DNA quantification
guides accurate next-generation sequencing mutation detection in
formalin-fixed, paraffin-embedded tumor biopsies. Genome Med.
5:772013. View
Article : Google Scholar : PubMed/NCBI
|